Ultrasonic Measurements on β Cyclodextrin/Hydroxyapatite Composites for Potential Water Depollution
Abstract
:1. Introduction
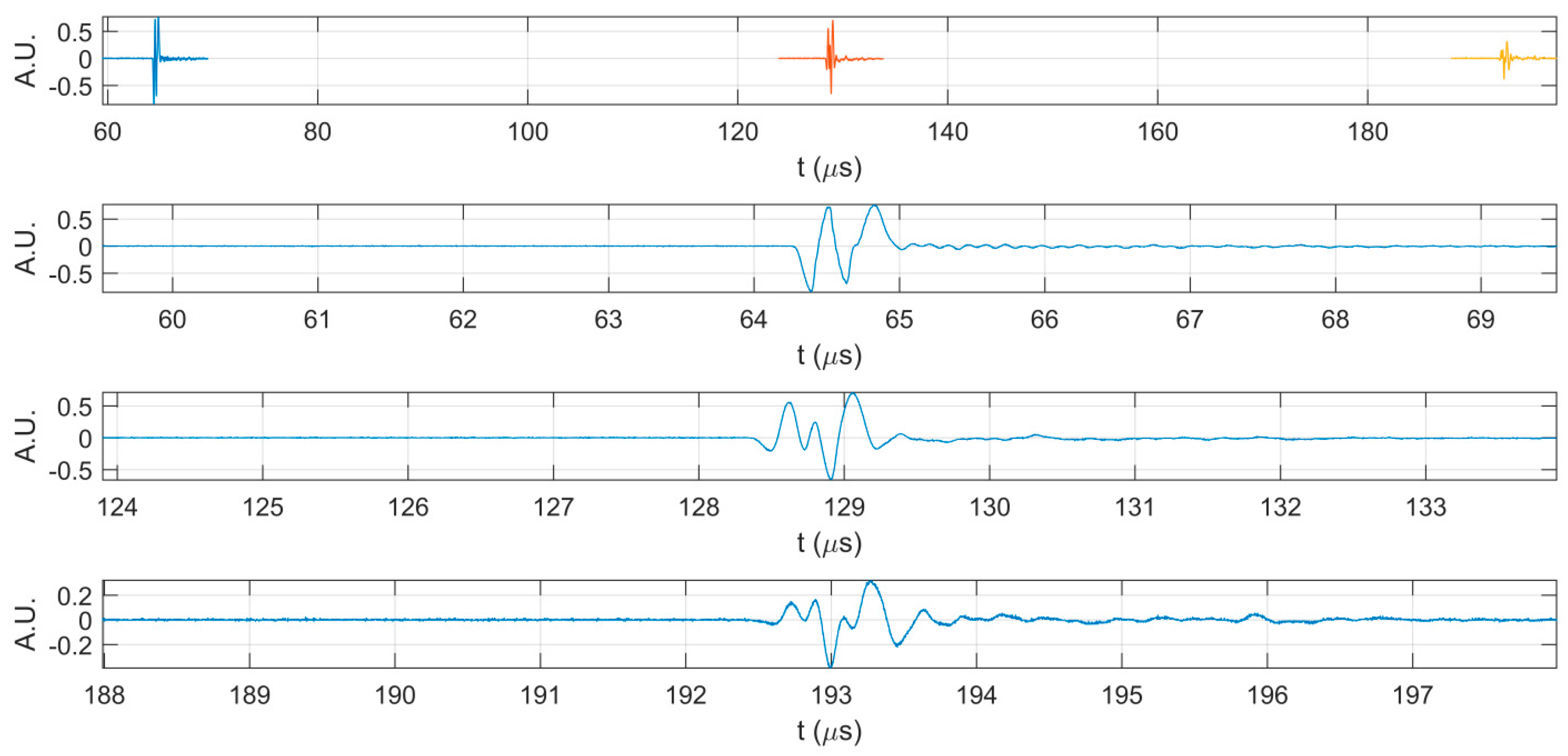
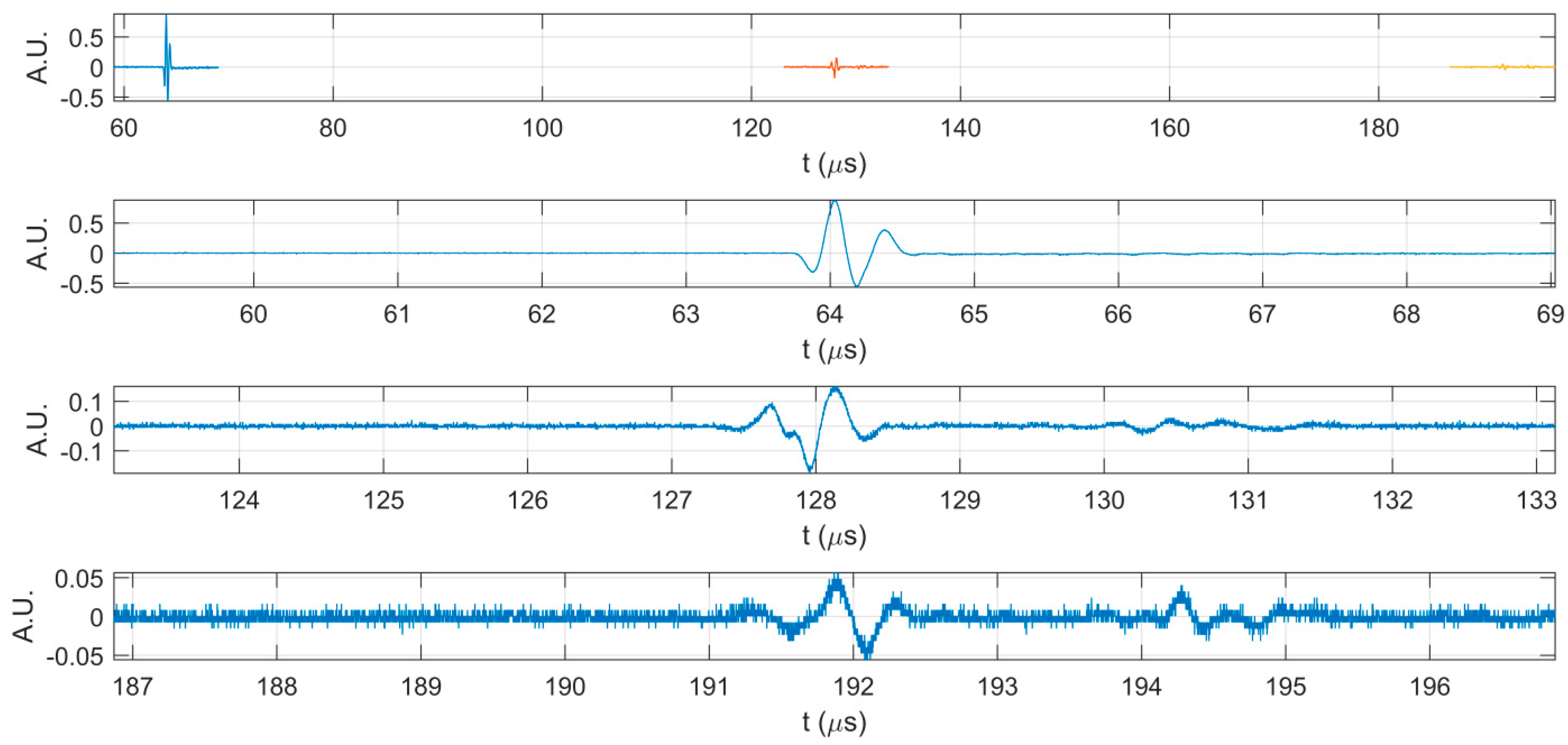
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of β-Cyclodextrin/Hydroxyapatite (CD-HAp) Composites
4.3. Adsorption Experiments
4.4. Physico-Chemical Characterization
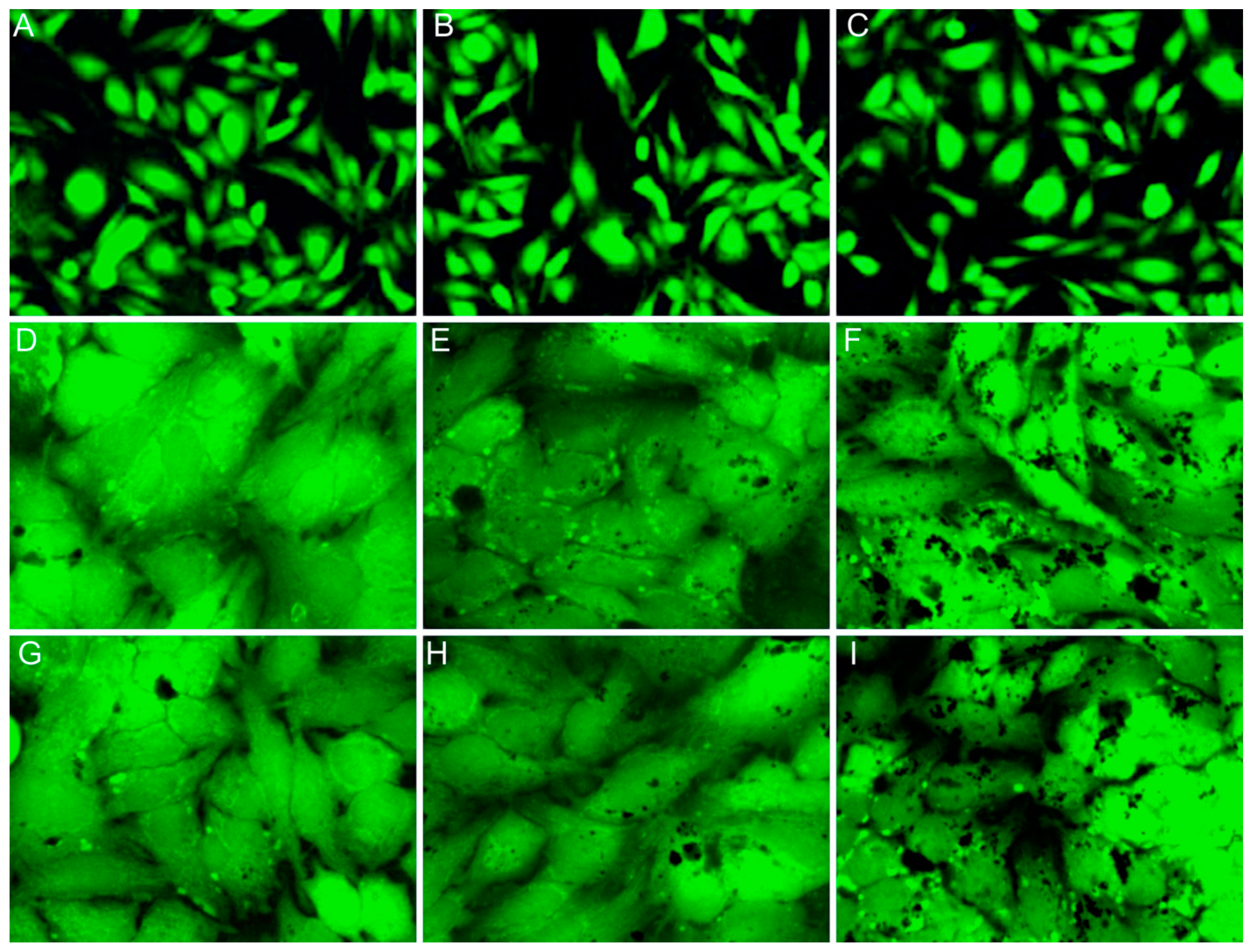
4.5. Effect of CD-HAp Nanocomposites on HeLa Cells
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fierascu, I.; Fierascu, R.C.; Popa, O.; Babeanu, N. Synthesized materials for decontamination of heavy metals polluted aqueous solutions. Rom. Biotech. Lett. 2014, 19, 9196–9202. [Google Scholar]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, 43, L327/1.
- Khraisheh, M.A.M.; Al-degs, Y.S.; Mcminn, W.A.M. Remediation of wastewater containing heavy metals using raw and modified diatomite. Chem. Eng. J. 2004, 99, 177–184. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Altunkaynak, Y.; Güzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Alemayehu, E.; Lennartz, B. Adsorptive removal of nickel from water using volcanic rocks. Appl. Geochem. 2010, 25, 1596–1602. [Google Scholar] [CrossRef]
- Dabrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Mok, S. Biomass-derived materials in the remediation of heavy-metal contaminated water: Removal of Cadmium(II) and Copper(II) from aqueous solutions. Water Environ. Res. 2011, 83, 874–881. [Google Scholar]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Mihalcea, B.M. Semiclassical dynamics for an ion confined within a nonlinear electromagnetic trap. Phys. Scr. 2011, 2011, T143. [Google Scholar] [CrossRef]
- Zamani, S.; Salahi, E.; Mobasherpour, I. Removal of Nickel from aqueous solution by nano hydroxyapatite originated from Persian Gulf corals. Can. Chem. Trans. 2013, 1, 173–190. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Costescu, A.; Motelica-Heino, M.; Predoi, D. Porous Methyltrimethoxysilane Coated Nanoscale-Hydroxyapatite for Removing Lead Ions from Aqueous Solutions. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.A.; García-Montelongo, J.; Garcia Casillas, P.E.; Farias-Mancilla, J.R.; Monreal Romero, H. Preparation of hydroxyapatite nanoparticles facilitated by the presence of β-cyclodextrin. J. Alloys Compd. 2012, 536S, S432–S436. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Motelica-Heino, M.; Predoi, D. Evaluation of samarium doped hydroxyapatite, ceramics for medical application: Antimicrobial activity. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Chapon, P.; Le Coustumer, P.; Predoi, D. Antimicrobial activity of thin solid films of silver doped hydroxyapatite prepared by sol-gel method. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Costescu, A.; Ciobanu, C.S.; Iconaru, S.L.; Ghita, R.V.; Chifiriuc, C.M.; Marutescu, L.G.; Predoi, D. Fabrication, Characterization, and Antimicrobial Activity, Evaluation of Low Silver Concentrations in Silver-Doped Hydroxyapatite Nanoparticles. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on Europium-Doped Hydroxyapatite Nanoparticles by Fourier Transform Infrared Spectroscopy and Their Antimicrobial Properties. J. Spectrosc. 2013, 2013. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Sm:HAp Nanopowders Present Antibacterial Activity against Enterococcus faecalis. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Predoi, D. A study on iron oxide nanoparticles coated with dextrin obtained by coprecipitation. Dig. J. Nanomater. Biostruct. 2007, 2, 169–173. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Popa, C.L.; Albu, M.; Bartha, C.; Costescu, A.; Luculescu, C.; Trusca, R.; Antohe, S. Structural characterization and optical properties of hydroxyapatite/collagen matrix. Rom. Rep. Phys. 2016, 68, 1149–1158. [Google Scholar]
- Predoi, D. Physico-chemical studies of sucrose thin films. Dig. J. Nanomater. Bios. 2010, 5, 373–377. [Google Scholar]
- Mihalcea, B.M. Nonlinear harmonic boson oscillator. Phys. Scr. 2010, 2010, T140. [Google Scholar] [CrossRef]
- Raita, S.; Cornila, N.; Danacu, V.; Belu, C.; Georgescu, B.; Rosu, P.; Barbuceanu, F. Morphological studies on the liver instruthio camelus. Anat. Histol. Embryol. 2014, 43, 74–75. [Google Scholar]
- Mihalcea, B.M. Quantum parametric oscillator in a radiofrequency trap. Phys. Scr. 2009, 2009, T135. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Girod-Fullana, S.; Charvillat, C.; Ternet-Fontebasso, H.; Dufour, P.; Dexpert-Ghys, J.; Santran, V.; Bordère, J.; Pipy, B.; Bernad, J.; et al. Biomimetic nanocrystalline apatites: Emerging perspectives in cancer diagnosis and treatment. Int. J. Pharm. 2012, 423, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Yu, R.; Yao, J.; Mao, D.; Xing, C.; Wang, D. Removal of Cd2+ from aqueous solutions by hydroxyapatite. Catal. Today 2008, 139, 94–99. [Google Scholar] [CrossRef]
- Milonjić, S.K. Comments on “factors influencing the removal of divalent cations by hydroxyapatite”. J. Hazard. Mater. 2009, 162, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Taki, T.; Sato, T. Sorption of dissolved lead from shooting range soils using hydroxyapatite amendments synthesized from industrial byproducts as affected by varying pH conditions. J. Environ. Manag. 2009, 90, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Turculet, C.; Le Coustumer, P.; Bleotu, C.; Chifiriuc, M.C.; Lazar, V.; Surugiu, A.; Badea, M.; Iordache, F.M.; Prodan, A.M. Biological studies on dextrin coated iron oxide nanoparticles. Rom. Rep. Phys. 2016, 68, 1536–1544. [Google Scholar]
- Raileanu, M.; Crisan, M.; Petrache, C.; Crisan, D.; Jitanu, A.; Zaharescu, M.; Predoi, D.; Kuncser, V.; Filoti, G. Sol-Gel FexOy-SiO2 Nanocomposites. Rom. J. Phys. 2005, 50, 595–606. [Google Scholar]
- Radulescu, C.; Stihi, C.; Dulama, I.D.; Chelarescu, E.D.; Bretcan, P.; Tanislav, D. Assessment of Heavy Metals Content in Water and Mud of Several Salt Lakes from Romania by Atomic Absorption Spectrometry. Rom. J. Phys. 2015, 60, 246–256. [Google Scholar]
- Kyoung, D.S.; Young-Jin, K. Morphological structure and characteristics of hydroxyapatite/β-cyclodextrin composite nanoparticles synthesized at different conditions. Mat. Sci. Eng. C 2013, 33, 499–506. [Google Scholar]
- Liu, H.; Liu, C.; Yang, X.; Zeng, S.; Xiong, Y.; Xu, W. Uniformly sized β-cyclodextrin molecularly imprinted microspheres prepared by a novel surface imprinting technique for ursolic acid. Anal. Chim. Acta 2008, 628, 87–94. [Google Scholar] [CrossRef]
- Sunita, P.V.; Chandra, P.S. Development and evaluation of cyclodextrin complexed hydroxyapatite nanoparticles for preferential albumin adsorption. Colloids Surf. B 2011, 85, 221–228. [Google Scholar] [CrossRef]
- McClements, D.J. Ultrasonic Measurements in Particle Size Analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Prodan, A.M.; Le Coustumer, P.; Predoi, D. Synthesis and Antibacterial and Antibiofilm Activity of Iron Oxide Glycerol Nanoparticles Obtained by Coprecipitation Method. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron Oxide Magnetic Nanoparticles: Characterization and Toxicity Evaluation by In Vitro and In Vivo Assays. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Bonini, M.; Rossi, S.; Karlsson, G.; Almgren, M.; Lo Nostro, P.; Baglioni, P. Selfassembly of—Cyclodextrin in water. Part 1: Cryo-TEM and dynamic and static light scattering. Langmuir 2006, 22, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Lucio, D.; Irache, J.M.; Font, M.; Martínez-Ohárriz, M.C. Nanoaggregation of inclusion complexes of glibenclamide with cyclodextrins. Int. J. Pharm. 2017, 519, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, T.; Liu, Z.; Guo, Y.; Li, C.; Dong, C.; Shuang, S. An ultra-sensitive sensor based on β-cyclodextrin modified magnetic graphene oxide for detection of tryptophan. J. Electroanal. Chem. 2016, 781, 363–370. [Google Scholar] [CrossRef]
- Jun, Y.W.; Huh, Y.-M.; Choi, J.-S.; Lee, J.-H.; Song, H.-T.; Kim, S.; Yoon, S.; Kim, K.-S.; Shin, J.-S.; Suh, J.-S.; et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, N.; Pons, J.N.; Roger, J.; Bee, A. Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J. Colloid Interface Sci. 1997, 194, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Adjadj, L.P.; Hipp, A.K.; Storti, G.; Morbidelli, M. Characterization of dispersions by ultrasound spectroscopy. In Proceedings of the 5th International Symposium on Ultrasonic Doppler Methods for Fluid Mechanics and Fluid Engineering, ETH Zurich, Switzerland, 12–14 September 2006; pp. 9–13. [Google Scholar]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Cho, D.W.; Jeon, B.H.; Chon, C.M.; Kim, Y.; Schwartz, F.W.; Lee, E.S.; Song, H. A novel chitosan/clay/magnetite composite for adsorption of Cu(II) and As(V). Chem. Eng. J. 2012, 200–202, 654–662. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Colloid and Capillary Chemistry; Metheum: London, UK, 1926; p. 993. [Google Scholar]
- Ishra, P.C.; Patel, R.K. Removal of lead and zinc ions from water by low cost adsorbents. J. Hazard. Mater. 2009, 168, 319–325. [Google Scholar] [CrossRef]
- Fernández-Nava, Y.; Ulmanu, M.; Anger, I.; Marañón, E.; Castrillón, L. Use of Granular Bentonite in the Removal of Mercury (II), Cadmium (II) and Lead (II) from Aqueous Solutions. Water Air Soil Pollut. 2011, 215, 239–249. [Google Scholar] [CrossRef]
- Vázquez, I.; Rodríguez-Iglesias, J.; Marñón, E.; Castrillon, L.; Álvarez, M. Removal of residual phenols from coke wastewater by adsorption. J. Hazard. Mater. 2007, 147, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.G.; Flippen, R.B. Particle Size Analysis. Anal. Chem. 1995, 67, 257R–272R. [Google Scholar] [CrossRef]
- Loh, X.J.; Wu, Y.-L.; Joseph Seow, W.T.; Irzuan Norimzan, M.N.; Zhang, Z.-X.; Xu, F.-J.; Kang, E.-T.; Neoh, K.-G.; Li, J. Micellization and phase transition behavior of thermosensitive poly(N-isopropylacrylamide)–poly(ɛ-caprolactone)–poly(N-isopropylacrylamide) triblock copolymers. Polymer 2008, 49, 5084–5094. [Google Scholar] [CrossRef]
- Li, K.G.; Chen, J.T.; Bai, S.S.; Wen, X.; Song, S.Y.; Yu, Q.; Li, J.; Wang, Y.Q. Intracellular oxidative stress and cadmium ions release induce cytotoxicity of unmodified cadmium sulfide quantum dots. Toxicol. In Vitro 2009, 23, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Oubrahim, H.; Stadtman, E.R.; Chock, P.B. Mitochondria play no roles in Mn(II)- induced apoptosis in HeLa cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9505–9510. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Adsorptive removal of Pb2+, Co2+ and Ni2+ by hydroxyapatite/chitosan composite from aqueous solution. J. Taiwan Inst. Chem. E 2012, 43, 125–131. [Google Scholar] [CrossRef]
- Lee, Y.J.; Elzinga, E.J.; Reeder, R.J. Sorption mechanisms of zinc on hydroxyapatite: Systematic uptake studies and EXAFS spectroscopy analysis. Environ. Sci. Technol. 2005, 39, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, L.Q.; Rhue, D.R.; Appel, C.S. Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ. Pollut. 2004, 131, 435–444. [Google Scholar] [CrossRef] [PubMed]
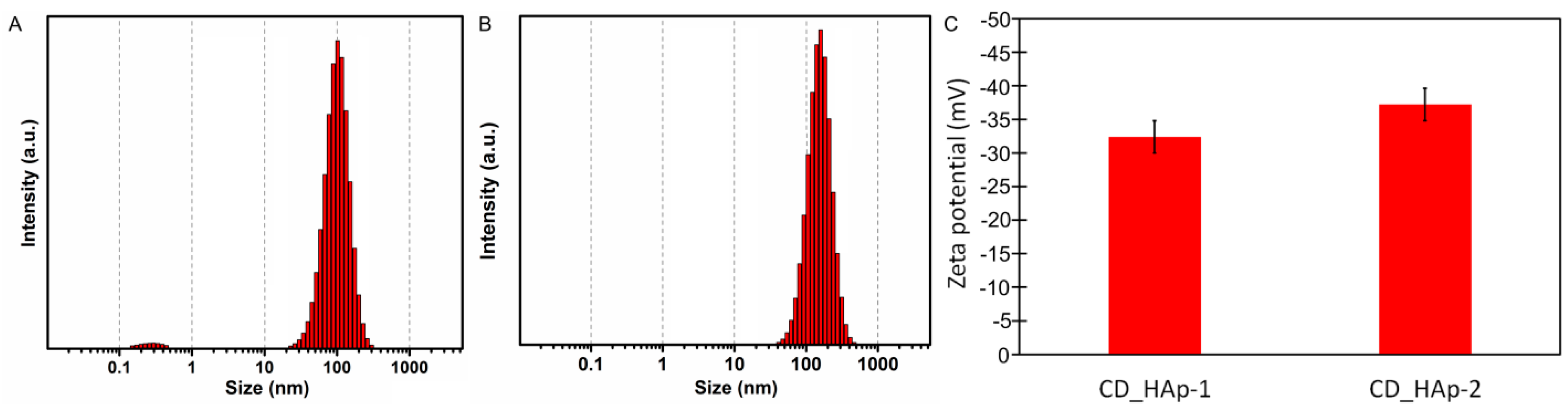

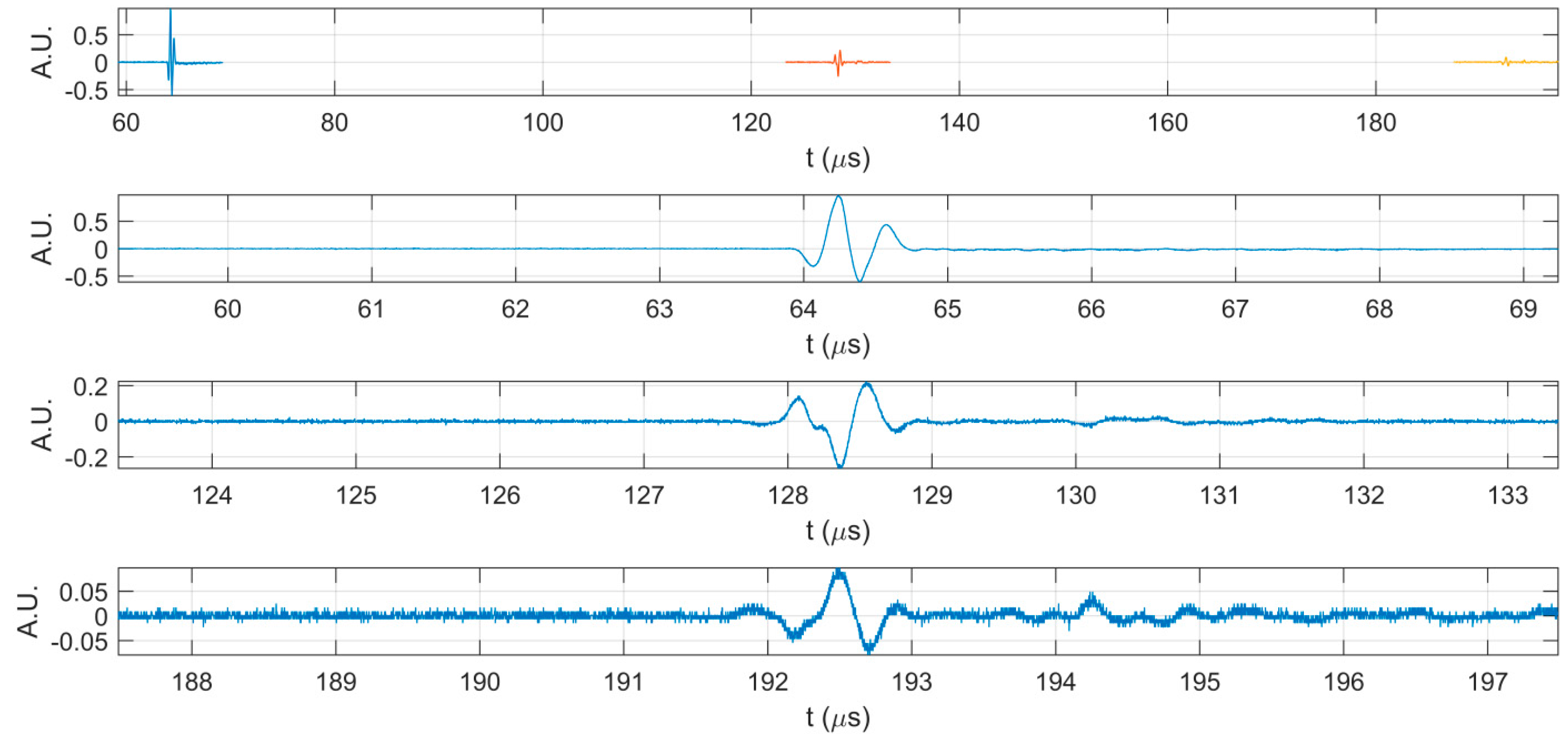
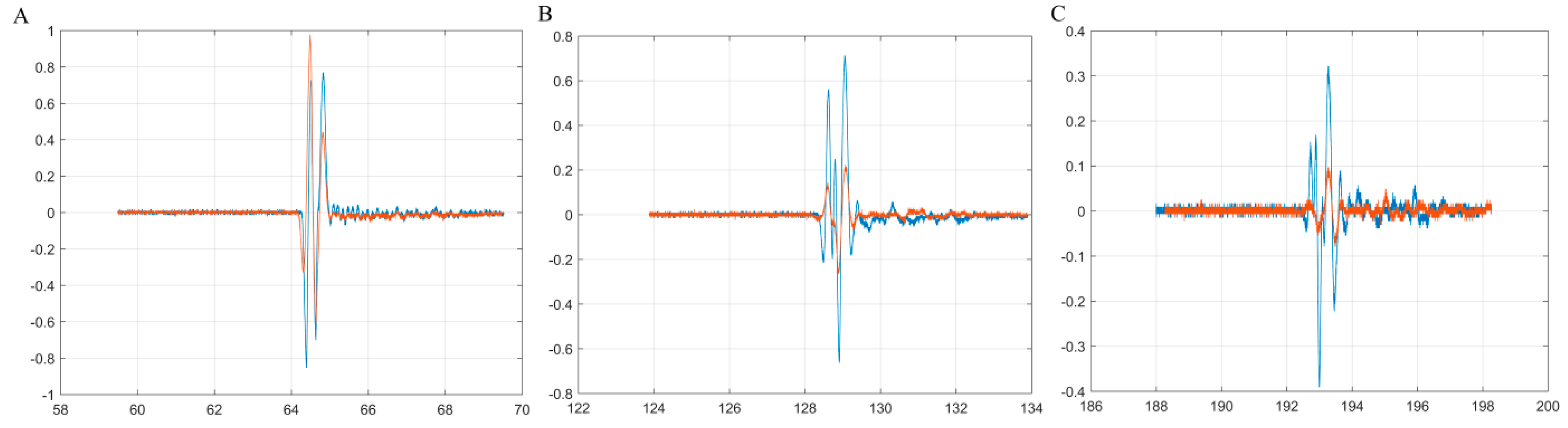

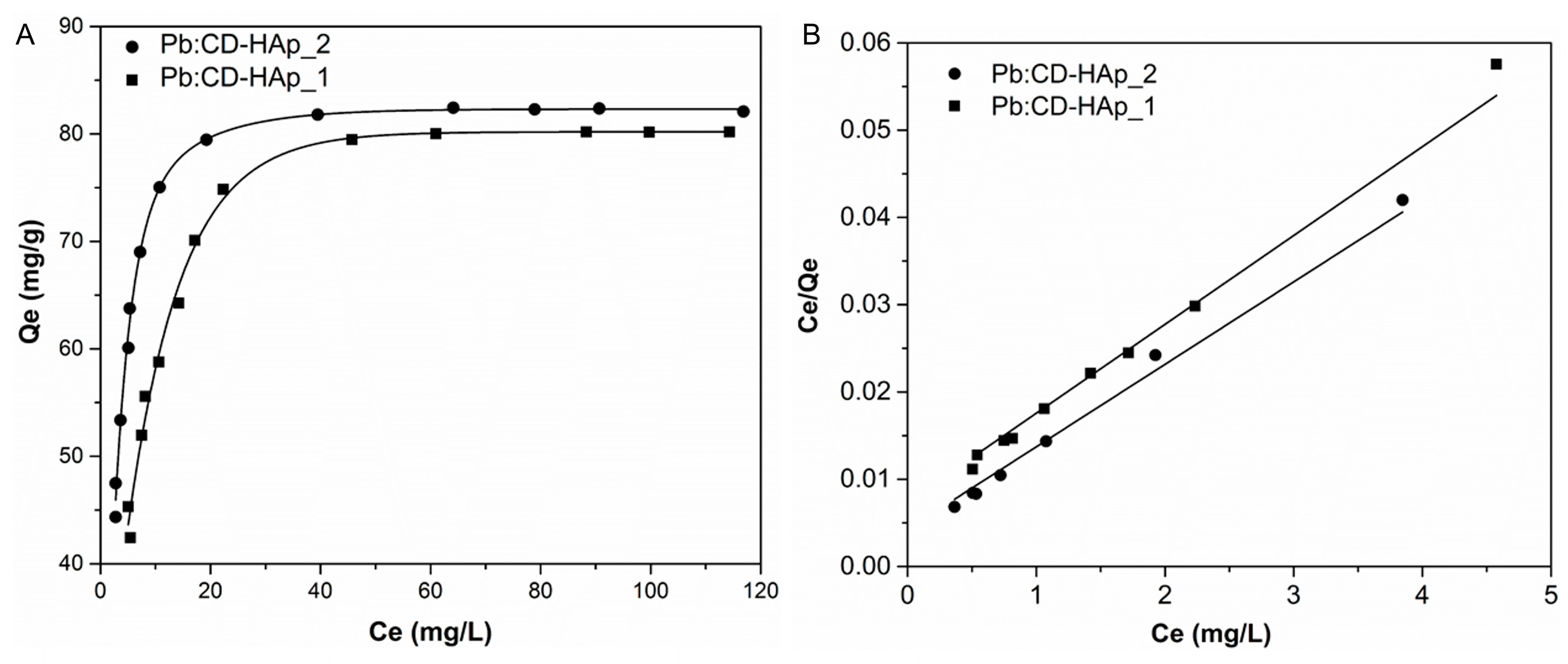


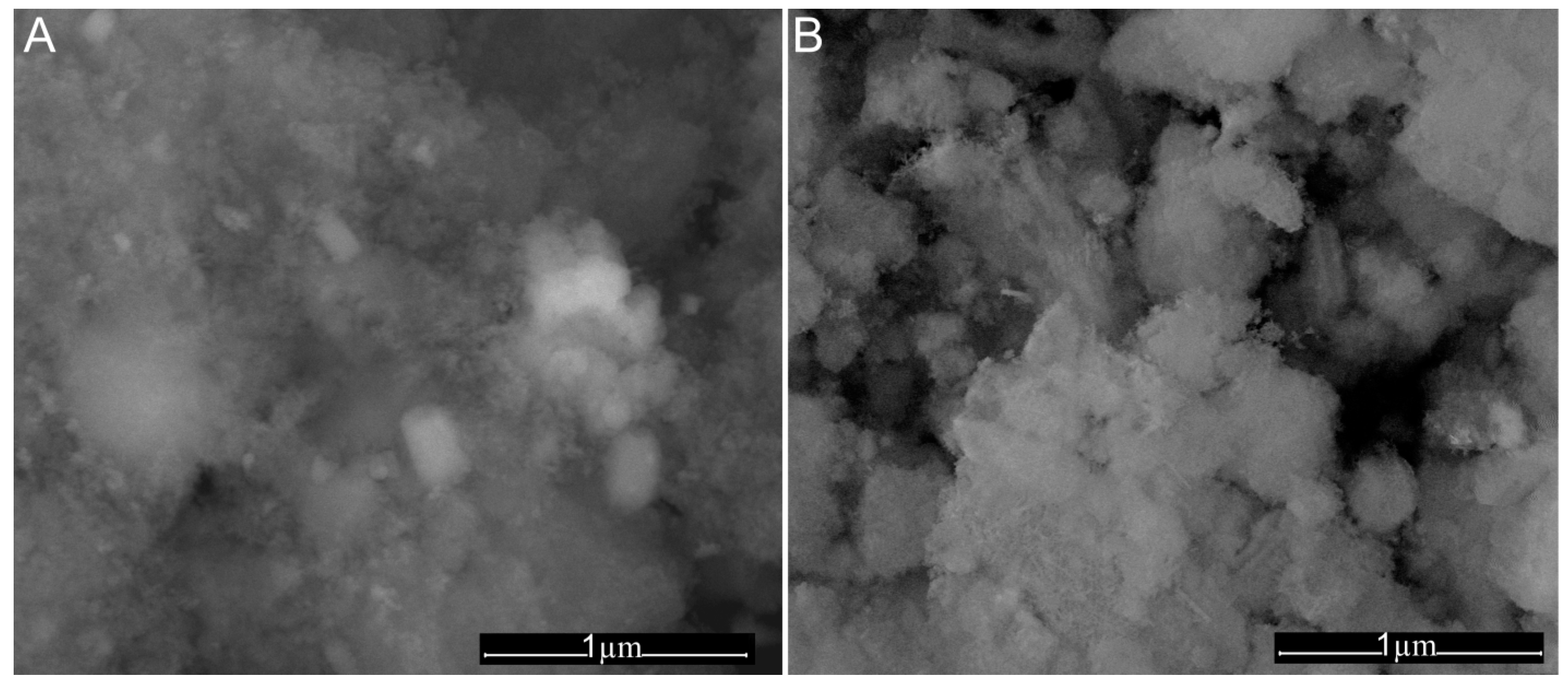
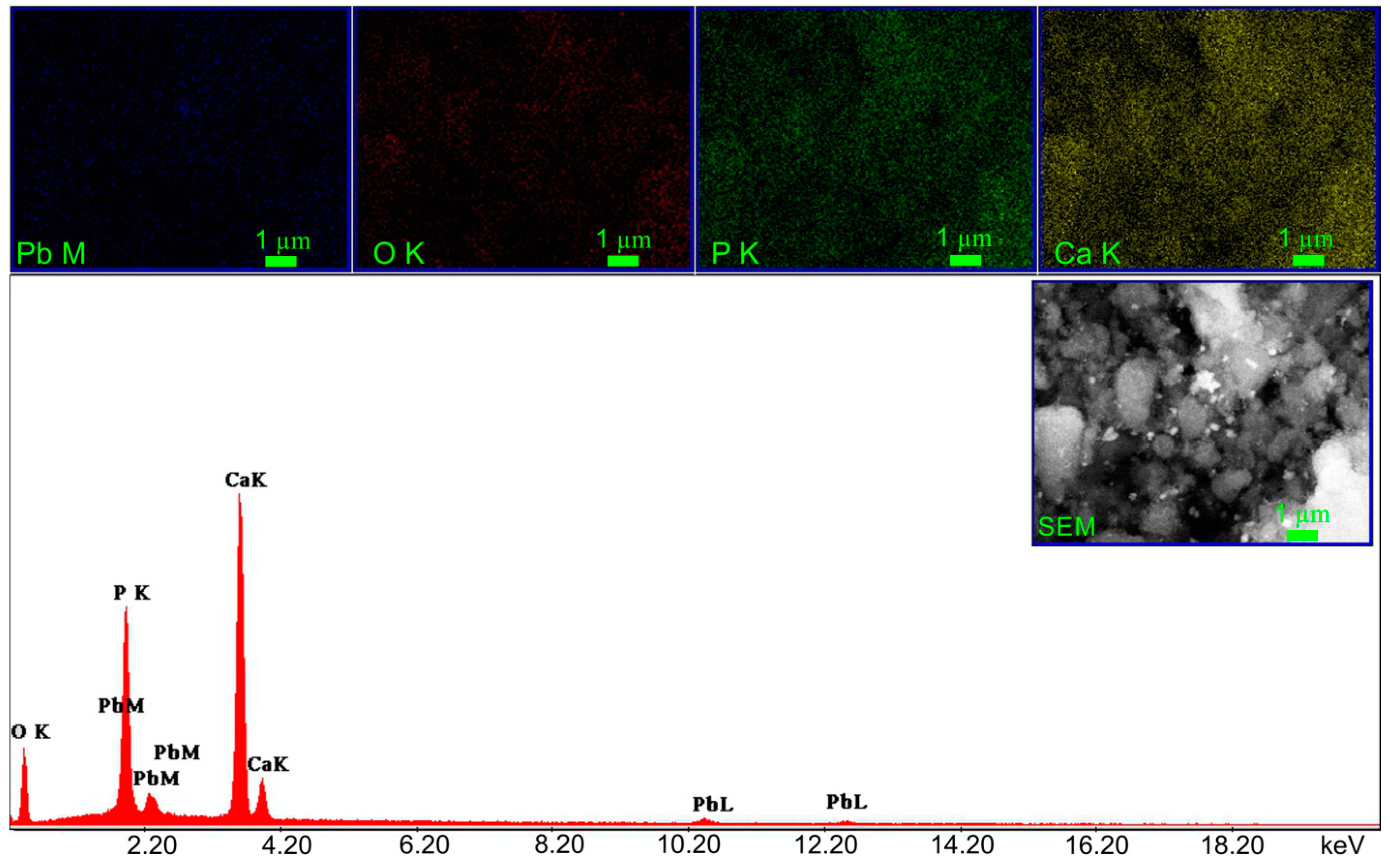


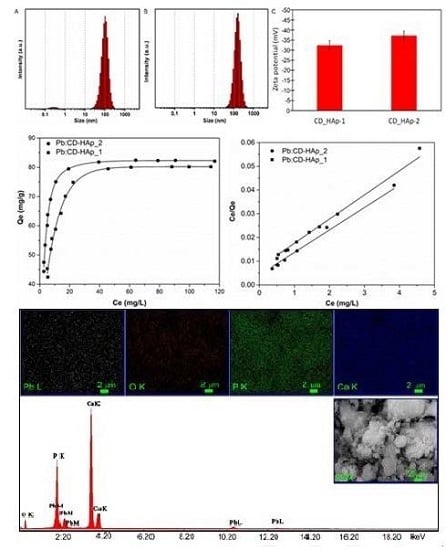
| Sample | SBET (m2/g) | Vp (nm) | Dp (cm3/g) |
|---|---|---|---|
| CD-HAp_1 | 102.54 | 0.3958 | 14.26 |
| CD-HAp_2 | 155.71 | 0.4568 | 12.49 |
| Pollutant | Sample | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | n | 1/n | KF (mg/g (K/mg)1/n) | R2 | ||
| Pb2+ | CD-HAp_1 | 98.232 | 1.378 | 0.99 | 3.088 | 0.324 | 57.971 | 0.981 |
| CD-HAp_2 | 105.820 | 2.218 | 0.992 | 2.917 | 0.343 | 75.733 | 0.984 | |
| Sample | US (m2/g) | SEM (nm) | DLS (nm) |
|---|---|---|---|
| CD-HAp_1 | 23.5 ± 2 | 20.8 ± 1 | 97.7 ± 4 |
| CD-HAp_2 | 16.8 ± 4 | 15.2 ± 2 | 163 ± 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Predoi, M.V.; Iconaru, S.L.; Ech Cherif El Kettani, M.; Leduc, D.; Prodan, A.M. Ultrasonic Measurements on β Cyclodextrin/Hydroxyapatite Composites for Potential Water Depollution. Materials 2017, 10, 681. https://doi.org/10.3390/ma10060681
Predoi D, Predoi MV, Iconaru SL, Ech Cherif El Kettani M, Leduc D, Prodan AM. Ultrasonic Measurements on β Cyclodextrin/Hydroxyapatite Composites for Potential Water Depollution. Materials. 2017; 10(6):681. https://doi.org/10.3390/ma10060681
Chicago/Turabian StylePredoi, Daniela, Mihai Valentin Predoi, Simona Liliana Iconaru, Moncef Ech Cherif El Kettani, Damien Leduc, and Alina Mihaela Prodan. 2017. "Ultrasonic Measurements on β Cyclodextrin/Hydroxyapatite Composites for Potential Water Depollution" Materials 10, no. 6: 681. https://doi.org/10.3390/ma10060681