Controlling Morphology and Aggregation in Semiconducting Polymers: The Role of Solvents on Lasing Emission in Poly[2-methoxy-5-(2′-ethyl-hexyloxy)-1,4-phenylene-vinylene]
Abstract
:1. Introduction
2. Materials and Methods
2.1. Semiconducting Layer Preparation
2.2. Waveguide Structure Fabrication
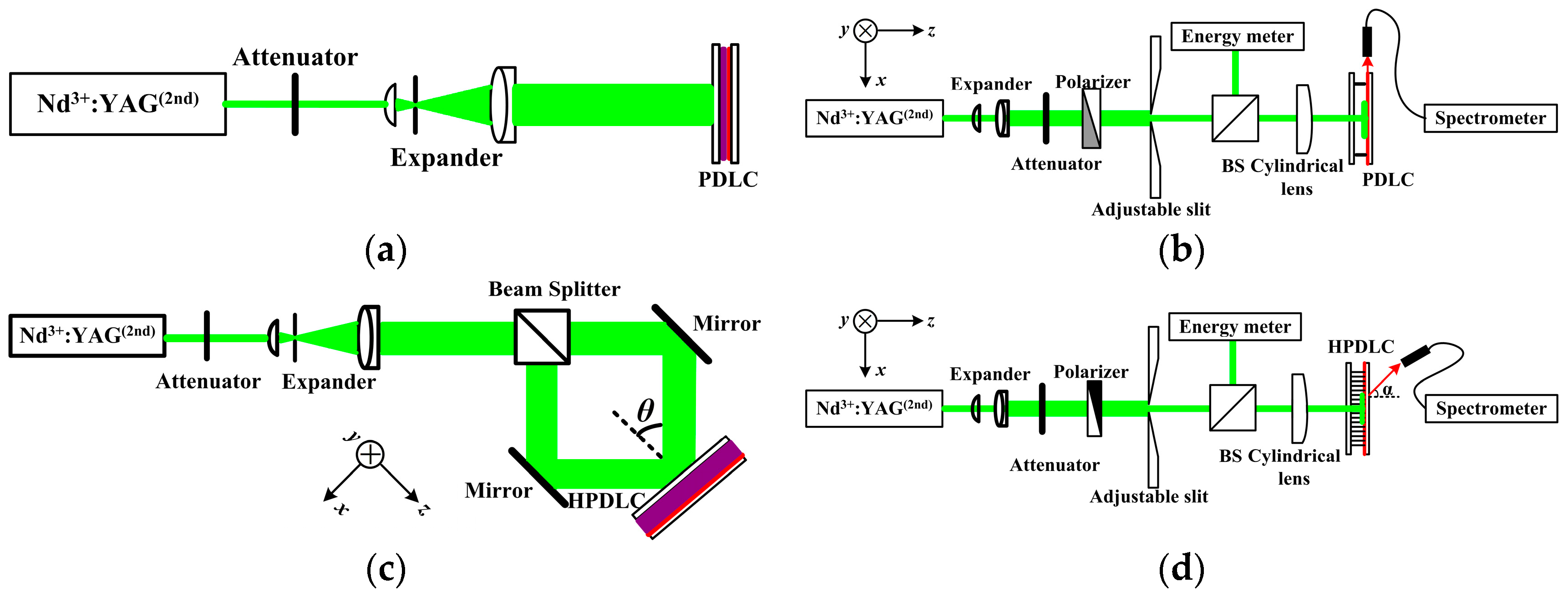
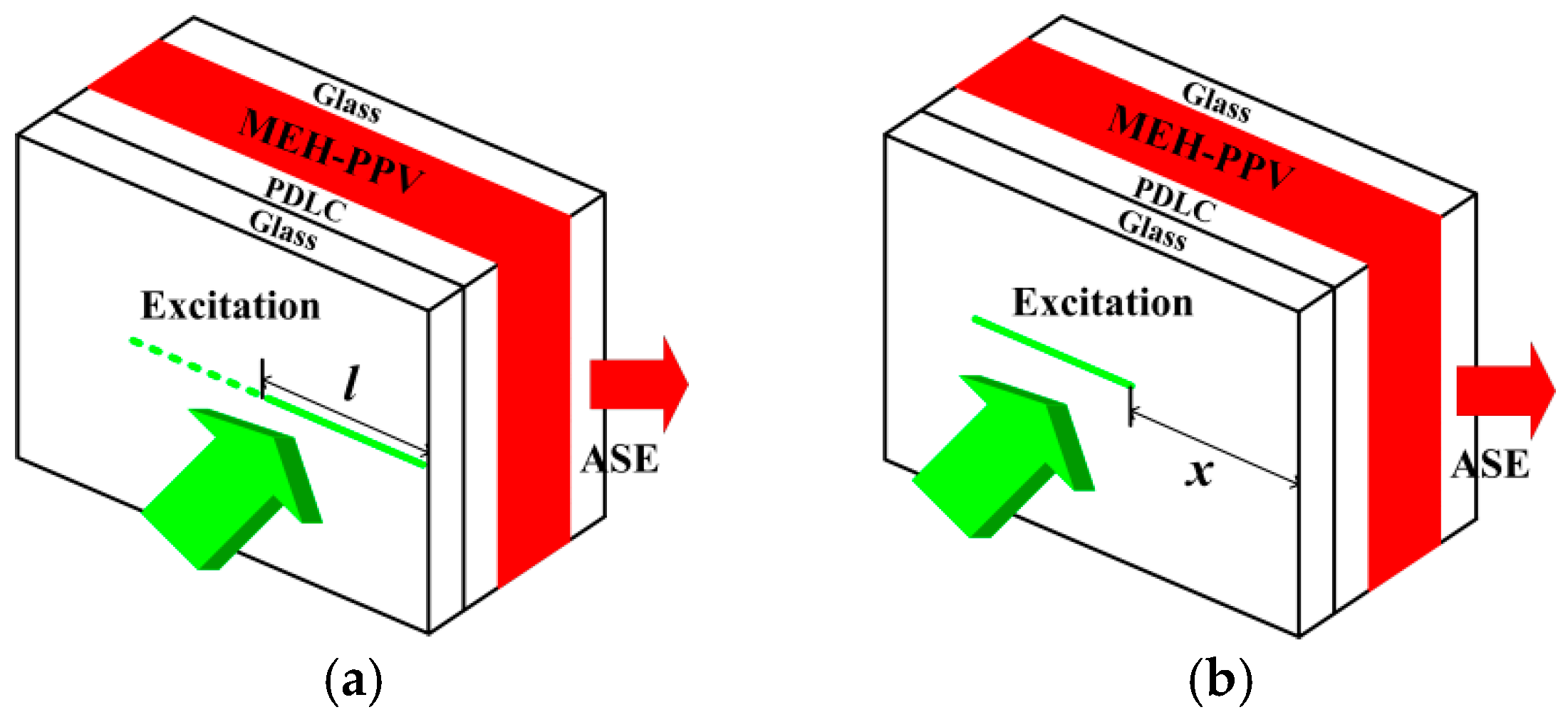
2.3. ASE Characterization
2.4. Laser Fabrication and Characterization
3. Results and Discussion
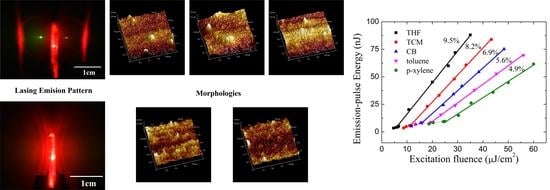
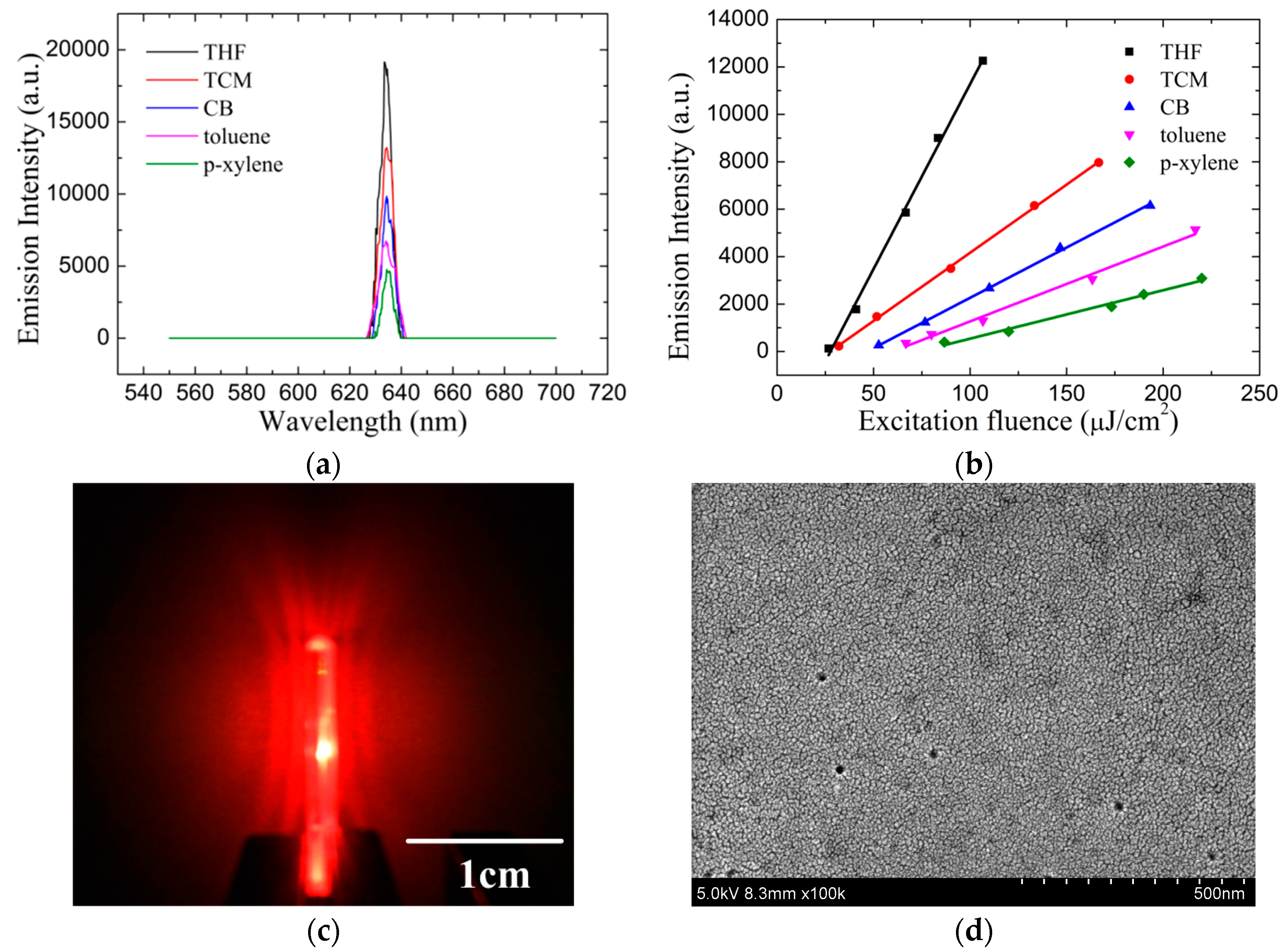
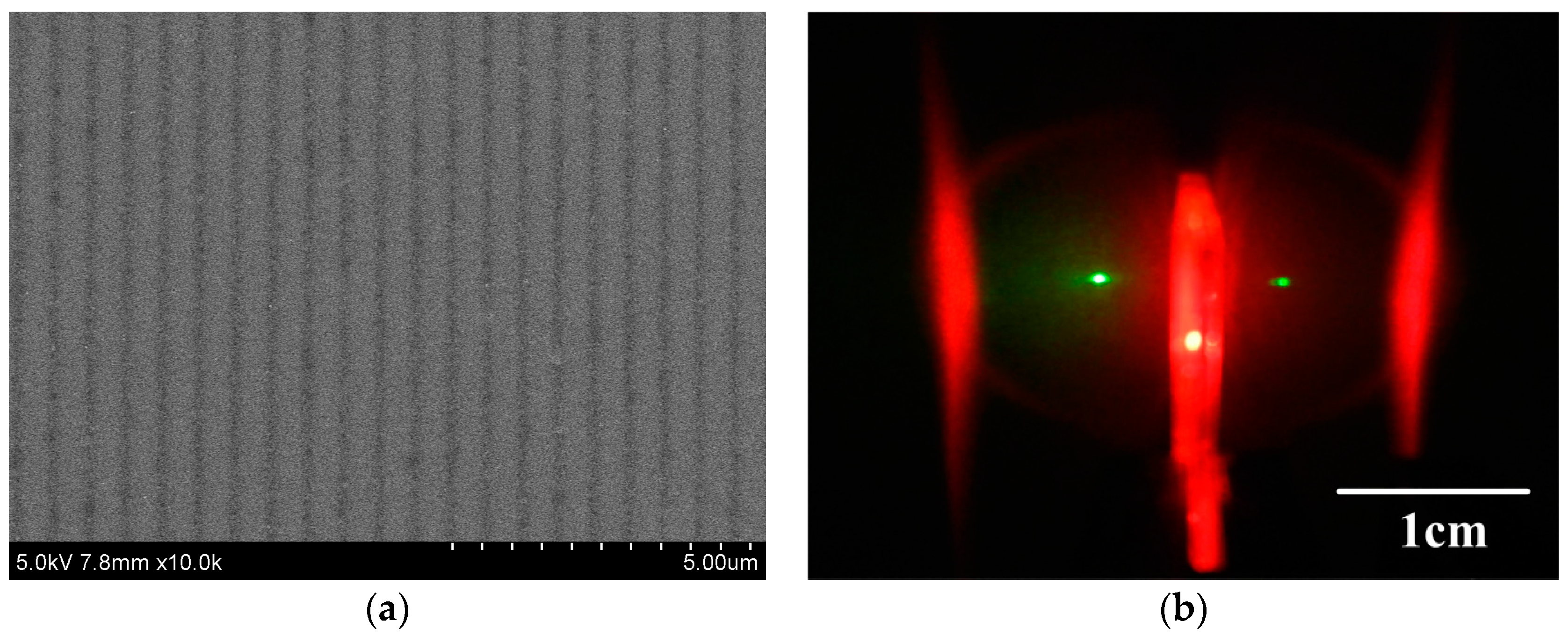
3.1. Film Morphologies
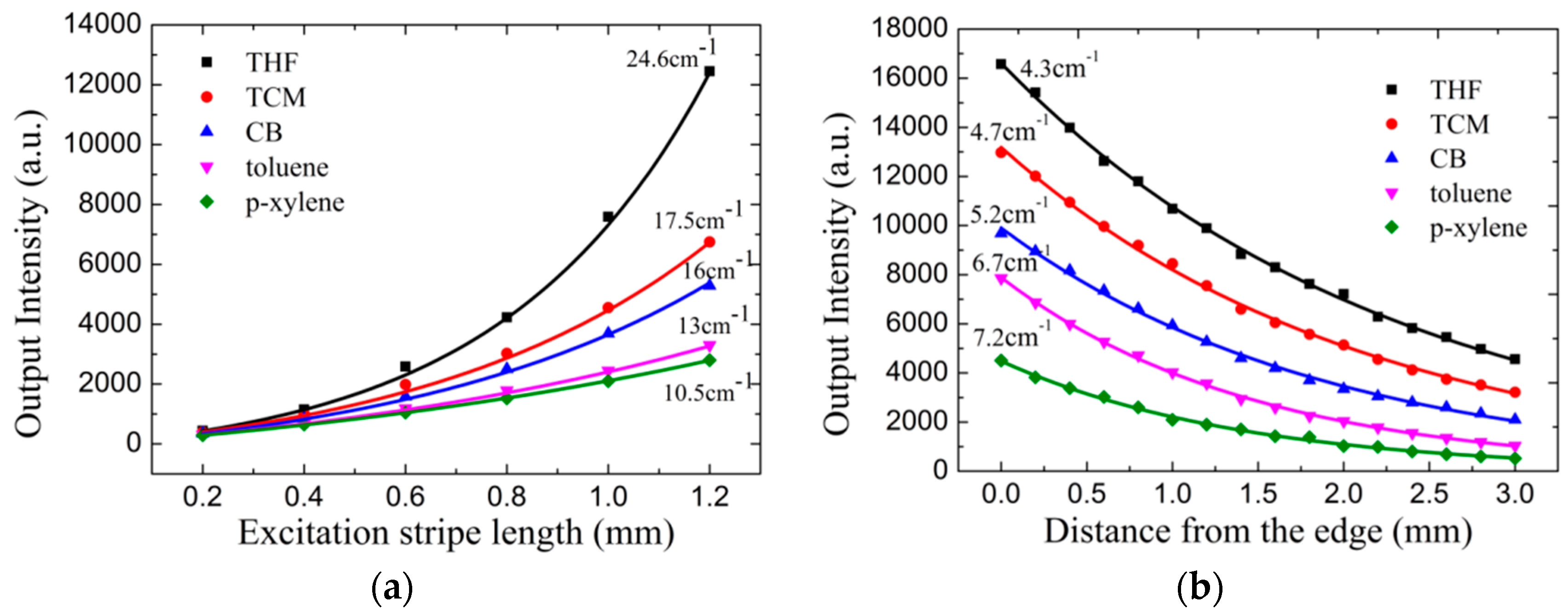
3.2. Film ASE Characterization
3.3. Optical Gain and Losses
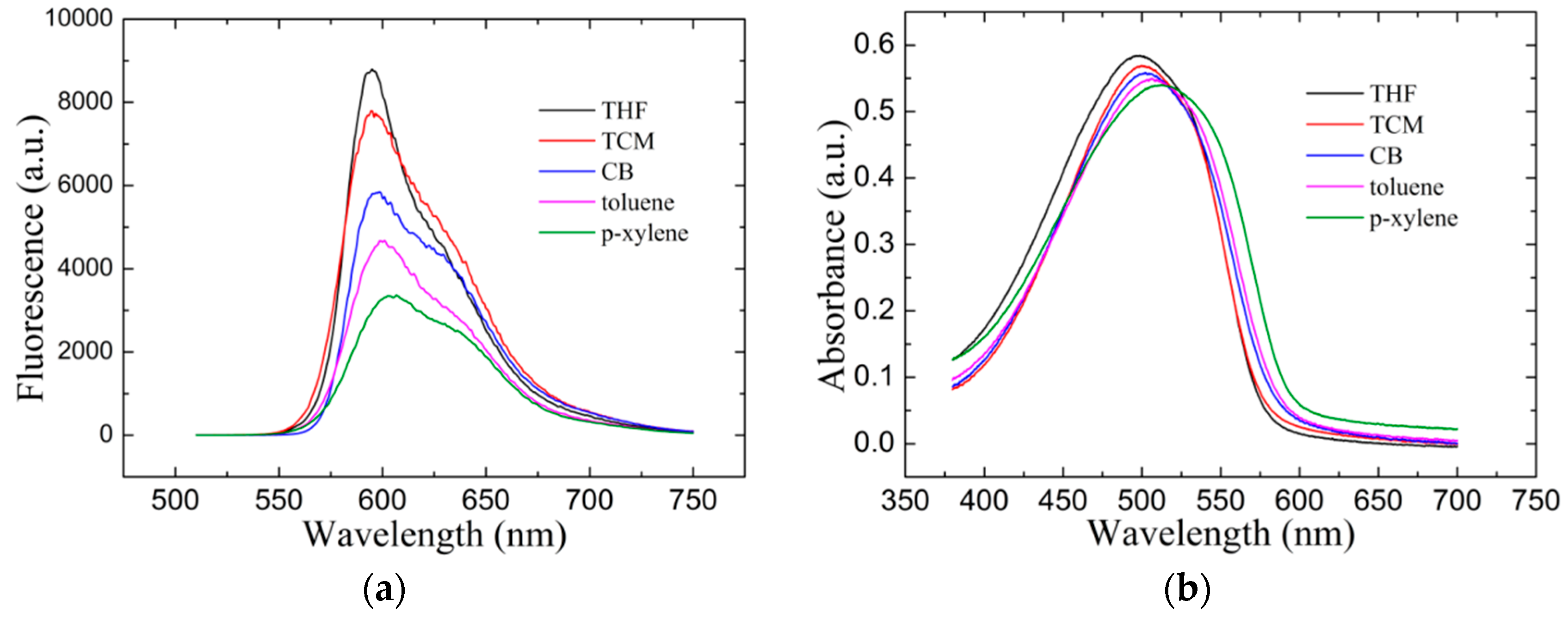
3.4. Pure Film Spectra Characterization
3.5. Lasing Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chenais, S.; Forget, S. Recent advances in solid-state organic lasers. Polym. Int. 2012, 61, 390–406. [Google Scholar] [CrossRef]
- Grivas, C.; Pollnau, M. Organic solid-state integrated amplifiers and lasers. Laser Photonics Rev. 2012, 6, 419–462. [Google Scholar] [CrossRef]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments on Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [PubMed]
- Hide, F.; DiazGarcia, M.A.; Schwartz, B.J.; Andersson, M.R.; Pei, Q.B.; Heeger, A.J. Semiconducting polymers: A new class of solid-state laser materials. Science 1996, 273, 1833–1836. [Google Scholar] [CrossRef]
- Mhibik, O.; Forget, S.; Ott, D.; Venus, G.; Divliansky, I.; Glebov, L.; Chenais, S. An ultra-narrow linewidth solution-processed organic laser. Light Sci. Appl. 2016, 5, e16206. [Google Scholar] [CrossRef]
- Wang, Y.; Morawska, P.O.; Kanibolotsky, A.L.; Skabara, P.J.; Turnbull, G.A.; Samuel, I.D.W. LED pumped polymer laser sensor for explosives. Laser Photonics Rev. 2013, 7, L71–L76. [Google Scholar] [CrossRef] [PubMed]
- Vannahme, C.; Dufva, M.; Kristensen, A. High frame rate multi-resonance imaging refractometry with distributed feedback dye laser sensor. Light Sci. Appl. 2015, 4, e269. [Google Scholar] [CrossRef]
- Fang, Y.R.; Sun, M.T. Nanoplasmonic waveguides: Towards applications in integrated nanophotonic circuits. Light Sci. Appl. 2015, 4, e294. [Google Scholar] [CrossRef]
- Huser, T.; Yan, M. Solvent-related conformational changes and aggregation of conjugated polymers studied by single molecule fluorescence spectroscopy. J. Photochem. Photobiol. A 2001, 144, 43–51. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Yee, R.Y.; Schwartz, B.J. Solution processing of conjugated polymers: The effects of polymer solubility on the morphology and electronic properties of semiconducting polymer films. J. Photochem. Photobiol. A 2001, 144, 21–30. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Martini, I.B.; Liu, J.; Schwartz, B.J. Controlling interchain interactions in conjugated polymers: The effects of chain morphology on exciton-exciton annihilation and aggregation in MEH-PPV films. J. Phys. Chem. B 2000, 104, 237–255. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.J.; Ma, L.P.; Yang, Y. Device performance and polymer morphology in polymer light emitting diodes: The control of device electrical properties and metal/polymer contact. J. Appl. Phys. 2000, 88, 605–609. [Google Scholar] [CrossRef]
- Yang, C.Y.; Hide, F.; Diaz-Garcia, M.A.; Heeger, A.J.; Cao, Y. Microstructure of thin films of photoluminescent semiconducting polymers. Polymer 1998, 39, 2299–2304. [Google Scholar] [CrossRef]
- Schaller, R.D.; Snee, P.T.; Johnson, J.C.; Lee, L.F.; Wilson, K.R.; Haber, L.H.; Saykally, R.J.; Nguyen, T.Q.; Schwartz, B.J. Nanoscopic interchain aggregate domain formation in conjugated polymer films studied by third harmonic generation near-field scanning optical microscopy. J. Chem. Phys. 2002, 117, 6688–6698. [Google Scholar] [CrossRef]
- Lampert, Z.E.; Reynolds, C.L.; Papanikolas, J.M.; Aboelfotoh, M.O. Controlling Morphology and Chain Aggregation in Semiconducting Conjugated Polymers: The Role of Solvent on Optical Gain in MEH-PPV. J. Phys. Chem. B 2012, 116, 12835–12841. [Google Scholar] [CrossRef] [PubMed]
- Manaka, T.; Iwamoto, M. Optical second-harmonic generation measurement for probing organic device operation. Light Sci. Appl. 2016, 5, e16040. [Google Scholar] [CrossRef]
- Costela, A.; García, O.; Cerdán, L.; García-Moreno, I.; Sastre, R. Amplified spontaneous emission and optical gain measurements from pyrromethene 567 doped polymer waveguides and quasi-waveguides. Opt. Express 2008, 16, 7023–7036. [Google Scholar] [CrossRef] [PubMed]
- Bunning, T.J.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L. Holographic polymer-dispersed liquid crystals (H-PDLCs). Annu. Rev. Mater. Sci. 2000, 30, 83–115. [Google Scholar] [CrossRef]
- Huang, W.; Diao, Z.; Liu, Y.; Peng, Z.; Yang, C.; Ma, J.; Xuan, L. Distributed feedback polymer laser with an external feedback structure fabricated by holographic polymerization technique. Org. Electron. 2012, 13, 2307–2311. [Google Scholar] [CrossRef]
- Liu, M.H.; Liu, Y.G.; Zhang, G.Y.; Peng, Z.H.; Li, D.Y.; Ma, J.; Xuan, L. Organic holographic polymer dispersed liquid crystal distributed feedback laser from different diffraction orders. J. Phys. D Appl. Phys. 2016, 49, 465102. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Kwong, R.C.; Thompson, M.E.; Schwartz, B.J. Improving the performance of conjugated polymer-based devices by control of interchain interactions and polymer film morphology. Appl. Phys. Lett. 2000, 76, 2454–2456. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Doan, V.; Schwartz, B.J. Conjugated polymer aggregates in solution: Control of interchain interactions. J. Chem. Phys. 1999, 110, 4068–4078. [Google Scholar] [CrossRef]
- Calzado, E.M.; Ramírez, M.G.; Boj, P.G.; García, M.A.D. Thickness dependence of amplified spontaneous emission in low-absorbing organic waveguides. Appl. Opt. 2012, 51, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- DiazGarcia, M.A.; Hide, F.; Schwartz, B.J.; Andersson, M.R.; Pei, Q.B.; Heeger, A.J. Plastic lasers: Semiconducting polymers as a new class of solid-state laser materials. Synth. Met. 1997, 84, 455–462. [Google Scholar] [CrossRef]
- Tammer, M.; Monkman, A.P. Measurement of the anisotropic refractive indices of spin cast thin poly(2-methoxy-5-(2′-ethyl-hexyloxy)-p-phenylenevinylene) (MEH-PPV) films. Adv. Mater. 2002, 14, 210–212. [Google Scholar] [CrossRef]
- McBranch, D.; Campbell, I.H.; Smith, D.L.; Ferraris, J.P. Optical determination of chain orientation in electroluminescent polymer-films. Appl. Phys. Lett. 1995, 66, 1175–1177. [Google Scholar] [CrossRef]
- Ye, Z. Waveguide Optics; Science Press: Beijing, China, 2007; Chapter 2; p. 16. [Google Scholar]
- McGehee, M.D.; Gupta, R.; Veenstra, S.; Miller, E.K.; Diaz-Garcia, M.A.; Heeger, A.J. Amplified spontaneous emission from photopumped films of a conjugated polymer. Phys. Rev. B 1998, 58, 7035. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Yang, Y. Device performance and polymer morphology in polymer light emitting diodes: The control of thin film morphology and device quantum efficiency. J. Appl. Phys. 2000, 87, 4254–4263. [Google Scholar] [CrossRef]
- Kogelnik, H.; Shank, C.V. Coupled-wave theory of distributed feedback lasers. J. Appl. Phys. 1972, 43, 2327–2335. [Google Scholar] [CrossRef]
- Riechel, S.; Lemmer, U.; Feldmann, J.; Benstem, T.; Kowalsky, W.; Scherf, U.; Gombert, A.; Wittwer, V. Laser modes in organic solid-state distributed feedback lasers. Appl. Phys. B-Lasers Opt. 2000, 71, 897–900. [Google Scholar] [CrossRef]
- Turnbull, G.A.; Andrew, P.; Barnes, W.L.; Samuel, I.D.W. Photonic mode dispersion of a two-dimensional distributed feedback polymer laser. Phys. Rev. B 2003, 67, 165107. [Google Scholar] [CrossRef]
- Lozano, G.; Rodriguez, S.R.K.; Verschuuren, M.A.; Rivas, J.G. Metallic nanostructures for efficient LED lighting. Light Sci. Appl. 2016, 5, e16080. [Google Scholar] [CrossRef]
- Shaw, P.E.; Ruseckas, A.; Peet, J.; Bazan, G.C.; Samuel, I.D.W. Exciton-Exciton Annihilation in Mixed-Phase Polyfluorene Films. Adv. Funct. Mater. 2010, 20, 155–161. [Google Scholar] [CrossRef]
- Lehnhardt, M.; Riedl, T.; Weimann, T.; Kowalsky, W. Impact of triplet absorption and triplet-singlet annihilation on the dynamics of optically pumped organic solid-state lasers. Phys. Rev. B. 2010, 81, 165206. [Google Scholar] [CrossRef]
- Rabe, T.; Gorrn, P.; Lehnhardt, M.; Tilgner, M.; Riedl, T.; Kowalsky, W. Highly Sensitive Determination of the Polaron-Induced Optical Absorption of Organic Charge-Transport Materials. Phys. Rev. Lett. 2009, 102, 137401. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Camposeo, A.; Del Carro, P.; Solaro, P.; Cingolani, R.; Boffi, P.; Pisignano, D. Rapid prototyping encapsulation for polymer light-emitting lasers. Appl. Phys. Lett. 2009, 94, 123305. [Google Scholar] [CrossRef]
- Herrnsdorf, J.; Guilhabert, B.; Chen, Y.; Kanibolotsky, A.L.; Mackintosh, A.R.; Pethrick, R.A.; Skabara, P.J.; Gu, E.; Laurand, N.; Dawson, M.D. Flexible blue-emitting encapsulated organic semiconductor DFB laser. Opt. Express 2010, 18, 25535–25545. [Google Scholar] [CrossRef] [PubMed]
- Vannahme, C.; Klinkhammer, S.; Christiansen, M.B.; Kolew, A.; Kristensen, A.; Lemmer, U.; Mappes, T. All-polymer organic semiconductor laser chips: Parallel fabrication and encapsulation. Opt. Express. 2010, 18, 24881–24887. [Google Scholar] [CrossRef] [PubMed]



| Materials | Chemical Structure | Polarity | Volatility (mg/hour) |
|---|---|---|---|
| THF |  | 4.2 | 687 |
| TCM |  | 4.4 | 323 |
| CB |  | 2.7 | 43 |
| toluene |  | 2.4 | 79 |
| p-xylene |  | 2.5 | 27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Liu, Y.; Peng, Z.; Yang, C.; Mu, Q.; Cao, Z.; Ma, J.; Xuan, L. Controlling Morphology and Aggregation in Semiconducting Polymers: The Role of Solvents on Lasing Emission in Poly[2-methoxy-5-(2′-ethyl-hexyloxy)-1,4-phenylene-vinylene]. Materials 2017, 10, 706. https://doi.org/10.3390/ma10070706
Liu M, Liu Y, Peng Z, Yang C, Mu Q, Cao Z, Ma J, Xuan L. Controlling Morphology and Aggregation in Semiconducting Polymers: The Role of Solvents on Lasing Emission in Poly[2-methoxy-5-(2′-ethyl-hexyloxy)-1,4-phenylene-vinylene]. Materials. 2017; 10(7):706. https://doi.org/10.3390/ma10070706
Chicago/Turabian StyleLiu, Minghuan, Yonggang Liu, Zenghui Peng, Chengliang Yang, Quanquan Mu, Zhaoliang Cao, Ji Ma, and Li Xuan. 2017. "Controlling Morphology and Aggregation in Semiconducting Polymers: The Role of Solvents on Lasing Emission in Poly[2-methoxy-5-(2′-ethyl-hexyloxy)-1,4-phenylene-vinylene]" Materials 10, no. 7: 706. https://doi.org/10.3390/ma10070706