Sc-Decorated Porous Graphene for High-Capacity Hydrogen Storage: First-Principles Calculations
Abstract
:1. Introduction
2. Calculation Details
3. Results and Discussion
3.1. Single Sc Atom Decorated PG
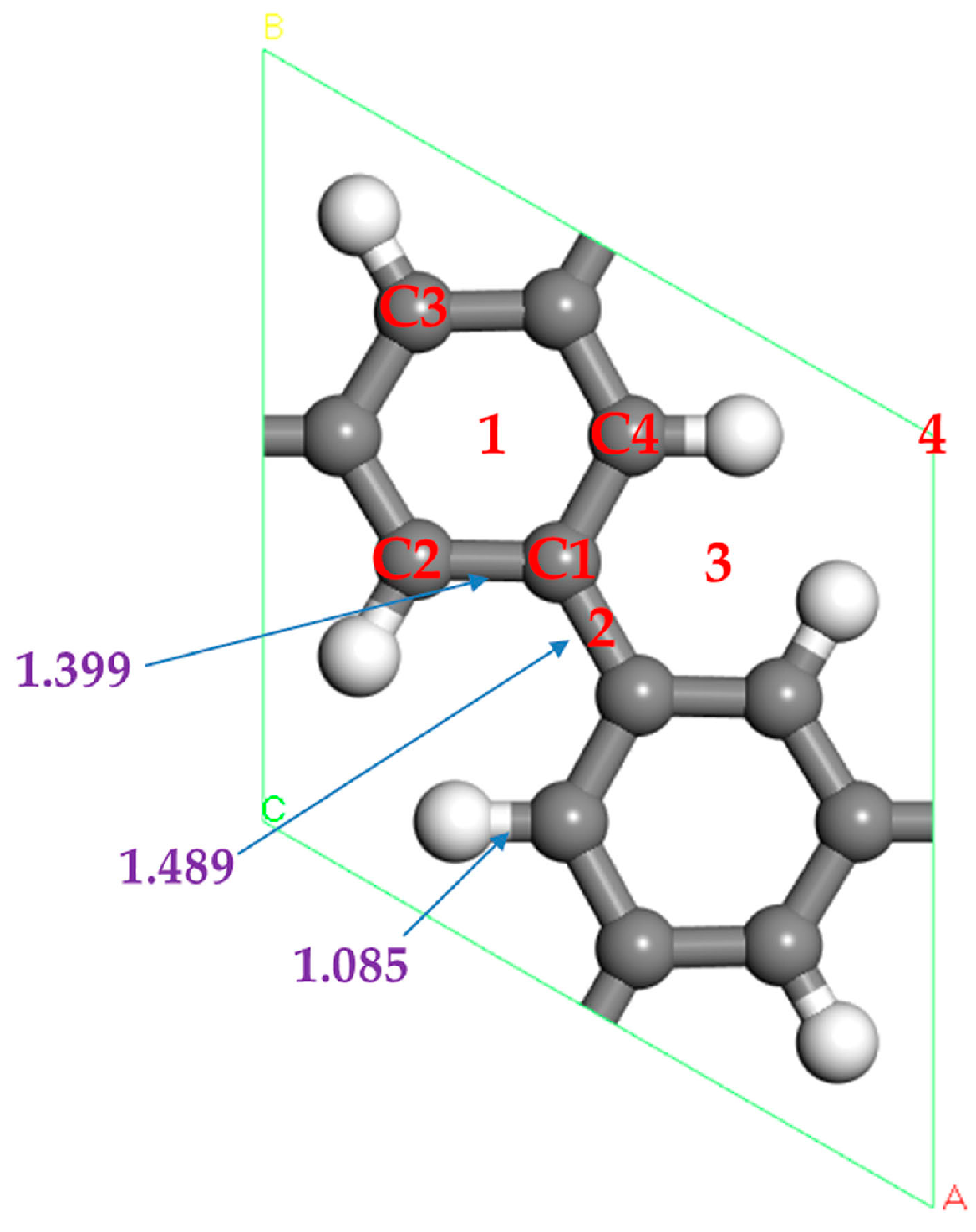
3.1.1. The Adsorption Structure of Single Sc Atom Decorated PG
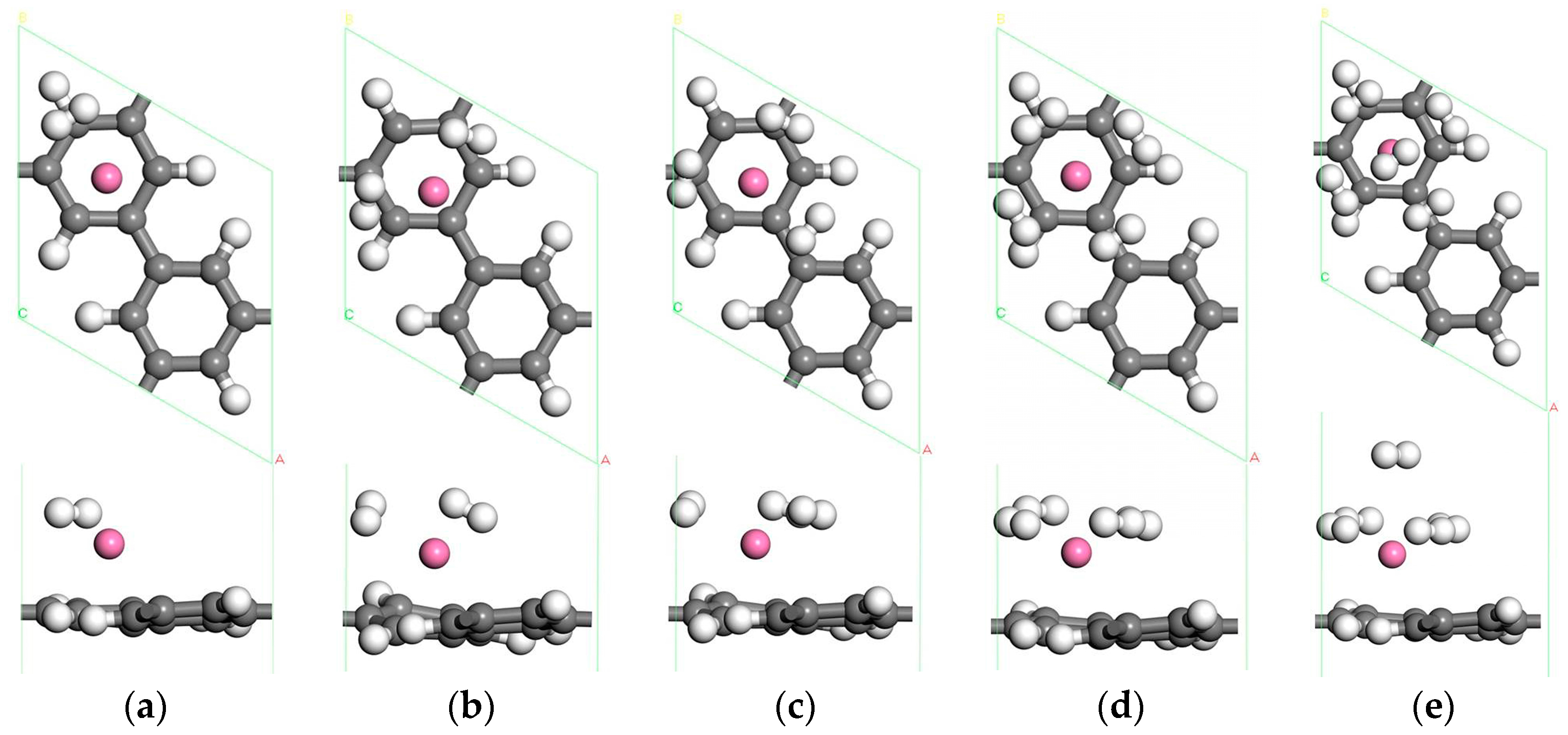
3.1.2. The Adsorption of H2 Molecules on Single Sc-Decorated PG
3.2. Two Sc Atoms Decorated PG
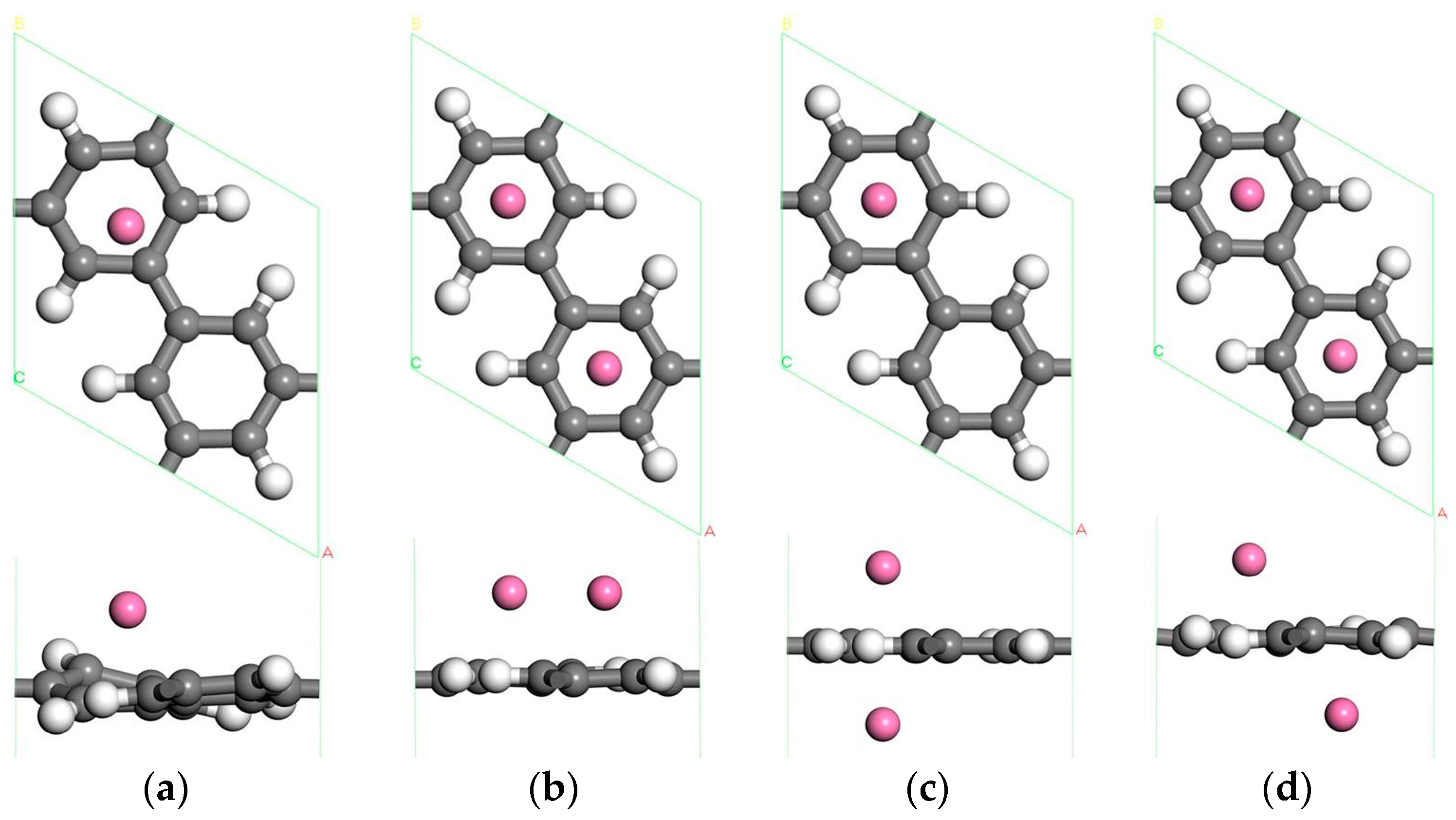
3.2.1. The Adsorption Structure of Two Sc Atoms Decorated PG
3.2.2. The Hydrogen Storage Capacity of Two Sc Atoms Modified PG System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Masika, E.; Mokaya, R. Exceptional gravimetric and volumetric hydrogen storage for densified zeolite templated carbons with high mechanical stability. Energy Environ. Sci. 2014, 7, 427–434. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, Y.; Li, M.; Yuan, P.; Sun, Q.; Jia, Y. Li and Ca Co-decorated carbon nitride nanostructures as high-capacity hydrogen storage media. J. Appl. Phys. 2011, 110, 94311. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Li, J.C.; Jiang, Q. Hydrogen Adsorption on Eu/SWCNT Systems: A DFT Study. J. Phys. Chem. C 2010, 114, 7733–7737. [Google Scholar] [CrossRef]
- Sahaym, U.; Norton, M.G. Advances in the application of nanotechnology in enabling a ‘hydrogen economy’. J. Mater. Sci. 2008, 43, 5395–5429. [Google Scholar] [CrossRef]
- Ataca, C.; Aktürk, E.; Ciraci, S.; Ustunel, H. High-capacity hydrogen storage by metallized graphene. Appl. Phys. Lett. 2008, 93, 43123. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Jhi, S.-H.; Lim, S.; Park, N. Crossover between multipole Coulomb and Kubas interactions in hydrogen adsorption on metal-graphene complexes. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef]
- Chandrakumar, K.R.S.; Ghosh, S.K. Alkali-Metal-Induced Enhancement of Hydrogen Adsorption in C60 Fullerene: An ab Initio Study. Nano Lett. 2008, 8, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Q.; Jena, P. Functionalized heterofullerenes for hydrogen storage. Appl. Phys. Lett. 2009, 94, 13111. [Google Scholar] [CrossRef]
- Seenithurai, S.; Pandyan, R.K.; Kumar, S.V.; Saranya, C.; Mahendran, M. Al-decorated carbon nanotube as the molecular hydrogen storage medium. Int. J. Hydrogen Energy 2014, 39, 11990–11998. [Google Scholar] [CrossRef]
- Du, A.; Zhu, Z.; Smith, S.C. Multifunctional Porous Graphene for Nanoelectronics and Hydrogen Storage: New Properties Revealed by First Principle Calculations. J. Am. Chem. Soc. 2010, 132, 2876–2877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, Z.; Liu, Y.; You, D.; Wu, K.; Lv, J.; Wang, X.; Deng, K.; Rao, D.; Lu, R. Lithium decoration of three dimensional boron-doped graphene frameworks for high-capacity hydrogen storage. Appl. Phys. Lett. 2015, 106, 63901. [Google Scholar] [CrossRef]
- Hussain, T.; Pathak, B.; Ramzan, M.; Maark, T.A.; Ahuja, R. Calcium doped graphane as a hydrogen storage material. Appl. Phys. Lett. 2012, 100, 183902. [Google Scholar] [CrossRef]
- Song, N.; Wang, Y.; Zheng, Y.; Zhang, J.; Xu, B.; Sun, Q.; Jia, Y. New template for Li and Ca decoration and hydrogen adsorption on graphene-like SiC: A first-principles study. Comput. Mater. Sci. 2015, 99, 150–155. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, N.; Li, J.; Liu, E.; He, C.; Shi, C. Hydrogen spillover storage on Ca-decorated graphene. Int. J. Hydrogen Energy 2012, 37, 11835–11841. [Google Scholar] [CrossRef]
- Lebon, A.; Carrete, J.; Gallego, L.J.; Vega, A. Ti-decorated zigzag graphene nanoribbons for hydrogen storage. A van der Waals-corrected density-functional study. Int. J. Hydrogen Energy 2015, 40, 4960–4968. [Google Scholar] [CrossRef]
- Chu, S.; Hu, L.; Hu, X.; Yang, M.; Deng, J. Titanium-embedded graphene as high-capacity hydrogen-storage media. Int. J. Hydrogen Energy 2011, 36, 12324–12328. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; He, Y.; Cheng, H.-P. Titanium-decorated graphene for high-capacity hydrogen storage studied by density functional simulations. J. Phys. Condens. Matter 2010, 22, 445301. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.I.; Leiva, E.P.M. Density functional theory study of a graphene sheet modified with titanium in contact with different adsorbates. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Kim, G.; Jhi, S.-H.; Park, N.; Louie, S.G.; Cohen, M.L. Optimization of metal dispersion in doped graphitic materials for hydrogen storage. Phys. Rev. B 2008, 78. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.; Pan, R.; An, Y. A first-principles study of Sc-decorated graphene with pyridinic-N defects for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 3106–3113. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.A.; Szpunar, B.; Beye, A.C. Hydrogen adsorption and storage on Palladium—Functionalized graphene with NH-dopant: A first principles calculation. Appl. Surf. Sci. 2017, 392, 362–374. [Google Scholar] [CrossRef]
- Faye, O.; Eduok, U.; Szpunar, J.; Szpunar, B.; Samoura, A.; Beye, A. Hydrogen storage on bare Cu atom and Cu-functionalized boron-doped graphene: A first principles study. Int. J. Hydrogen Energy 2017, 42, 4233–4243. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Wang, R. Prediction of hydrogen storage on Y-decorated graphene: A density functional theory study. Appl. Surf. Sci. 2014, 296, 204–208. [Google Scholar] [CrossRef]
- Sivek, J.; Sahin, H.; Partoens, B.; Peeters, F.M. Adsorption and absorption of boron, nitrogen, aluminum, and phosphorus on silicene: Stability and electronic and phonon properties. Phys. Rev. B 2013, 87. [Google Scholar] [CrossRef]
- Vogt, P.; De Padova, P.; Quaresima, C.; Avila, J.; Frantzeskakis, E.; Asensio, M.C.; Resta, A.; Ealet, B.; Le Lay, G. Silicene: Compelling Experimental Evidence for Graphenelike Two-Dimensional Silicon. Phys. Rev. Lett. 2012, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. Strain and chirality effects on the mechanical and electronic properties of silicene and silicane under uniaxial tension. Phys. Lett. A 2012, 376, 3546–3550. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, Z.; Li, Y.; Chen, Z.; Zeng, H. Atomically Thin Arsenene and Antimonene: Semimetal-Semiconductor and Indirect-Direct Band-Gap Transitions. Angew. Chem. Int. Ed. 2015, 54, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhou, Y.; Bi, H.; Yin, K.; Wan, S.; Sun, L. Large-range Control of the Microstructures and Properties of Three-dimensional Porous Graphene. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Bieri, M.; Treier, M.; Cai, J.; Aït-Mansour, K.; Ruffieux, P.; Gröning, O.; Gröning, P.; Kastler, M.; Rieger, R.; Feng, X.; et al. Porous graphenes: Two-dimensional polymer synthesis with atomic precision. Chem. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, H.; Deng, K.; Tang, W.; Kan, E. Improved permeability and selectivity in porous graphene for hydrogen purification. Phys. Chem. Chem. Phys. 2014, 16, 25755–25759. [Google Scholar] [CrossRef] [PubMed]
- Reunchan, P.; Jhi, S.-H. Metal-dispersed porous graphene for hydrogen storage. Appl. Phys. Lett. 2011, 98, 93103. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, Y.; Kang, L.; Zhang, C.; Wang, D.; Wang, C.; Zhang, M.; Wu, X. First-principles investigation of hydrogen storage capacity of Y-decorated porous graphene. Appl. Surf. Sci. 2017, 399, 463–468. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.-T.; He, J.; Tian, Y. Ab initio investigations of optical properties of the high-pressure phases of ZnO. Phys. Rev. B 2005, 71. [Google Scholar] [CrossRef]
- Rao, D.; Lu, R.; Meng, Z.; Wang, Y.; Lu, Z.; Liu, Y.; Chen, X.; Kan, E.; Xiao, C.; Deng, K.; et al. Electronic properties and hydrogen storage application of designed porous nanotubes from a polyphenylene network. Int. J. Hydrogen Energy 2014, 39, 18966–18975. [Google Scholar] [CrossRef]
- Brunetto, G.; Autreto, P.A.S.; Machado, L.D.; Santos, B.I.; Dos Santos, R.P.B.; Galvão, D.S. Nonzero Gap Two-Dimensional Carbon Allotrope from Porous Graphene. J. Phys. Chem. C 2012, 116, 12810–12813. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Shen, P.; Chen, Z. Two-dimensional polyphenylene: Experimentally available porous graphene as a hydrogen purification membrane. Chem. Commun. 2010, 46, 3672. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.M.; Peeters, F.M. High-capacity hydrogen storage in Al-adsorbed graphene. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Bhattacharya, S.; Majumder, C.; Das, G.P. Transition-Metal Decoration Enhanced Room-Temperature Hydrogen Storage in a Defect-Modulated Graphene Sheet. J. Phys. Chem. C 2010, 114, 10297–10301. [Google Scholar] [CrossRef]
- Ataca, C.; Aktürk, E.; Ciraci, S. Hydrogen storage of calcium atoms adsorbed on graphene: First-principles plane wave calculations. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef]
- Chen, M.; Yang, X.-B.; Cui, J.; Tang, J.-J.; Gan, L.-Y.; Zhu, M.; Zhao, Y.-J. Stability of transition metals on Mg(0001) surfaces and their effects on hydrogen adsorption. Int. J. Hydrogen Energy 2012, 37, 309–317. [Google Scholar] [CrossRef]
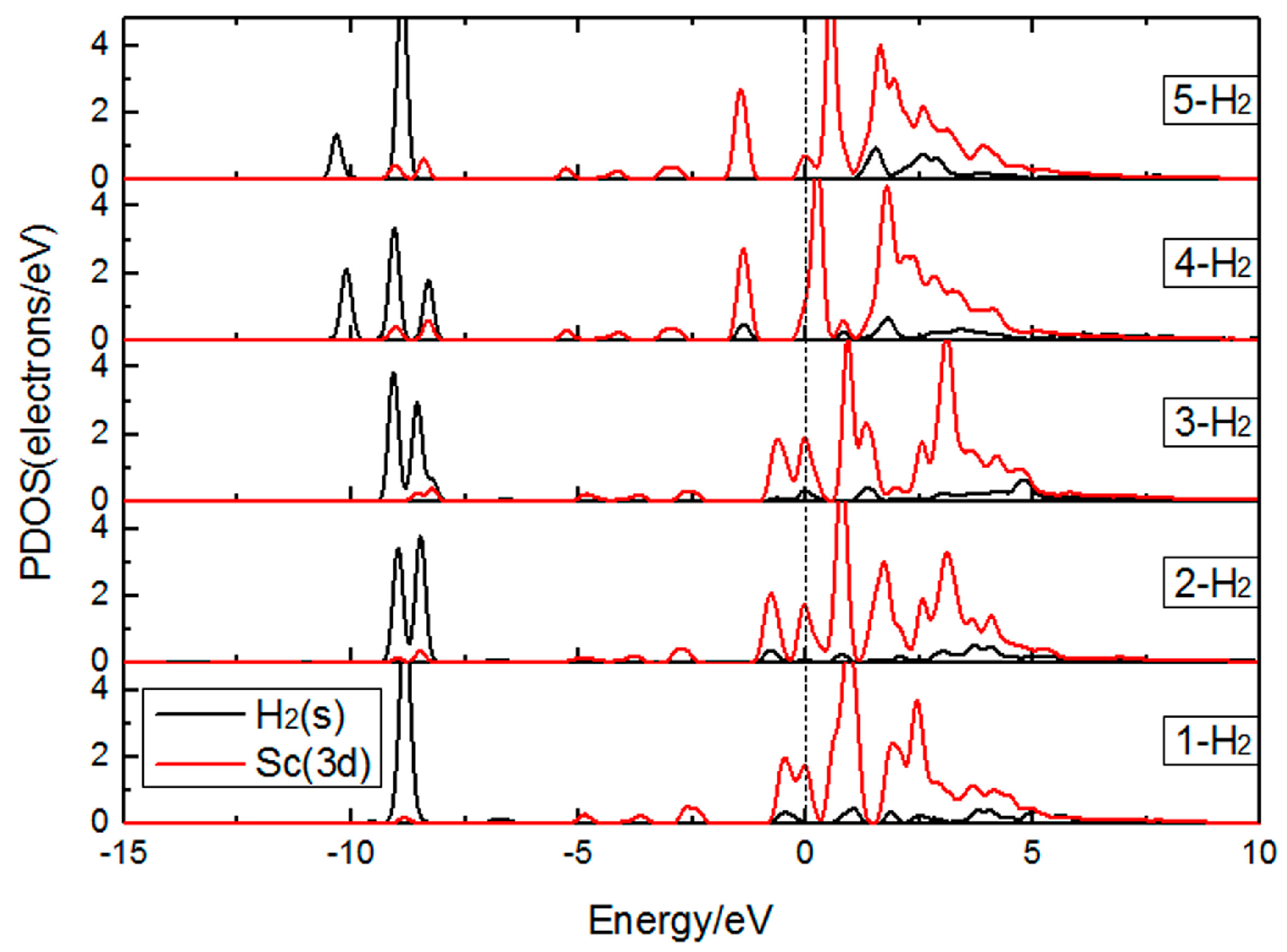

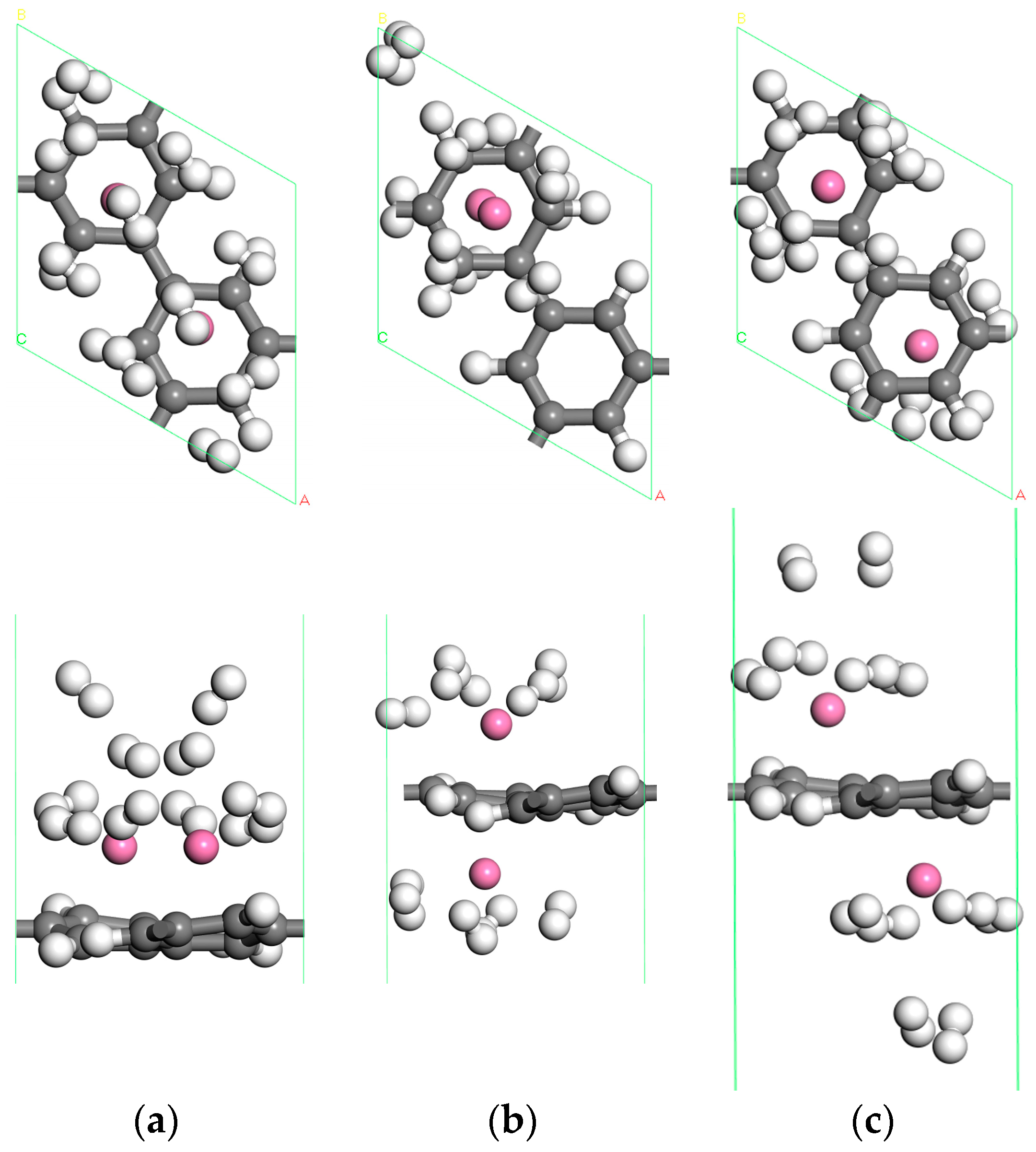
| Number of H2 | (eV) | (eV) | (Å) |
|---|---|---|---|
| 1H2 | −0.401 | −0.401 | 2.348 |
| 2H2 | −0.718 | −0.559 | 2.217 |
| 3H2 | −0.422 | −0.514 | 2.291 |
| 4H2 | −0.174 | −0.429 | 2.451 |
| 5H2 | −0.093 | −0.361 | 2.477 |
| Atom | Before Adsorption (e) | After Adsorption (e) | ||||||
|---|---|---|---|---|---|---|---|---|
| s | p | d | Charge | s | p | d | Charge | |
| H1 | 1.00 | - | - | - | 1.13 | - | - | –0.13 |
| H2 | 1.00 | - | - | - | 1.13 | - | - | –0.13 |
| C2 | 1.19 | 3.19 | - | –0.38 | 1.19 | 3.23 | - | –0.42 |
| C3 | 1.20 | 3.28 | - | –0.48 | 1.18 | 3.15 | - | –0.33 |
| C4 | 1.19 | 3.20 | - | –0.39 | 1.19 | 3.23 | - | –0.42 |
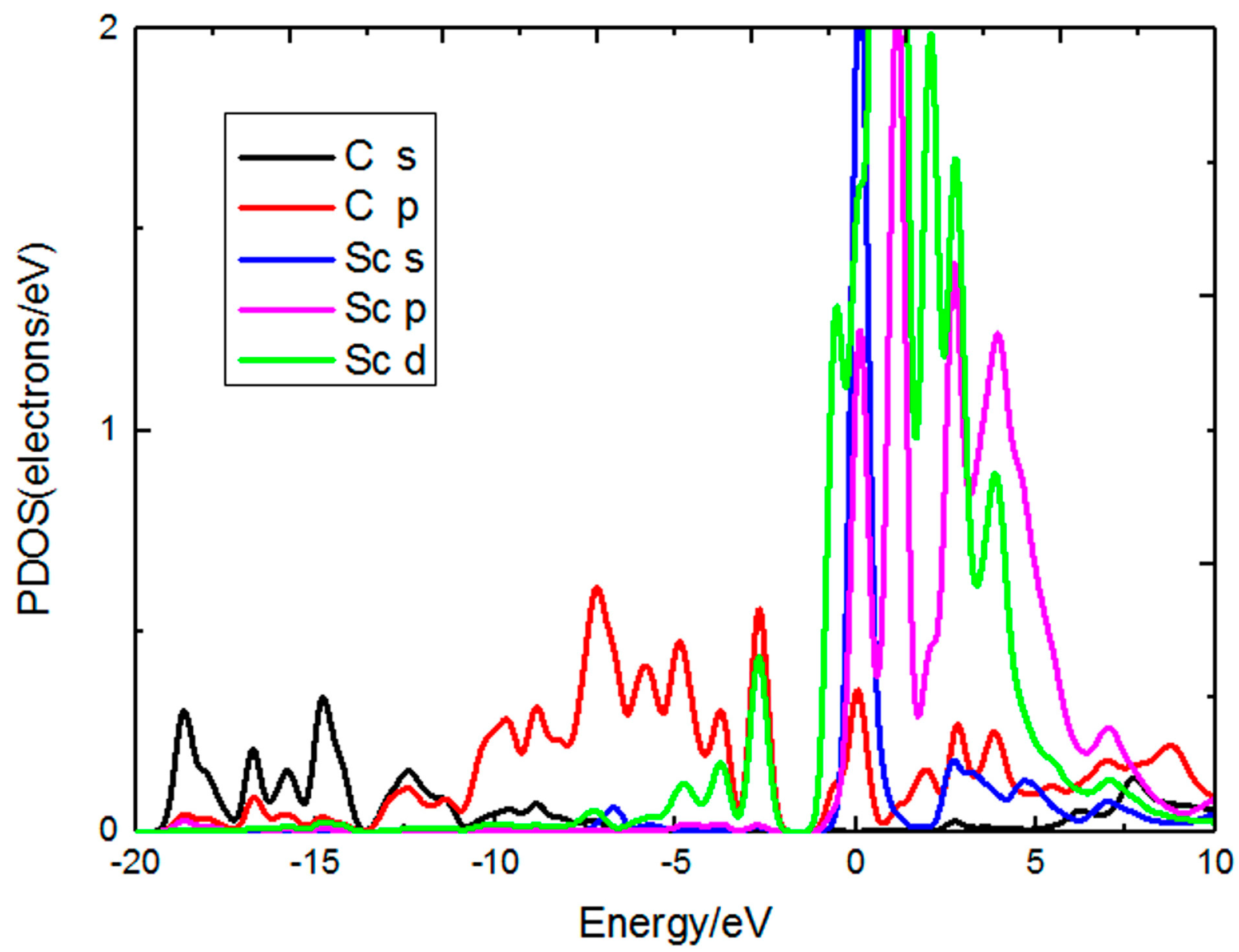
| Sc | 0.18 | 5.84 | 1.65 | 1.33 | 0.04 | 5.63 | 1.77 | 1.56 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, J.; Yuan, L.; Zhang, M.; Zhang, C. Sc-Decorated Porous Graphene for High-Capacity Hydrogen Storage: First-Principles Calculations. Materials 2017, 10, 894. https://doi.org/10.3390/ma10080894
Chen Y, Wang J, Yuan L, Zhang M, Zhang C. Sc-Decorated Porous Graphene for High-Capacity Hydrogen Storage: First-Principles Calculations. Materials. 2017; 10(8):894. https://doi.org/10.3390/ma10080894
Chicago/Turabian StyleChen, Yuhong, Jing Wang, Lihua Yuan, Meiling Zhang, and Cairong Zhang. 2017. "Sc-Decorated Porous Graphene for High-Capacity Hydrogen Storage: First-Principles Calculations" Materials 10, no. 8: 894. https://doi.org/10.3390/ma10080894





