2.1. SH-NBBs Coatings
SH-NBBs solutions prepared via ISWP at a different reaction time were used for coating cotton and paper substrates. On the basis of the NMR study performed for elucidating the SH-NBBs growth in solution [
15], three reaction times were selected as representative of different SSQs architectures. After 6 h reaction the condensation degree is limited and SH-NBBs are represented by small linear and cyclic species mainly based on T
2 units, i.e., according to the
29Si NMR notation, SiCO
3 species with two bridging oxygen and one terminal OH function. These species after 16 h start to rearrange into fully condensed T
3 units (with three bridging oxygen) mainly in ladder–type architectures. At 80 h SH-NBBs are mainly composed of condensed units with closed cages, i.e., octakis (3-mercaptopropylsilsesquioxane), as the dominant structure.
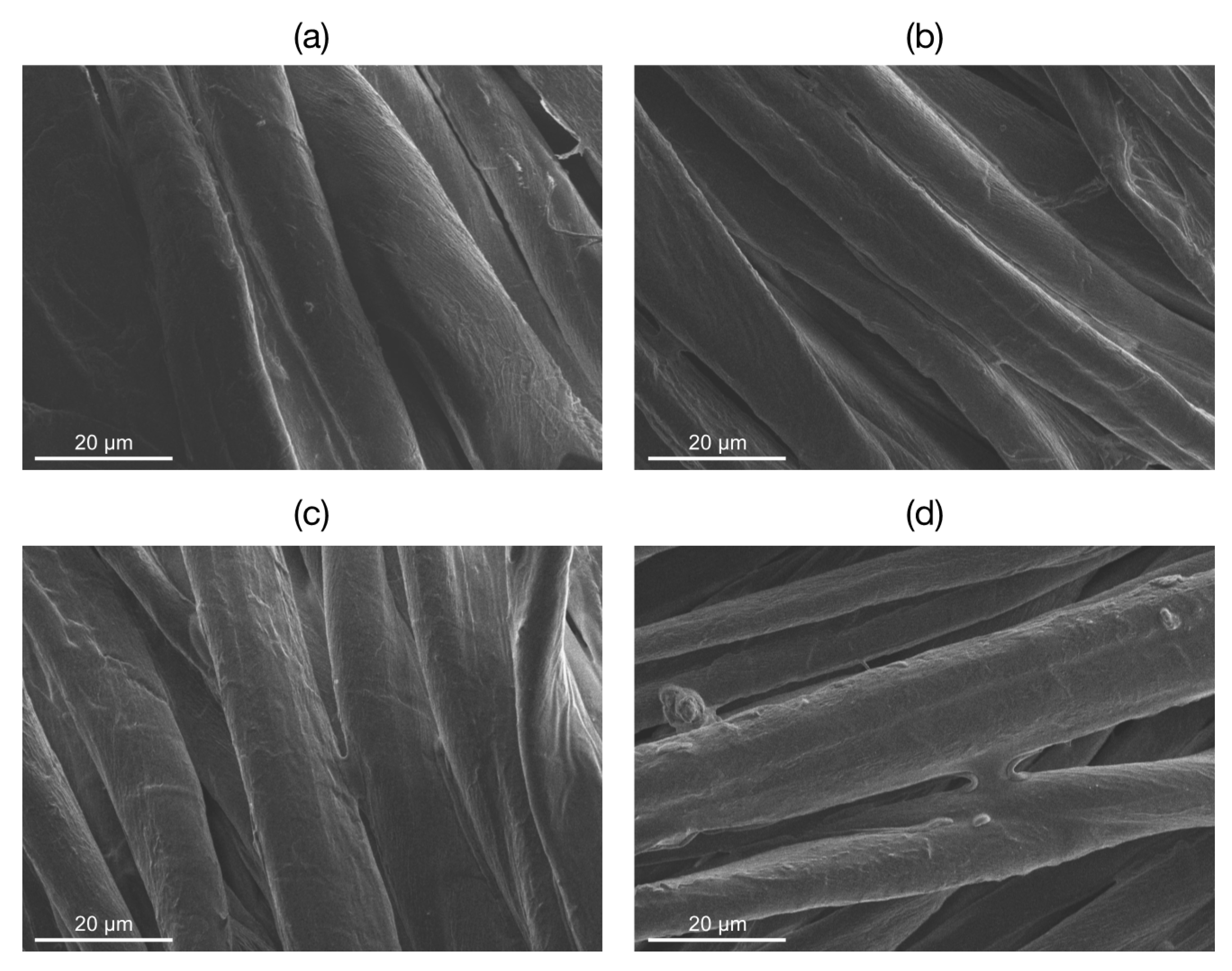
The raw cotton and the cotton samples coated with SH-NBBs sols obtained after 6, 16, and 80 h reaction were characterized by Scanning Electron Microscopy, in order to assess the morphological changes undergone by the material surface during the first step of the process (
Figure 2). In raw cotton, both the tubular and the secondary micro-fibrillar structures of the cotton fibers [
23] can be observed. In
Cotton-NBB samples the presence of a coating appears superimposed to the wrinkled surface textures and the appearance of sub-micrometric bumps, clumps, and bridge-like structures among the fibers can be clearly observed. From
Figure 1, the filming ability of SH-NBBs solutions appear good independently of the duration of the ISWP reaction, thus assuring the effective coating of the cotton fibers in all samples.
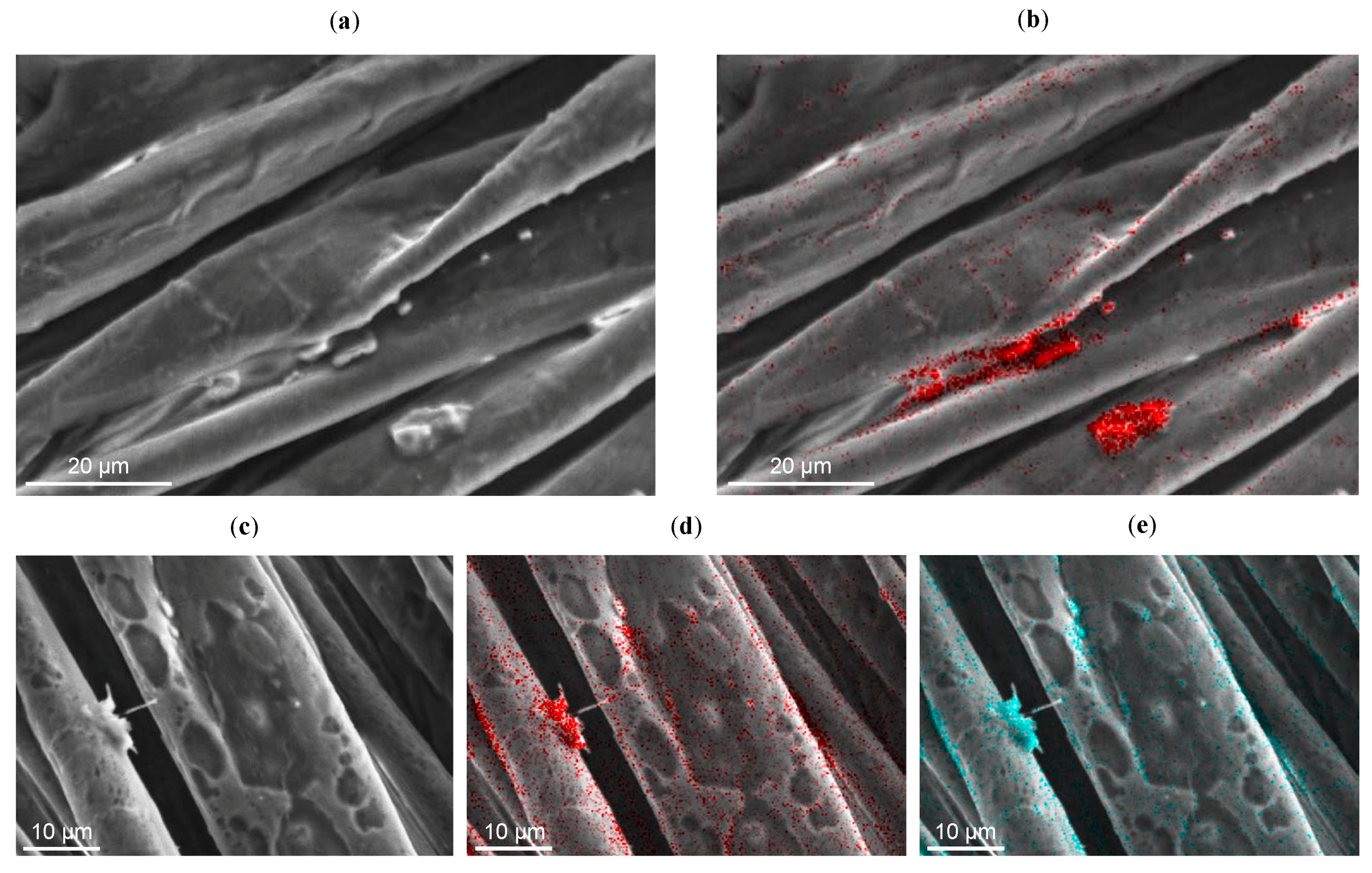
To ascertain the presence and homogeneity of SH-NBBs coating on the fibers, EDX spectra were acquired on raw and coated cotton samples. In the case of SH-NBBs-coated samples, the signals due to Si and S, which are indicative of the presence of thiol-functionalized silsesquioxanes, can be clearly detected in the survey EDX spectra. On the contrary, the amount of Cl, which is indicative of chloroacetic acid and its esters i.e., the side products of the ISWP synthesis, appears very low and a clear correlation with the coating cannot be made on the basis of the acquired maps.
In
Cotton-NBB16h, from the element maps obtained analyzing different selected zones, the Si and S signals do not appear uniform but are stronger in conjunction with the bridge-like structures. The SEM-EDX analysis of
Cotton–NBB80h (
Figure 3a,b) points out that, in comparison with
Cotton-NBB16h, S and Si are more visible in the areas of the fibers where bridge-like structures or lumps are not present. These results can be probably explained by taking into account the viscosity of the SH-NBBs solutions, which increases with an increasing reaction time as a result of oligomers growth.
This evidence is confirmed by the EDX results (that were recorded on a sample, exposed for a long time to the focused electron beam, and showed clear signs of damage compatible with coating degradation).
Figure 3c,d and e suggest that the coating prior to degradation was more homogeneous than what was concluded on the basis of
Figure 3a,b.
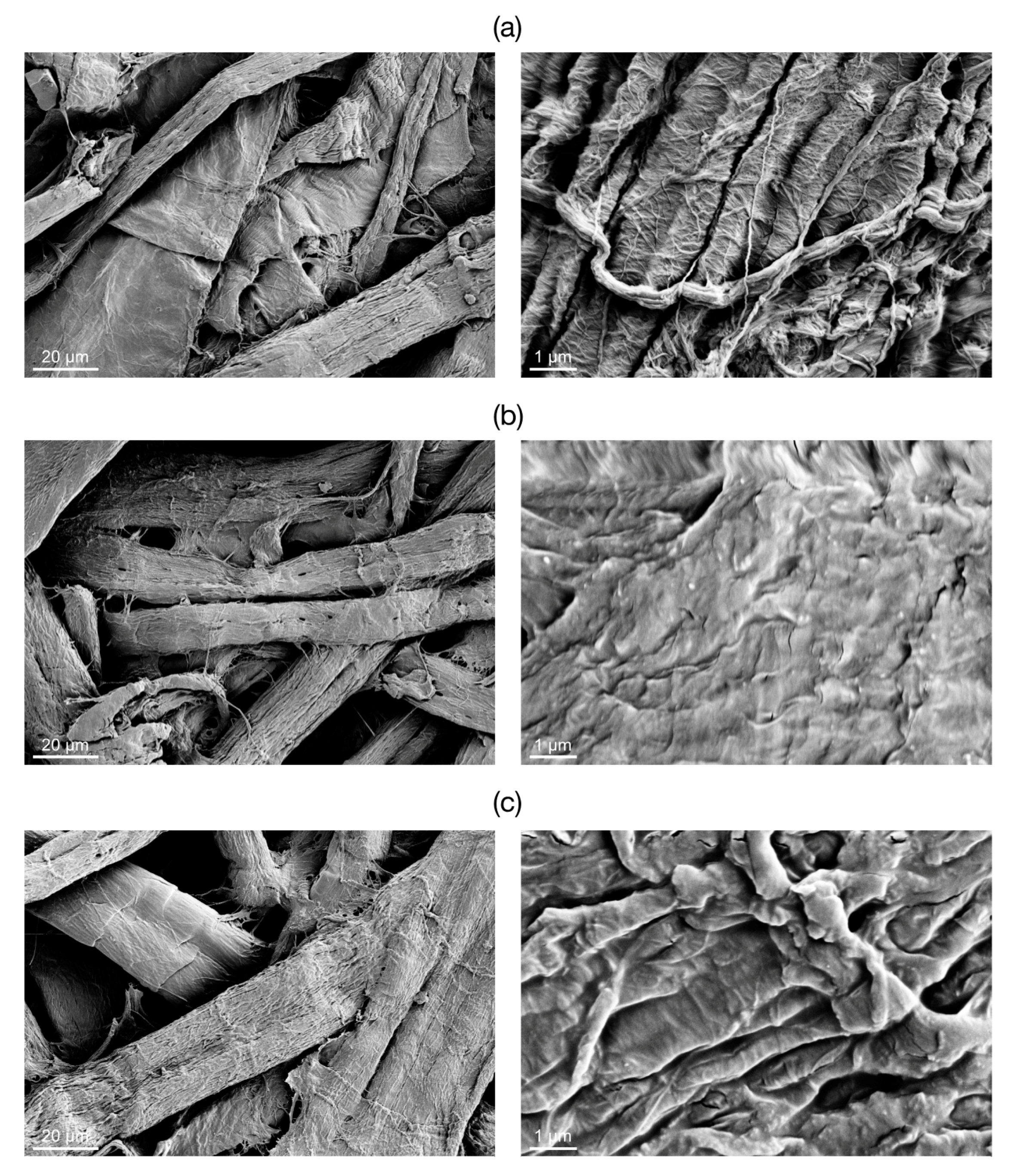
The SEM images of the raw paper substrate and
Paper-NBB samples are shown in
Figure 4. Owing to the high heterogeneity of the substrate, it is difficult to clearly distinguish signs of the NBB coating from the low magnification images (
Figure 4 left). However, the images recorded at higher magnification (
Figure 4 right) show the SH-NBBs layer covering the disordered fibrillary surface texture of the raw paper substrate. This layer exhibits a sub-micrometric roughness that persists even after rinsing of the samples in the ultrasound bath (see
Section 3.2).
From the SEM study, both Cotton-NBB and Paper-NBB samples show that coating with SH-NBBs leads to changes in surface morphology with the appearance, in the case of cotton fabrics, of bridge-like structures connecting the fibers. However, from EDX spectra the presence of SH-NBBs is detected also in the other regions, which suggests that a fairly homogeneous layer is present on the majority of the natural substrates. Therefore, a quite good distribution of the thiol anchoring sites for the subsequent alkene functionalization is also expected.
In the case of Paper-NBB, it is worth noting that the coating roughness is enhanced as a consequence of its rinsing using an ultrasound bath to remove the SH-NBBs excess. While the substrate fibers coating is still preserved, the change in morphology could be beneficial for the wetting behavior. Indeed, the sub-micrometric texture coupled with the microstructure given by the cellulosic fibers of cotton and paper might give rise to hierarchical multi-scale roughness, suitable for achieving extreme wetting properties. Finally, it should be mentioned that, despite the presence of the SH-NBBs coating, both cotton and paper samples keep their macroscopic features, such as color and mechanical behavior, and exhibit quite negligible increases in weight.
The very low amount of deposited SSQs makes the structural characterization of the SH-NBBs coating and the study of the interface between substrate and coating very difficult. However, some hints on the structural features displayed by the coating were obtained by solid state NMR experiments. In particular, a
29Si NMR spectrum was recorded on a cotton sample, prepared ad hoc by repeated immersion steps in the SH-NBBs solution reacted for 80 h (
Supplementary Materials,
Figure S2); 36 k scans were acquired in a cross-polarization experiment (CPMAS). The main signal, due to fully condensed T
3 units with the second resonance related to T
2 units, can be observed in the spectrum. The CPMAS sequence does not lead to quantitative results since it is well known that the magnetization transfer enhances the intensity of proton-rich environments. Accordingly, the low intensity of the T
2 signal is an indication of the high crosslinking degree of the SSQs coating.
2.2. SH-NBBs Purification and Thiol-Ene Click Functionalization
As reported in the experimental section (
Section 3.2), after the immersion of cotton and paper substrates in the SH-NBBs solution the coated samples were rinsed in chloroform in order to remove the ungrafted SH-NBBs, the solutions were kept to dryness, and the obtained solids were dissolved in deuterated chloroform (
SH-NBB_c and
SH-NBB_p solutions) for the NMR analyses.
The structural features of SH-NBBs and ISWP by-products were already described in Borovin et al. [
15]. Accordingly, it has been proved that the synthesis of SH-NBBs leads to a final solution containing propyl- (PrClA) and methyl-chloroacetate (MeClA) residues, as a result of esterification and transalcoholysis reactions (
Figure 1), and chlorothioacetate derivatives. The latter compounds are formed both through direct reaction between McPTMS derivatives and ClAAc, or by transesterification between McPTMS derivatives and propyl or methyl chloroacetate [
15]. In order to remove the side-products from the pristine SH-NBBs solution, purification methods (as extraction or volatilization under reduced pressure) were proved to be not effective, representing an obstacle for further reactions. As a matter of fact, the attempt at running the thiol-ene coupling using the as prepared SH-NBBs solution was unsuccessful, contrary to the observed reactivity of the McPTMS precursor in 1-propanol solution.
Therefore, the NMR spectroscopy was used not only to verify the structural integrity of SH-NBBs recovered by rinsing in chloroform the coated samples, but also to assess both the purification process and the effectiveness of thiol-ene functionalization of the purified SH-NBBs.
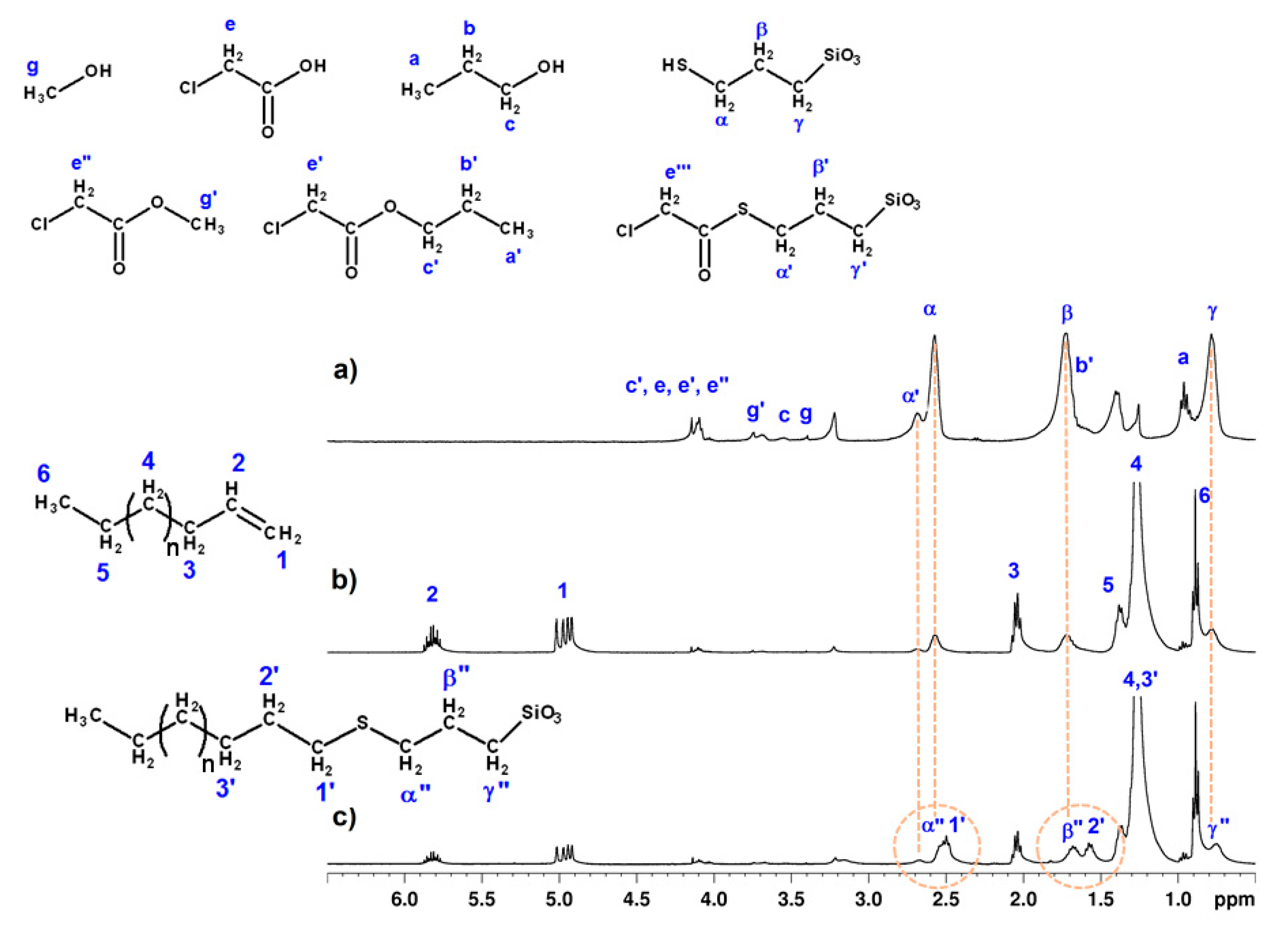
Figure 5a shows the
1H NMR spectrum of the
SH-NBB_c solution prepared by rinsing
Cotton-NBB80h, as an example of the results obtained with the different
SH-NBB_c and
SH-NBB_p solutions. The peak assignment is based on the numbering scheme shown in
Figure 5.
The most intense signals are attributed to α, β, and γ resonances of the mercaptopropyl chain, and together with α’, due to the chlorothioacetate derivative of the pristine propylsilane group [
15], confirm the presence of SH-NBBs in the rinsing residue and, according to the chemical shifts, prove the maintenance of the structural features displayed by the pristine SSQs. Moreover, the integrated areas of (α + α’), β, and γ resonances are an indication that the mercaptopropyl functionalities of the recovered SH-NBBs do not undergo any degradation.
The resonances due to the ISWP side products are still observable in the spectrum. In detail, signals c, e, e’, and e” are due to chloroacetic acid and its esters; in addition, PrClA gives rise to a’ and b’ resonances, the latter appearing as a shoulder of the signal, while MeClA gives rise to g’ resonance. The low intensity of c and g signals suggest the large removal of 1-propanol and methanol during cotton fabrics processing. Finally, other signals not ascribable to SH-NBBs or to ISWP side-products can be observed and have been tentatively assigned to products of cotton degradation.
In order to evaluate the amount of residual ClAAc derived compounds with respect to the SH-NBBs, the following parameter can be calculated (Equation (1)):
where the different quantities are the integral areas of the specified signals. The parameter calculated according to Equation (1) slightly overestimates the molar ratio of ClAAc and its esters with respect to SH-NBBs, since the resonances due to SH-NBBs (α and α’), ClAAc (e), and MeClA (e”) each contribute two protons to the signals, while PrClA contributes four protons (c’ and e’). The calculated R
Cl values (
Table 1) for
SH-NBB_c and
SH-NBB_p solutions indicate the effectiveness of the SH-NBBs purification following the adopted procedure. Indeed, taking into account the 6:1 molar ratio between ClAAc and McPTMS used for the SH-NBBs synthesis, the nominal R
Cl for the as-prepared solutions would assume values of 6 and 12, if only ClAAc and MeClA or PrClA were present, respectively. Conversely, in
SH-NBB_c and
SH-NBB_p, R
Cl assume values ranging from 0.061 to 0.165, resulting in a more than fifty-fold reduction in the relative amount of ClAAc and its esters.
In order to quantitatively evaluate the reliability of the purification step, the percentage of unreacted available thiol groups can be calculated according to Equation (2):
The SH
av values (
Table 1) are generally close to 100% for both paper and cotton supports, with the exception of the purified SH-NBBs 80 h that present a loss of thiol functionalities higher than what was previously reported for the as-prepared SH-NBBs solutions [
15]. However, this result can be related to the fact that the solutions were kept at room temperature during the coating procedure, thus allowing the thioesterification reaction to proceed.
From the above results, the SH-NBBs purification appears effective, and the process is independent of the coated substrate and highly reproducible. In conclusion, the employed NBB coating procedure of cotton and paper produces recyclable wastes, from which the purified SH-NBBs can be easily collected and further reacted.
The
SH-NBB_c and
SH-NBB_p solutions were mixed with 1-decene or 1-tetradecene to study the NBBs reactivity in the thiol-ene coupling run without any photoinitiator addition.
Figure 5b,c shows the
1H NMR spectra of
SH-NBBs_c (obtained from
Cotton-NBB80h) and C14 mixture before and after irradiation for 30 min with λ
max = 254 nm. According to the signal labeling presented in
Figure 5, in the mixture before irradiation C14 can be easily identified by the strong peaks at 1.27, 2.04, and 1.37 ppm due to the methylene protons 4, 3, and 5, respectively, in the alkyl chain, the methyl signal (6) at 0.88 ppm, and the two multiplets at 5.80 and 5.00 ppm due to the double bond end group (1 and 2). After UV irradiation, it is possible to observe a decrease in signal intensity of 1, 2, and 3 resonances and the corresponding appearance of signals 1’ at 2.50, 2’ at 1.57, and of resonance 3’ at 1.26 (overlapped with 4), which is proof of the conversion of C14 into the thioether product of thiol-ene coupling. Moreover, the formation of the thioether group leads to shielding of the α, β, and γ nuclei, with a consequent up-field shift of 0.03 ppm of the mercaptoproyl signals (α”, β”, and γ”). The reaction gives rise to methylene groups with similar neighboring, thus leading resonances 1’ and 2’ to appear overlapped to α” and β”, respectively.
Since deconvolution of α” and 1’ signals with Gaussian-Lorentzian line-shapes failed to obtain an acceptable fit, and integral areas of β” and 2’ are not reliable due to the presence of other signals in the same range (the yield of the reaction was calculated from the comparison of the spectra before and after irradiation). The intensities of α, α”, and 1’ resonances were normalized in the spectra with respect to the signal 6 at 0.88 ppm, which is unaffected by the reaction. The integrated areas are used in Equation (3) that gives the thiol-ene click reaction yield in %:
Upon irradiation at λ
max = 254 nm in the presence of C14, an almost complete conversion is detected for
SH-NBB_c and
SH-NBB_p at 6, 16, and 80 h, which gives a yield above 99% with the only exception of
SH-NBB_p at 16 h that reaches 93%. The same results are obtained for the reaction with 1-decene, indicating that the conversion reaction is achieved independently from the alkyl chain length. On the contrary, from the
1H NMR spectra no reaction is observed with the same samples irradiated at 365 nm or kept in the darkness (control samples). All the obtained results are summarized in
Table S1.
The
1H NMR study of purified SH-NBBs/alkene mixtures demonstrates that initiator-free thiol-ene coupling between SH-NBBs and selected alkenes in over-stoichiometric amounts occurs with near to quantitative conversion after irradiation with light in the middle UV range with λ
max = 254 nm. Moreover, in accordance with its click character, the observed thiol-ene reaction is very fast, achieving almost complete completion within 15 min, as demonstrated for
SH-NBBs_p/C14 mixtures. To the best of our knowledge, this constitutes the fastest and most efficient example of initiator-free thiol-ene coupling of silsesquioxane-based materials ever achieved [
24,
25].
While reactions conducted under irradiation at λ
max = 254 nm achieved excellent yields typical of click reactions, samples irradiated with λ
max = 365 nm did not react at all for the given exposure times (although, from this result it cannot be concluded that thiol-ene coupling cannot occur in those conditions; the reaction would certainly proceed much more slowly than for a λ
max = 254 nm irradiation, in accordance with the literature [
26,
27]).
2.3. Thiol-Ene Functionalization of SH-NBBs Coated Cotton and Paper
In the second functionalization step,
Cotton-NBB and
Paper-NBB samples were soaked in the pure alkene without the addition of any photoinitiator, and subsequently irradiated with λ
max = 254 nm. As reported above, the structural characterization of the coating by solid state NMR was possible only using the sample ad hoc prepared. With the purpose to check the occurrence of the click coupling between the alkene and the available thiol functions of the coatings,
1H MAS NMR spectra were recorded on the previous sample before and after the reaction. Comparing the spectra of raw and coated cotton (
Figure S3a,b), the overlapping of the relatively sharp peaks belonging to the NBBs and the broad cellulose signal can be appreciated. Moreover, the signal due to the substrate further broadens as a consequence of the interaction with the NBBs. The spectrum recorded on the coated sample soaked in C14 after UV irradiation (
Figure S3c) presents two sharp and intense peaks at 1.3 and 0.9 ppm, which prove the presence of the long alkyl chain; the absence of sharp resonances in the region 5–6 ppm, attributable to the double bond protons (
Figure S3c), confirms the occurrence of the click reaction.
In order to observe the effect of the click functionalization step on the coating, the SEM analysis was run on
Cotton-NBB click (
Figure S4) and
Paper-NBB click (
Figure S5) samples.
Cotton-NBB click samples appear to have lost the bridge-like connections between fibers observed in their parent coated samples, but at higher magnification the sub-micrometric features previously observed on the coatings (
Figure 2) appear clearly visible. Similar results are obtained for
Paper-NBB click samples. Therefore, even if some large-scale features are lost with the click functionalization, the coating integrity is generally assessed on the basis of the SEM analysis.
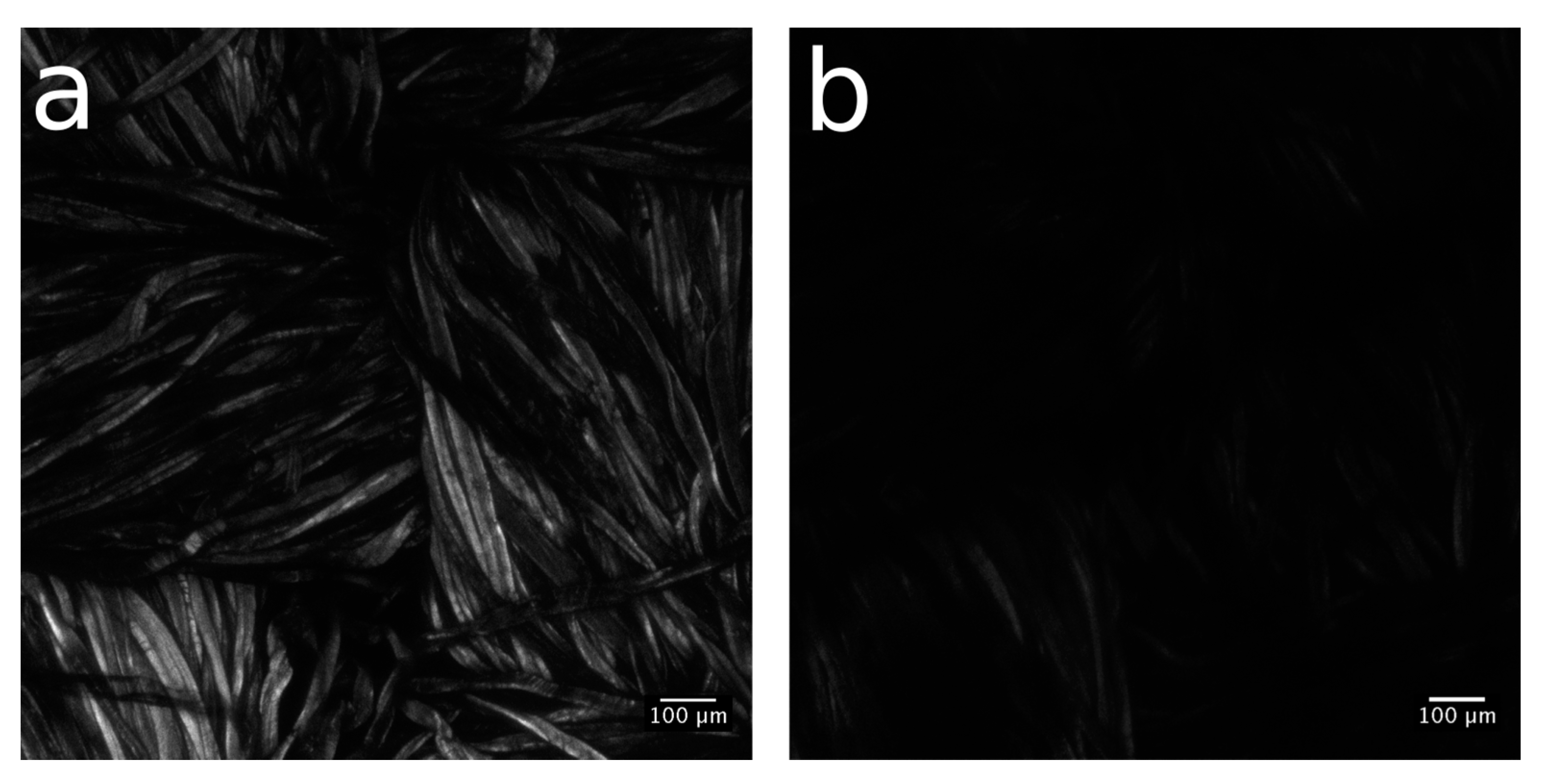
Aiming to estimate the spatial distribution of available thiol groups and, consequently, the extent of alkene functionalization, the confocal microscopy analysis of Cotton-NBB80h, Cotton-NBB80h C14 click and raw cotton reference samples was conducted after binding with 7-diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin fluorophore (CPM). An important characteristic of CPM is that it becomes fully fluorescent in the blue spectral range only when bound to thiols, while it is only weakly fluorescent in the absence of binding.
The raw cotton sample (shown in
Figure S6a) appears brighter and with a more homogeneous emission than the
Cotton-NBB80h sample (
Figure 6a), while practically no fluorescence is visible in
Cotton-NBB80h C14 click (
Figure 6b). The brightest emission of raw cotton is produced by the cotton autofluorescence, which lies in the same spectral region of CPM emission. As it is common for natural fibers, cotton fluorescence has its origin in residual nucleotides, such as nicotinamide adenine dinucleotide phosphate (NADPH), and in the structure of cellulose, though the molecular origin of the latter phenomenon is still poorly understood [
28,
29]. Therefore, while it is not possible to determine the extent of CPM binding to SH-NBBs, the lower emission of
Cotton-NBB80h can be reasonably ascribed to the quenching of cotton self-fluorescence resulting from the surface functionalization with SH-NBBs. Moreover, the evident reduced emission after click coupling (
Figure 6b) can be taken as proof of the presence of a further functionalization of the surface, which derives from the reaction of the thiol groups available on the coating with the alkene.
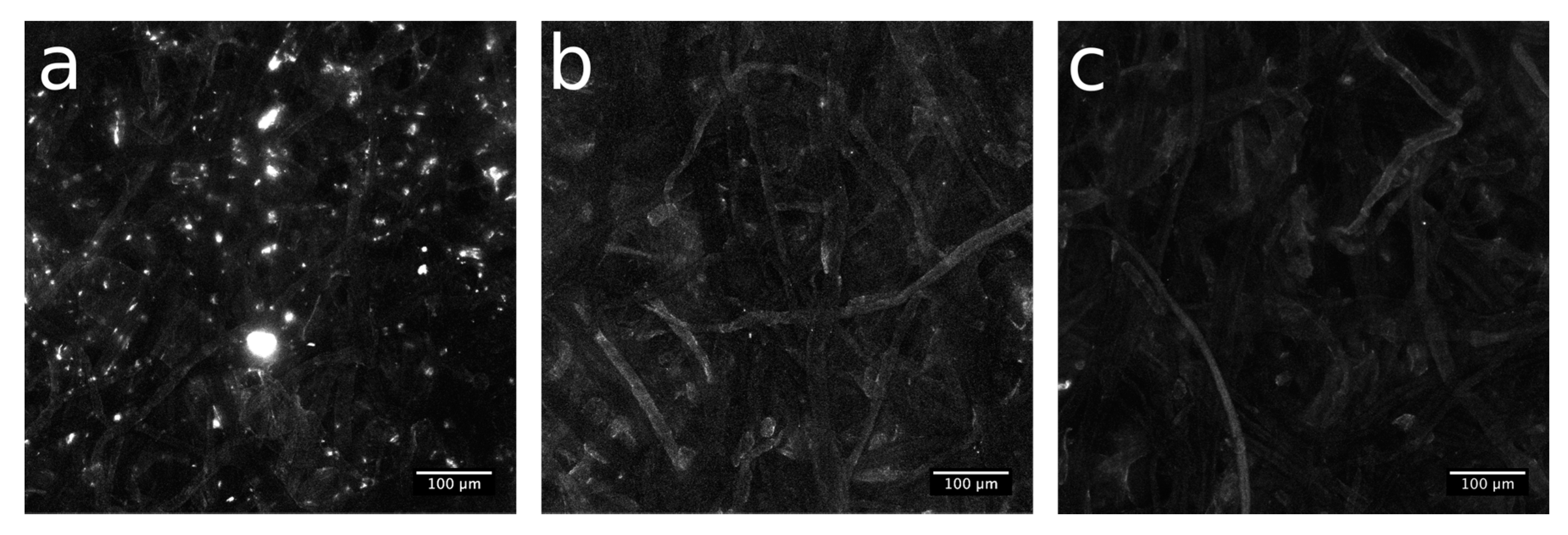
Even if raw paper exhibits a strong autofluorescence signal (
Figure S6b), the spatial distribution of available thiol groups in the NBB coating before and after the click coupling was evaluated by means of confocal microscopy, after reaction with fluorescein diacetate 5-maleimide (FDM). As visible in
Figure 7a,
Paper-NBB exhibits diffused fiber fluorescence, with high intensity bright spots that could be addressed to a high local confinement of the FDM dye. After the click reaction (15’ and 30’,
Figure 7b,c, respectively) the bright spots are switched off. Moreover, the overall fluorescence emission evidences a tendency to reduce with the UV exposure time and, thus, with the progression of the click reaction that reduces the available SH groups at the surface.
Owing to the different characteristics of the substrates, the performance of the two-step surface functionalization as a hydrophobization treatment was evaluated with different techniques for cotton and paper substrates.
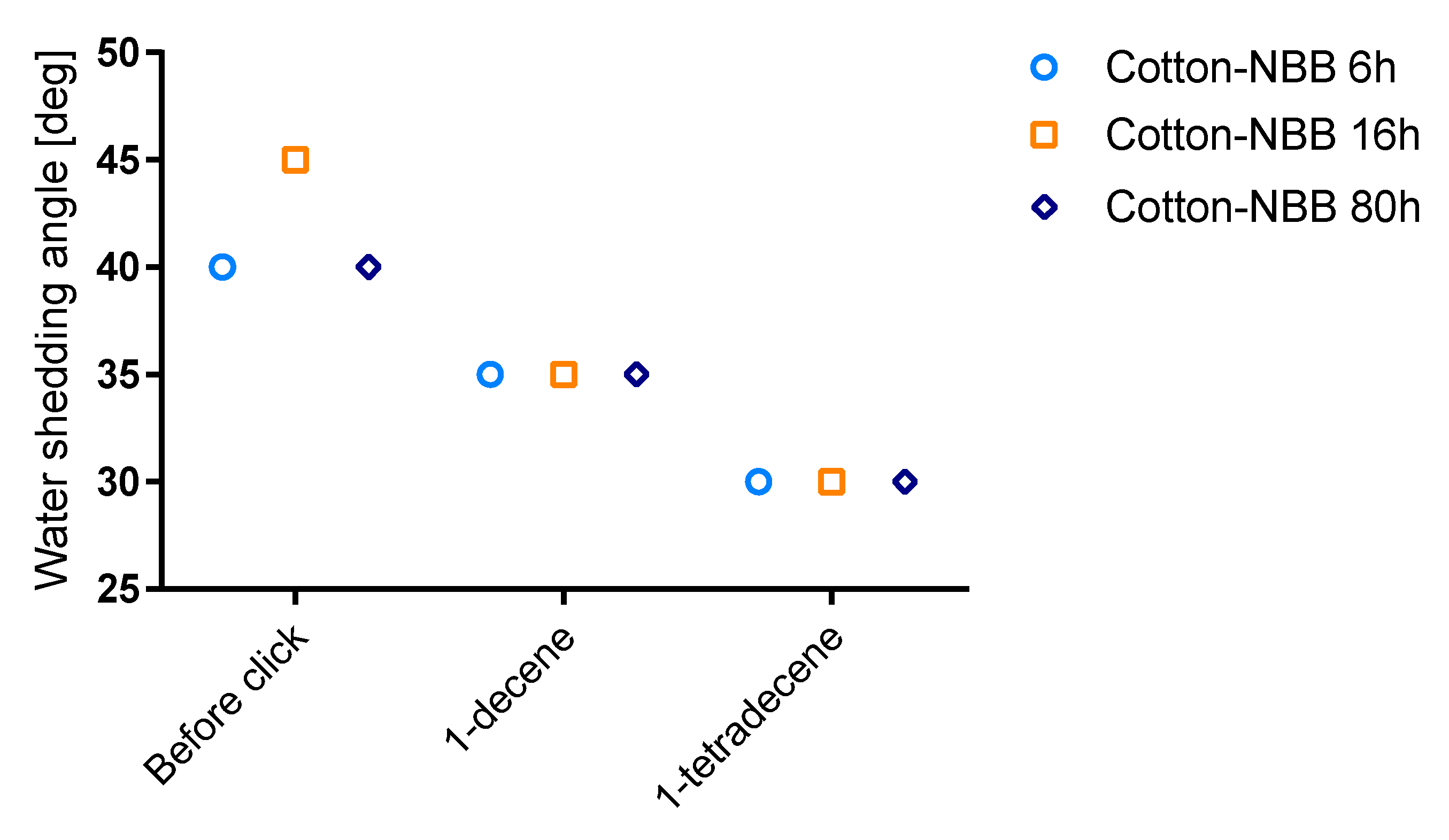
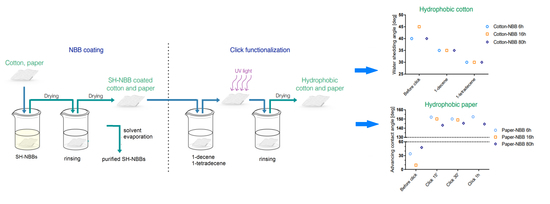
Wettability of cotton samples was evaluated by the means of water shedding angle (ω) measurements, a technique specifically developed for rough, non-reflective textile surfaces such as cotton [
30]. The results of the analysis are summarized in
Figure 8; raw cotton samples showed ω values higher than 70° and are not reported in the figure. ω decreases both after NBB coating and click functionalization of the samples. Only a small difference was observed among coated samples, with
Cotton-NBB6h and
Cotton-NBB80h showing better performance than
Cotton-NBB16h, but the difference disappeared after click functionalization. It is worth of noting that C14 functionalized samples appear to be more hydrophobic than those functionalized with C10.
However, when water droplets are pinned on
Cotton-NBB samples, after a very short time they are absorbed into the fabric. On the other hand, droplets pinned on the click functionalized samples remain indefinitely on the fabric surface. The behavior of
Cotton-NBB samples that show an instaneous hydrophobic character suggests that the coating, although characterized by sub-micrometric surface roughness, is not sufficient to permit a stable surface hydrophobicity. On the other hand, after click functionalization, the introduced long alkyl chains, together with the hierarchical roughness conferred by the coating, can satisfy both the chemical and morphological conditions for stable hydrophobicity of the surface. Indeed, at least for the samples reacted with C14, the obtained ω values are comparable with those reported in the literature for superhydrophobic cotton fabrics obtained by silicon nanofilament coating [
31].
The higher hydrophobicity of C14 functionalized samples could be related to the interactions occurring among the alkyl chains, which can increase with the functionalization density [
32]. The grafted alkyl chains are free to rotate and can fold to find the best accommodation over the material surface, thus offering a degree of hydrophobic coverage that is probably higher for C14 than for C10.
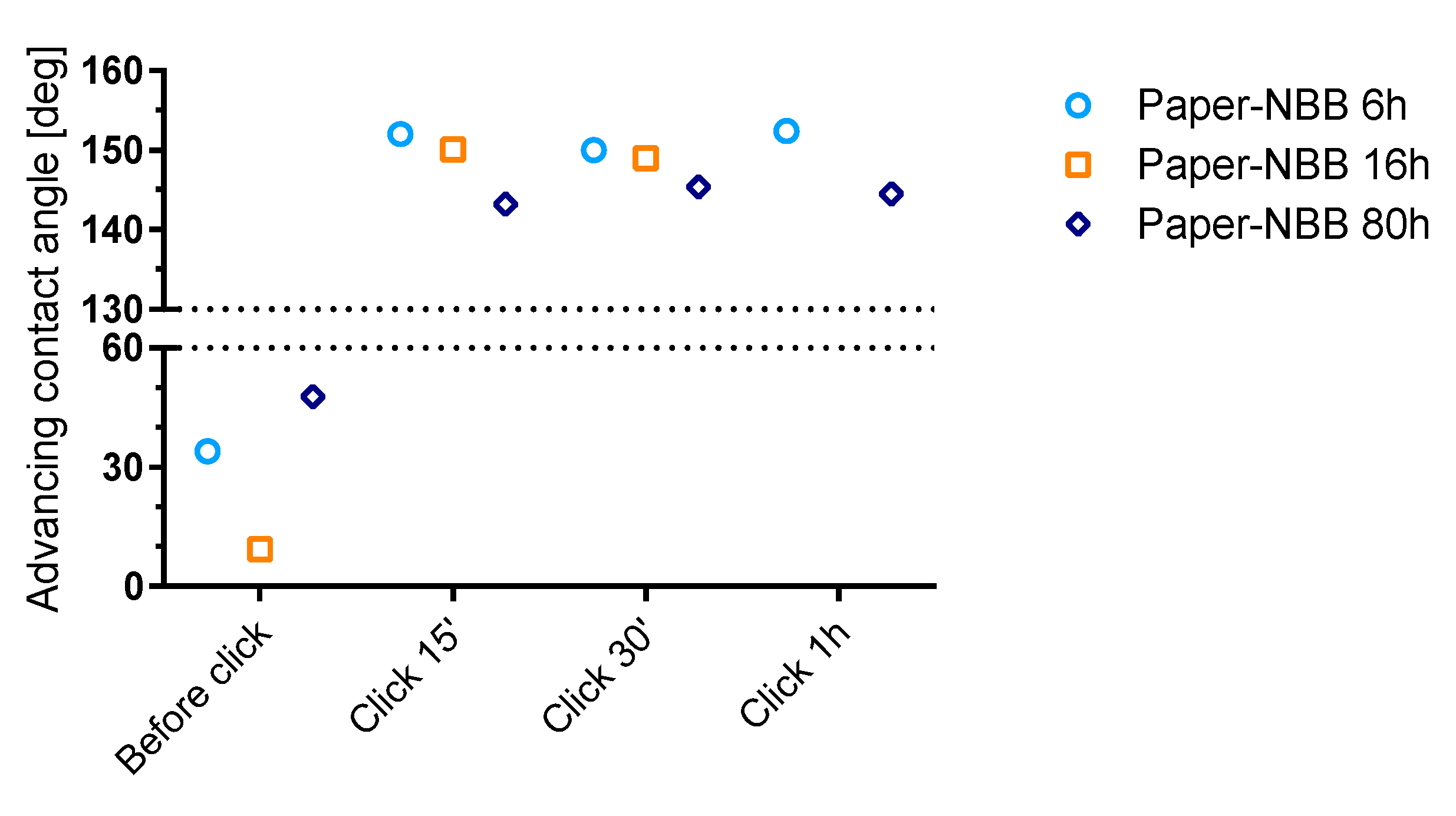
Water contact angle on paper samples was evaluated by the means of the Wilhemly technique. Force curves were analyzed to evaluate the advancing contact angle (
Figure 9). The receding angle (ϑ
rec) was impossible to evaluate because samples resulted completely wet after the advancing phases. This fact is explained by water adsorption due to defects on the sample edges and the capillary rise into the paper fibers network.
Raw paper samples, as expected, appeared to be completely hydrophilic with ϑ
adv compatible with 0° (not reported in the
Figure 9). ϑ
adv, which slightly increased after NBB coating, was found to significantly increase after click functionalization, reaching values greater than 140°.
The high values of ϑadv observed in Paper-NBB click samples, typical of highly hydrophobic material, confirm the effectiveness of the proposed two-step surface functionalization process as a hydrophobization procedure. As observed for cotton samples, it appears that no substantial difference exists between samples coated with SH-NBBs prepared at different reaction times, although Paper-NBB6h click shows the highest ϑadv values. Moreover, it can be clearly seen that no remarkable change is observable with increasing irradiation times, and 15 min is a sufficient time to achieve the maximum observed hydrophobicity. This result would be in good accordance with the fast kinetics of the thiol-ene coupling between SH-NBBs and the C14 observed in solution.
According to the results obtained on cotton and paper, the click functionalization step is effective in introducing hydrophobic alkyl chains on the material surface while keeping the surface roughness obtained as a result of the NBB coating step. Consequently, at the end of the two-step surface functionalization, samples possess both the chemical and morphological requirements for extreme wettability. Therefore, the two-step surface functionalization process here proposed is a viable process for obtaining the efficient hydrophobization of natural materials by combined sol-gel and initiator free thiol-ene chemistry. In perspective, the preparation of superhydrophobic materials without employing fluorinated compounds and based on cellulose appears as a good response to the request of low environmental impact materials for water remediation.