The Effect of Precipitate Evolution on Austenite Grain Growth in RAFM Steel
Abstract
:1. Introduction
2. Experimental Procedure
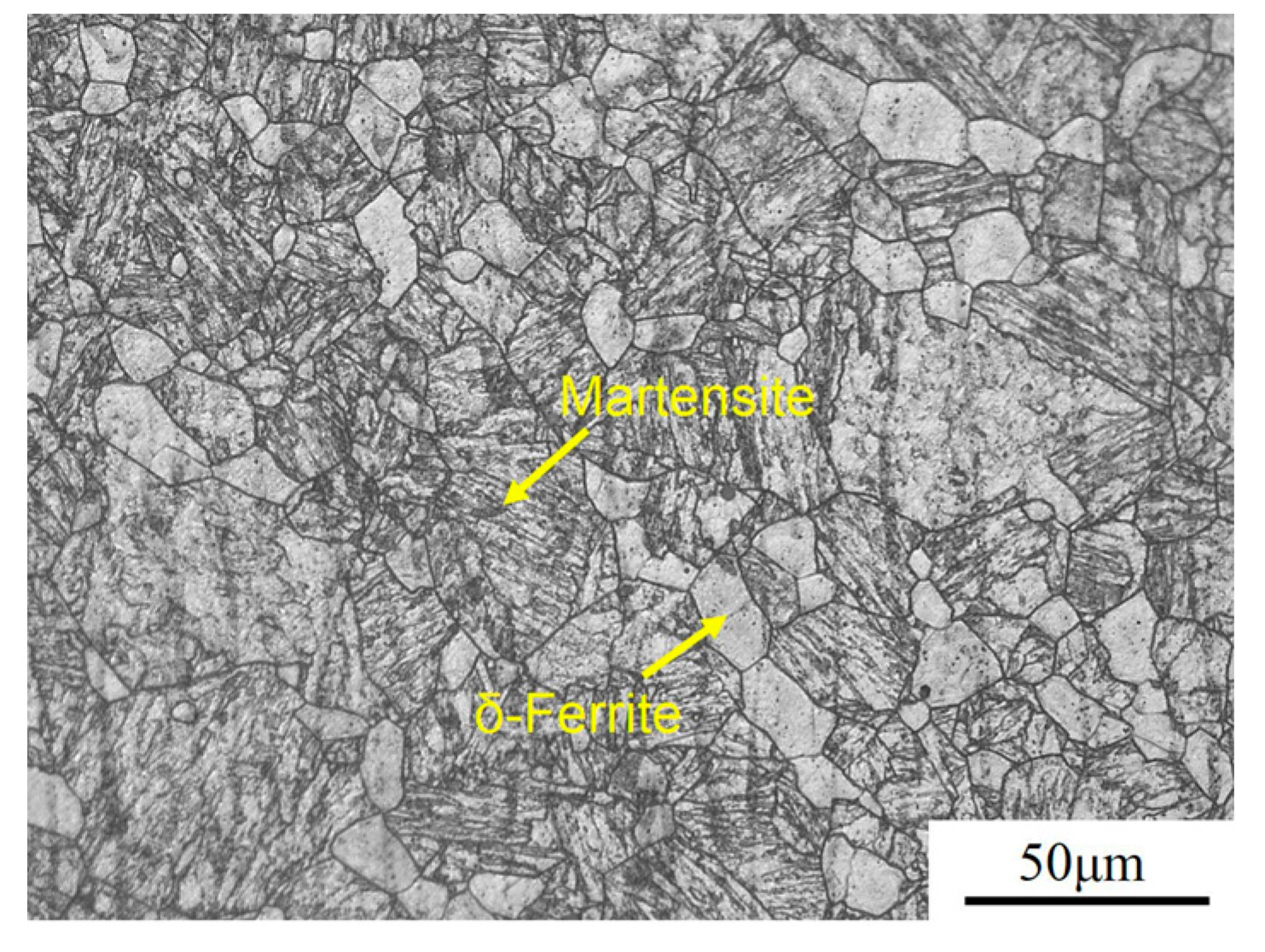
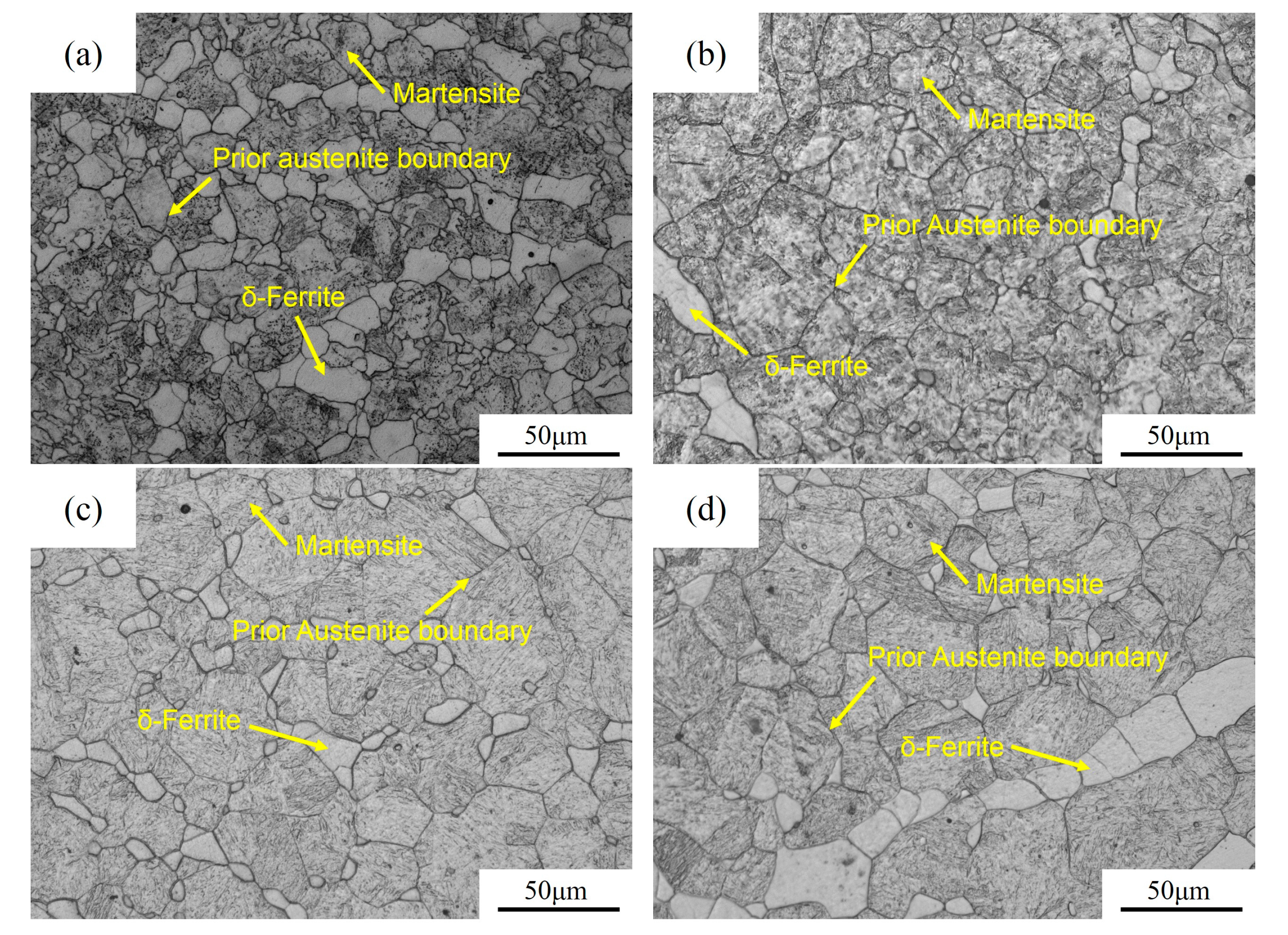
3. Results
4. Discussion
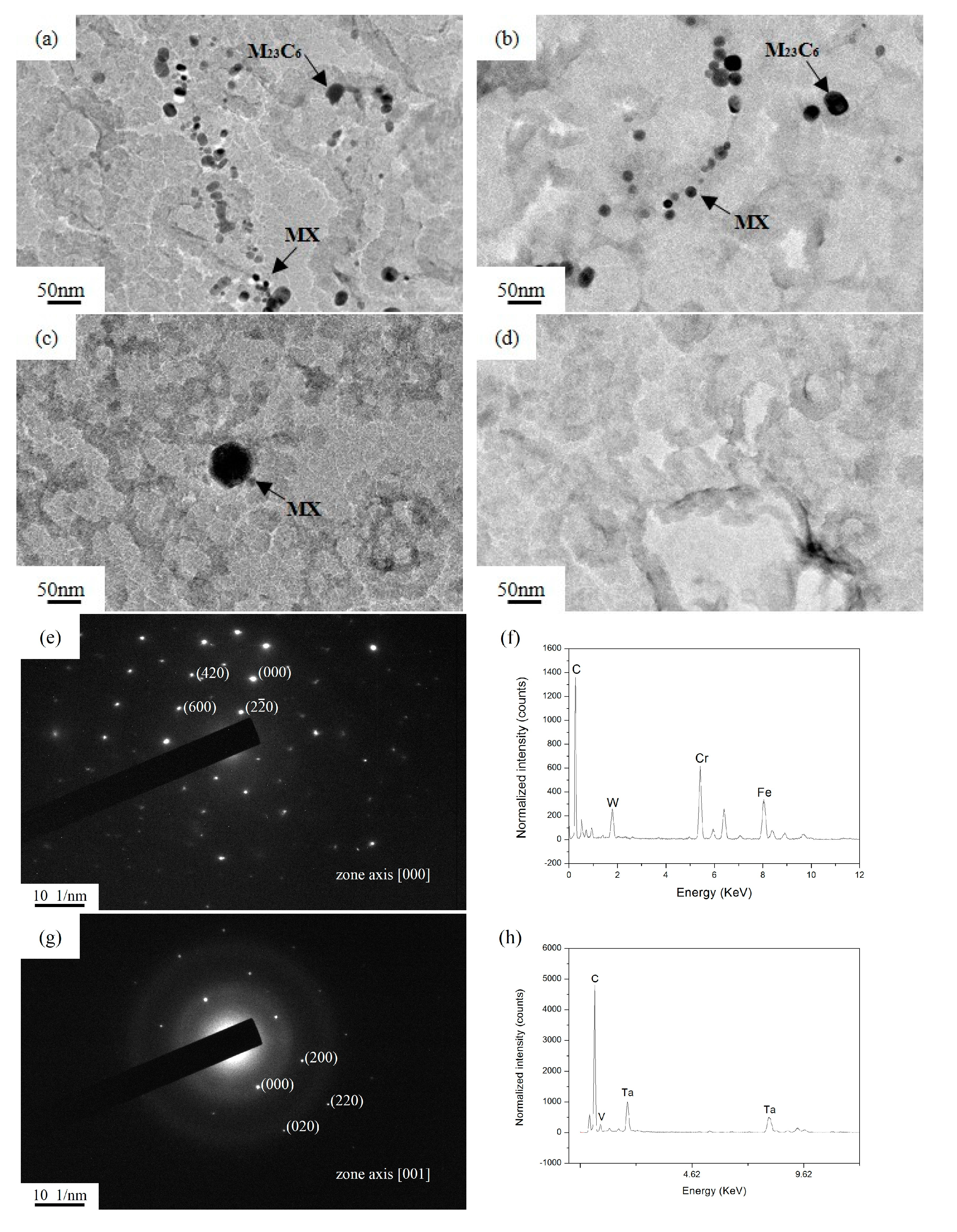
4.1. The Evolution of M23C6 and MX Precipitates
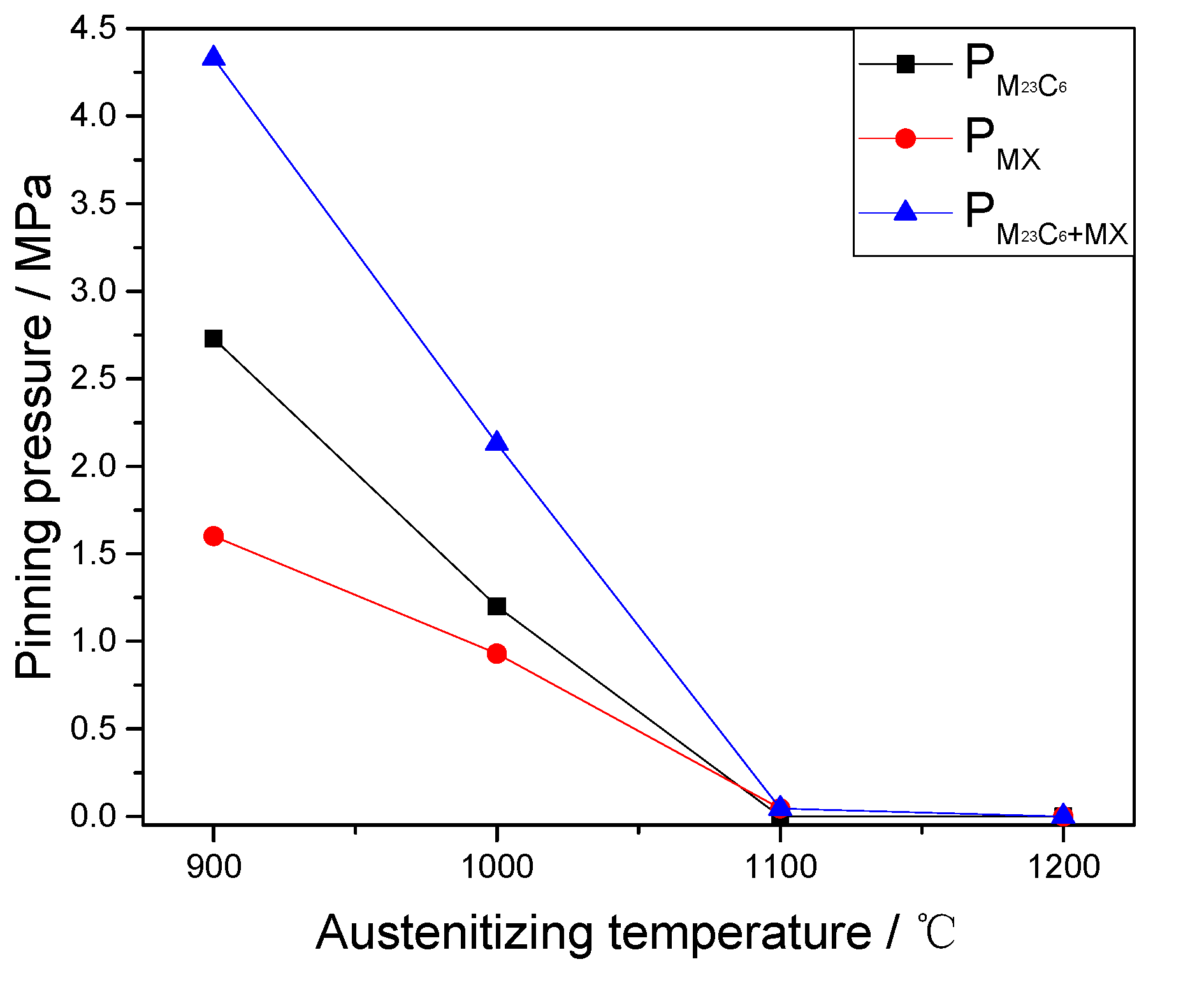
4.2. Effects of Precipitates on Austenite Sizes
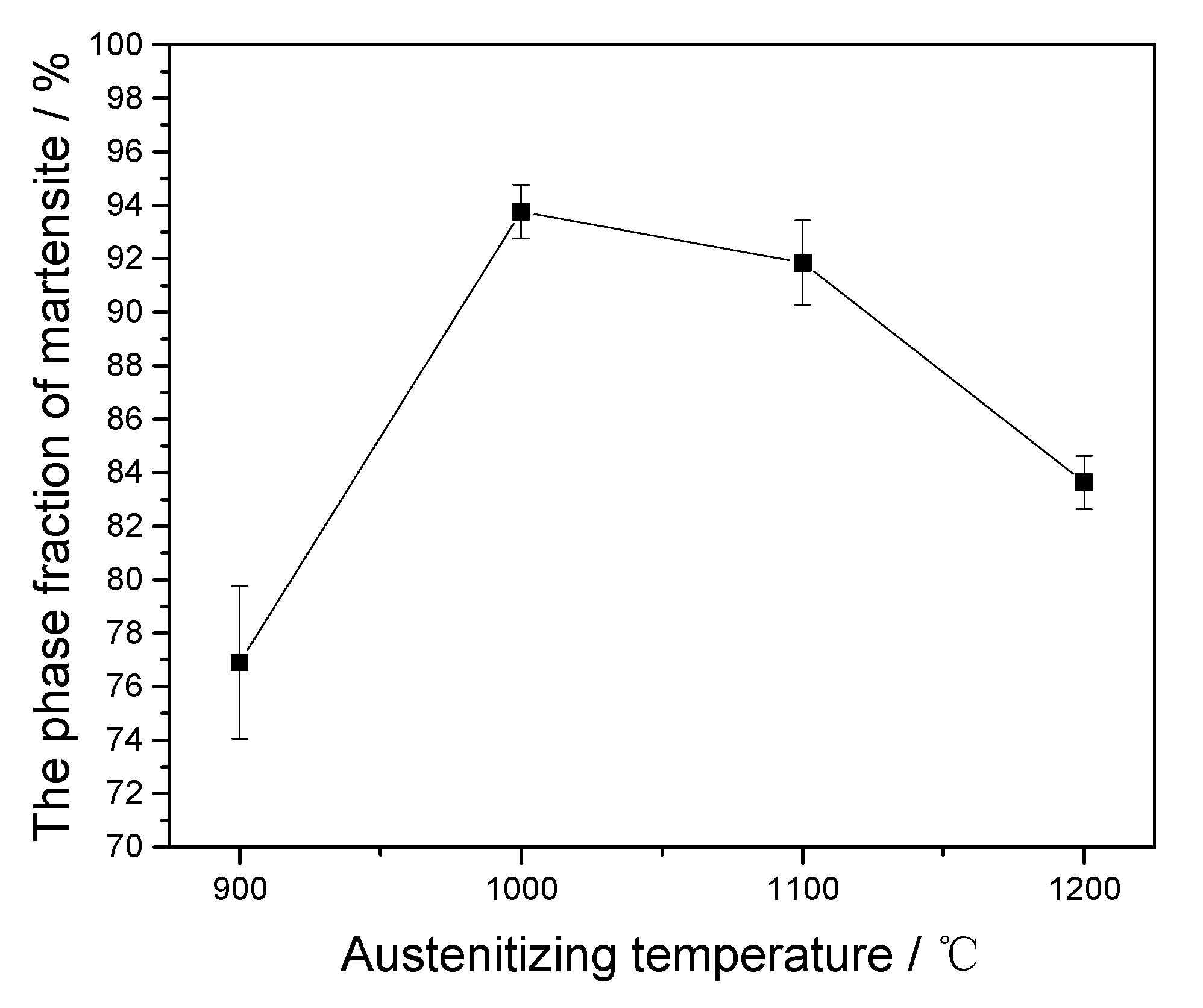
4.3. Effect of Precipitates on Austenite Phase Fraction
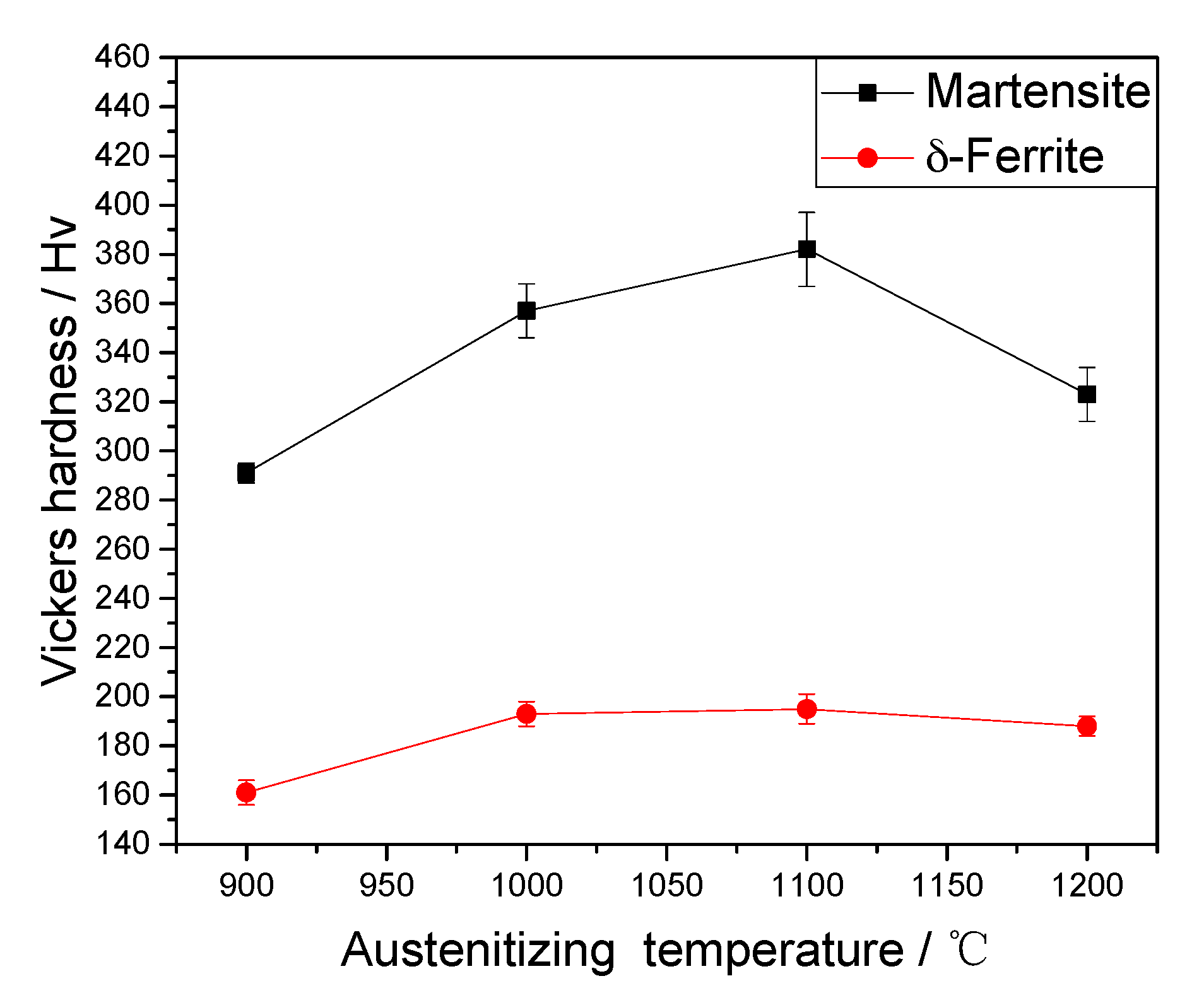
4.4. Vickers Hardness
5. Summary and Conclusions
- (1)
- The M23C6 and MX precipitates gradually coarsen and dissolve into the matrix as the austenitizing temperature increases. The M23C6 precipitates dissolve completely at 1100 °C, while the MX precipitates dissolve completely at 1200 °C.
- (2)
- The increase of austenite grain size is retarded due to the retarding of two different types of precipitate (M23C6 and MX) with the sizes of 9–70 nm by exerting a pinning pressure on the grain boundaries. The coarsening and dissolution of M23C6 and MX precipitates also result in the reduction and removal of the pinning pressure on grain boundaries, which contributes to the free growth of the austenite grains.
- (3)
- The austenite phase fraction increases first and then gradually decreases as the austenitizing temperature increases. The dissolution of the two types of precipitate has an important effect on the change of the austenite phase fraction.
- (4)
- With the increase of austenitizing temperatures, the hardness of δ-ferrite is nearly constant, while the hardness of martensite first increases and then decreases. The precipitate dissolution is propitious to the increase in the hardness of martensite, while the formation of δ-ferrite and prior austenite grain growth is disadvantageous to the increase in the hardness of martensite.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muroga, T.; Gasparotto, M.; Zinkle, S.J. Overview of materials research for fusion reactors. Fusion Eng. Des. 2002, 61, 13–25. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Wu, Y.; Yu, J.; Wan, F. The development of low activation martensitic steels for fusion reactor. Chin. J. Nucl. Sci. Eng. 2004, 24, 56–64. [Google Scholar]
- Klueh, R.L. Reduced-activation steels: Future development for improved creep strength. J. Nucl. Mater. 2008, 378, 159–166. [Google Scholar] [CrossRef]
- Ehrlich, K.; Kelzenberg, S.; Röhrig, H.D.; Schäfer, L.; Schirra, M. The development of ferritic-martensitic steels with reduced long-term activation. J. Nucl. Mater. 1994, 212, 678–683. [Google Scholar] [CrossRef]
- Van der Schaaf, B.; Gelles, D.S.; Jitsukawa, S.; Kimura, A.; Klueh, R.L.; Möslang, A.; Odette, G.R. Progress and critical issues of reduced activation ferritic/martensitic steel development. J. Nucl. Mater. 2000, 283, 52–59. [Google Scholar] [CrossRef]
- Białobrzeska, B.; Konat, Ł.; Jasiński, R. The Influence of Austenite Grain Size on the Mechanical Properties of Low-Alloy Steel with Boron. Metals 2017, 7, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, M.; Zheng, Y.; Liu, S.; Wang, W.; Zhang, Z. Finite-element thermal analysis and grain growth behavior of HAZ on argon Tungsten-arc welding of 443 stainless steel. Metals 2016, 6, 77. [Google Scholar] [CrossRef]
- Kohyama, A.; Hishinuma, A.; Gelles, D.S.; Klueh, R.L.; Dietz, W.; Ehrlich, K. Low-activation ferritic and martensitic steels for fusion application. J. Nucl. Mater. 1996, 233, 138–147. [Google Scholar] [CrossRef]
- Maalekian, M.; Radis, R.; Militzer, M.; Moreau, A.; Poole, W. In situ measurement and modelling of austenite grain growth in a Ti/Nbmicroalloyed steel. Acta Mater. 2012, 60, 1015–1026. [Google Scholar] [CrossRef]
- Hu, H.; Rath, B. On the time exponent in isothermal grain growth. Metall. Mater. Trans. 1970, 1, 3181–3184. [Google Scholar]
- Wang, H.R.; Wang, W. Coupled model for particle dissolution and coarsening in microalloyed steels. Mater. Sci. Technol. 2007, 23, 1305–1308. [Google Scholar] [CrossRef]
- Fu, L.M.; Wang, H.R.; Wang, W.; Shan, A.D. Austenite grain growth prediction coupling with drag and pinning effects in low carbon Nbmicroalloyed steels. Mater. Sci. Technol. 2011, 27, 996–1001. [Google Scholar] [CrossRef]
- Shahandeh, S.; Militzer, M. Grain boundary curvature and grain growth kinetics with particle pinning. Philos. Mag. 2013, 93, 3231–3247. [Google Scholar] [CrossRef]
- Roy, S.; Karmakar, A.; Mukherjee, S.; Kundu, S.; Srivastava, D.; Chakrabarti, D. Effect of starting microstructure on austenite grain sizes developed after reheating of HSLA steel. Mater. Sci. Technol. 2014, 30, 1142–1153. [Google Scholar] [CrossRef]
- Alogab, K.A.; Matlock, D.K.; Speer, J.G.; Kleebe, H.J. The influence of Niobium microalloying on austenite grain coarsening behavior of Ti-modified SAE 8620 Steel. ISIJ Int. 2007, 47, 307–316. [Google Scholar] [CrossRef]
- Adrian, H.; Pickering, F.B. Effect of titanium additions on austenite grain growth kinetics of medium carbon V–Nb steels containing 0.008–0.018% N. Mater. Sci. Technol. 1991, 7, 176–182. [Google Scholar] [CrossRef]
- Yan, X.F.; Zhang, H.T.; Wang, R.Z.; Pang, G.Y. Austenite Grain Coarsening and NbC Dissolution-Precipitation Behavior in Niobium-Bearing Steel 16Mn. J. Iron Steel Res. 2000, 12, 49–53. [Google Scholar]
- Zhou, X.S.; Liu, C.X.; Yu, L.M.; Liu, Y.C.; Li, H.J. Phase transformation behavior and microstructural control of high-Cr martensitic/ferritic heat-resistant steels for power and nuclear plants: A review. J. Mater. Sci. Technol. 2015, 31, 235–242. [Google Scholar] [CrossRef]
- Tian, D.W.; Karjalainen, L.P.; Qian, B.; Chen, X. Nonuniform distribution of carbonitride particles and its effect on prior austenite grain size in the simulated coarse-grained heat-affected zone of thermomechanical control-processed steels. Metall. Mater. Trans. A 1996, 27, 4031–4038. [Google Scholar] [CrossRef]
- Sha, Q.Y.; Sun, Z.Q. Grain growth behavior of coarse-grained austenite in aNb–V–Timicroalloyed steel. Mater. Sci. Eng. A 2009, 523, 77–84. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, G.Q.; Hu, B.F.; Wang, J.S.; Ma, W.B. Microstructure Stability of V and Ta Microalloyed 12% Cr Reduced Activation Ferrite/Martensite Steel during Long-term Aging at 650 °C. J. Mater. Sci. Technol. 2015, 31, 311–319. [Google Scholar] [CrossRef]
- Uhm, S.; Moon, J.; Lee, C.; Yoon, J.; Lee, B. Prediction model for the austenite grain size in the coarse grained heat affected zone of Fe-C-Mn steels: Considering the effect of initial grain size on isothermal growth behavior. ISIJ Int. 2004, 44, 30–1237. [Google Scholar] [CrossRef]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five decades of the Zener equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef]
- Maropoulos, S.; Karagiannis, S.; Ridley, N. The effect of austenitising temperature on prior austenite grain size in a low-alloy steel. Mater. Sci. Eng. A 2008, 483, 735–739. [Google Scholar] [CrossRef]
- Banerjee, K.; Militzer, M.; Perez, M.; Wang, X. Nonisothermalaustenite grain growth kinetics in a microalloyed X80 linepipe steel. Metall. Mater. Tran. A 2010, 41, 3161–3172. [Google Scholar] [CrossRef]
- Azghandi, S.H.M.; Ahmadabadi, V.G.; Zabett, A.; Fazeli, F. Modelling of austenite grain growth kinetics in a microalloyed steel (30MSV6) in the presence of carbonitride precipitates. Philos. Mag. 2014, 94, 2758–2775. [Google Scholar] [CrossRef]
- Zener, C. Grains, phases, and interfaces: An interpretation of microstructure. Metall. Mater. Trans. A 1948, 175, 15–51. [Google Scholar]
- Militzer, M.; Hawbolt, E.B.; Meadowcroft, T.R.; Giumelli, A. Austenite grain growth kinetics in Al-killed plain carbon steels. Metall. Mater. Tran. A 1996, 27, 3399–3409. [Google Scholar] [CrossRef]
- Chen, J.G.; Liu, Y.C.; Liu, C.X.; Yan, B.Y.; Li, H.J. Effects of tantalum on austenitic transformation kinetics of RAFM steel. J. Iron Steel Res. 2017, 24, 705–710. [Google Scholar] [CrossRef]
- Chen, J.G.; Liu, C.X.; Liu, Y.C.; Yan, B.Y.; Li, H.J. Effects of tantalum content on the microstructure and mechanical properties of low-carbon RAFM steel. J. Nucl. Mater. 2016, 479, 295–301. [Google Scholar] [CrossRef]
- Morito, S.; Saito, H.; Ogawa, T.; Furuhara, T.; Maki, T. Effect of austenite grain size on the morphology and crystallography of lath martensite in low carbon steels. ISIJ. Int. 2005, 45, 91–94. [Google Scholar] [CrossRef]
- Morito, S.; Yoshida, H.; Maki, T.; Huang, X. Effect of block size on the strength of lath martensite in low carbon steels. Mater. Sci. Eng. A 2006, 438, 237–240. [Google Scholar] [CrossRef]
- Abe, F. Precipitate design for creep strengthening of 9% Cr tempered martensitic steel for ultra-supercritical power plants. Sci. Technol. Adv. Mater. 2008, 9, 013002. [Google Scholar] [CrossRef] [PubMed]
- Petch, N.J. The Cleavage Strengh of Polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Di Schino, A.; Kenny, J.M. Grain refinement strengthening of a micro-crystalline high nitrogen austenitic stainless steel. Mater. Lett. 2003, 57, 1830–1834. [Google Scholar] [CrossRef]


| C | Cr | W | Mn | Si | V | Ta | Fe |
|---|---|---|---|---|---|---|---|
| 0.04 | 8.93 | 1.71 | 0.44 | 0.04 | 0.22 | 0.073 | Bal |
| Parameter | Precipitate | Austenitizing Temperature/°C | |||
|---|---|---|---|---|---|
| 900 | 1000 | 1100 | 1200 | ||
| M23C6 | 0.00650 | 0.00380 | — | — | |
| MX | 0.00089 | 0.00066 | 0.00015 | — | |
| /nm | M23C6 | 21.71 | 28.84 | — | — |
| MX | 5.07 | 6.50 | 30.49 | — | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Liu, Y.; Wang, Z.; Liu, C.; Si, Y.; Li, H.; Yu, J. The Effect of Precipitate Evolution on Austenite Grain Growth in RAFM Steel. Materials 2017, 10, 1017. https://doi.org/10.3390/ma10091017
Yan B, Liu Y, Wang Z, Liu C, Si Y, Li H, Yu J. The Effect of Precipitate Evolution on Austenite Grain Growth in RAFM Steel. Materials. 2017; 10(9):1017. https://doi.org/10.3390/ma10091017
Chicago/Turabian StyleYan, Biyu, Yongchang Liu, Zejun Wang, Chenxi Liu, Yonghong Si, Huijun Li, and Jianxing Yu. 2017. "The Effect of Precipitate Evolution on Austenite Grain Growth in RAFM Steel" Materials 10, no. 9: 1017. https://doi.org/10.3390/ma10091017




