Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Films Coloration (Visual and Image Analyzer Software)
2.2. Apparent Density
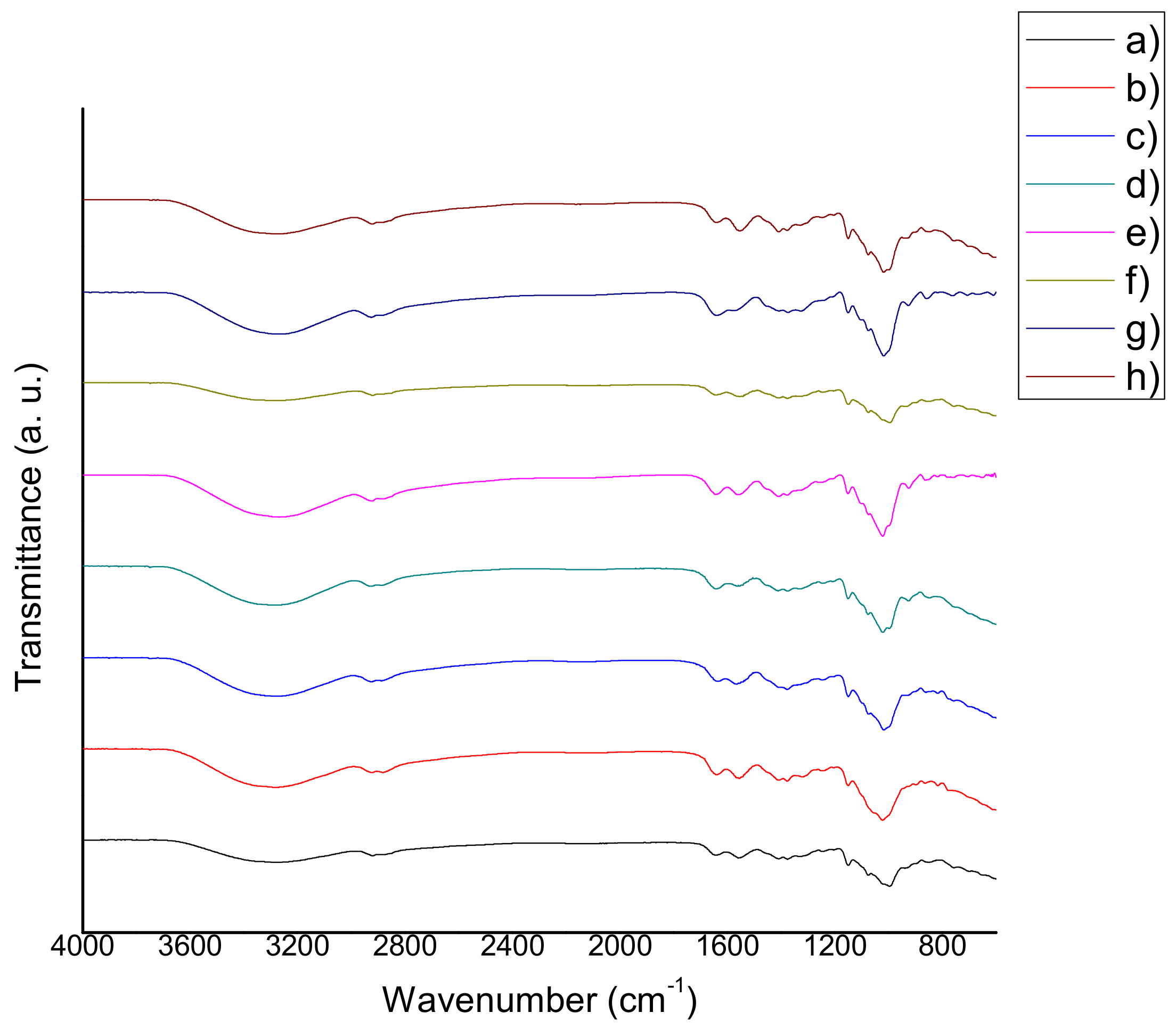
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Determination of Antioxidant Capacity (Reducing Capacity) of the Films
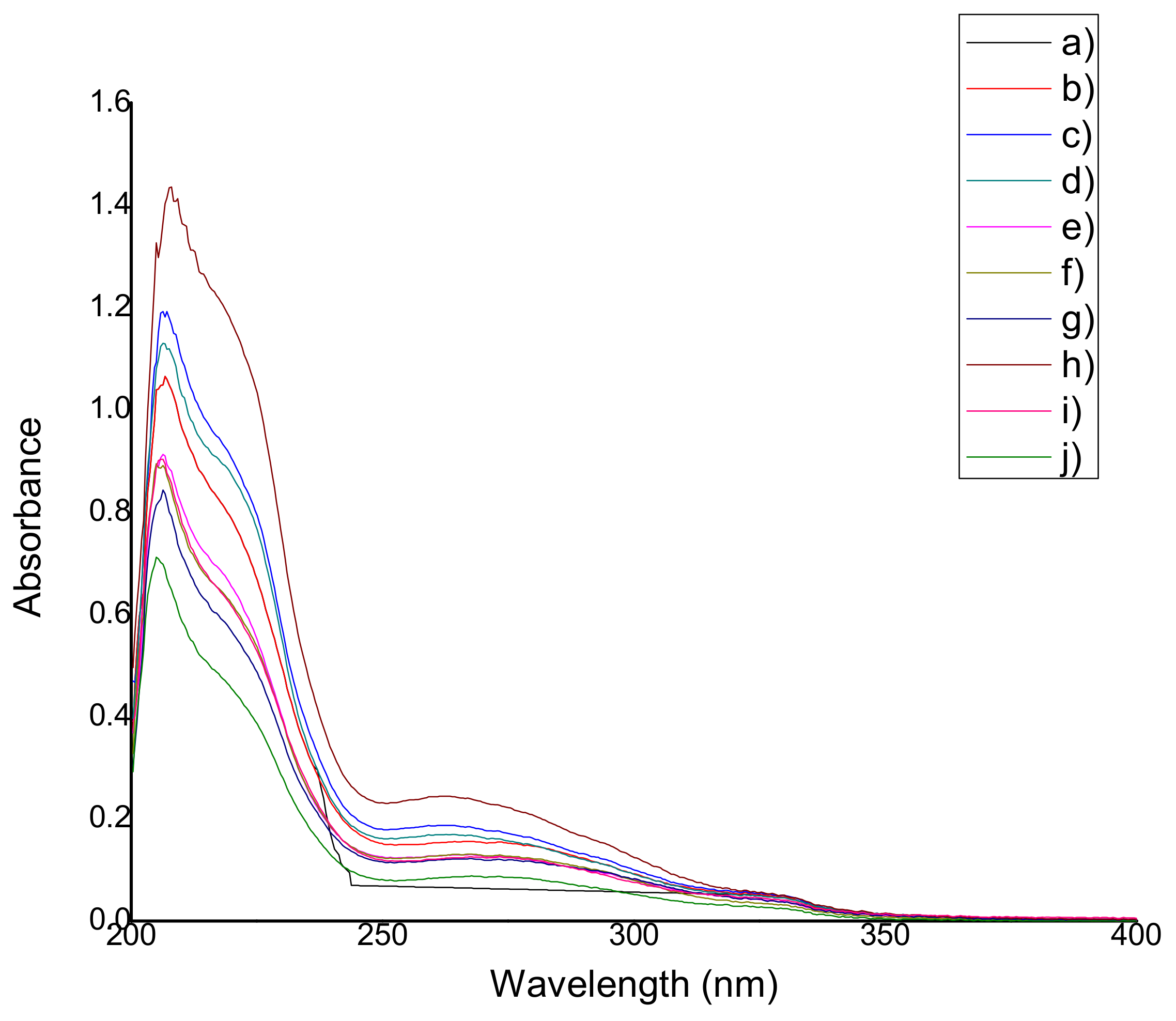
2.4.1. Qualification of Natural Antioxidants
2.4.2. Antioxidant Capacity (Reducing Capacity)
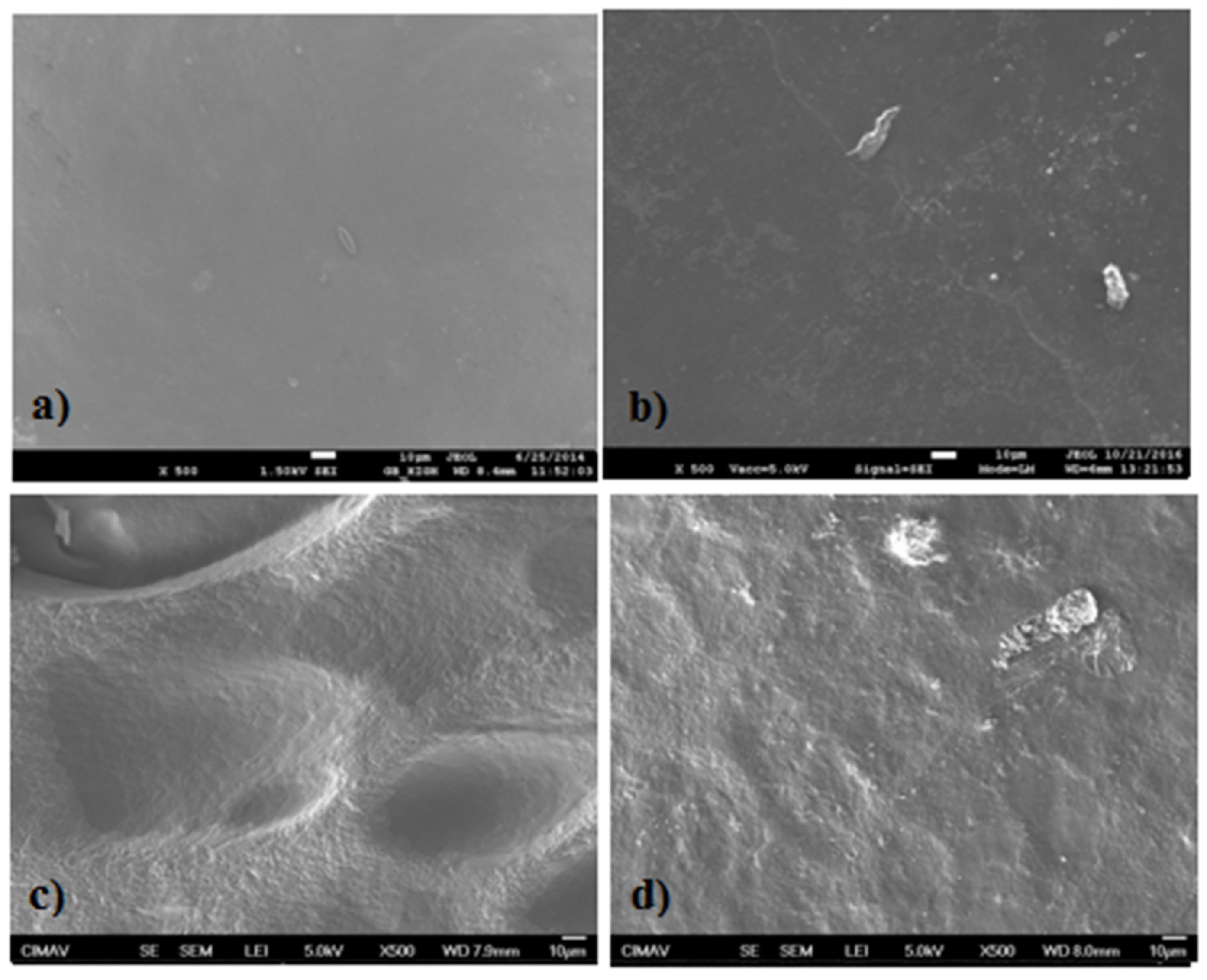
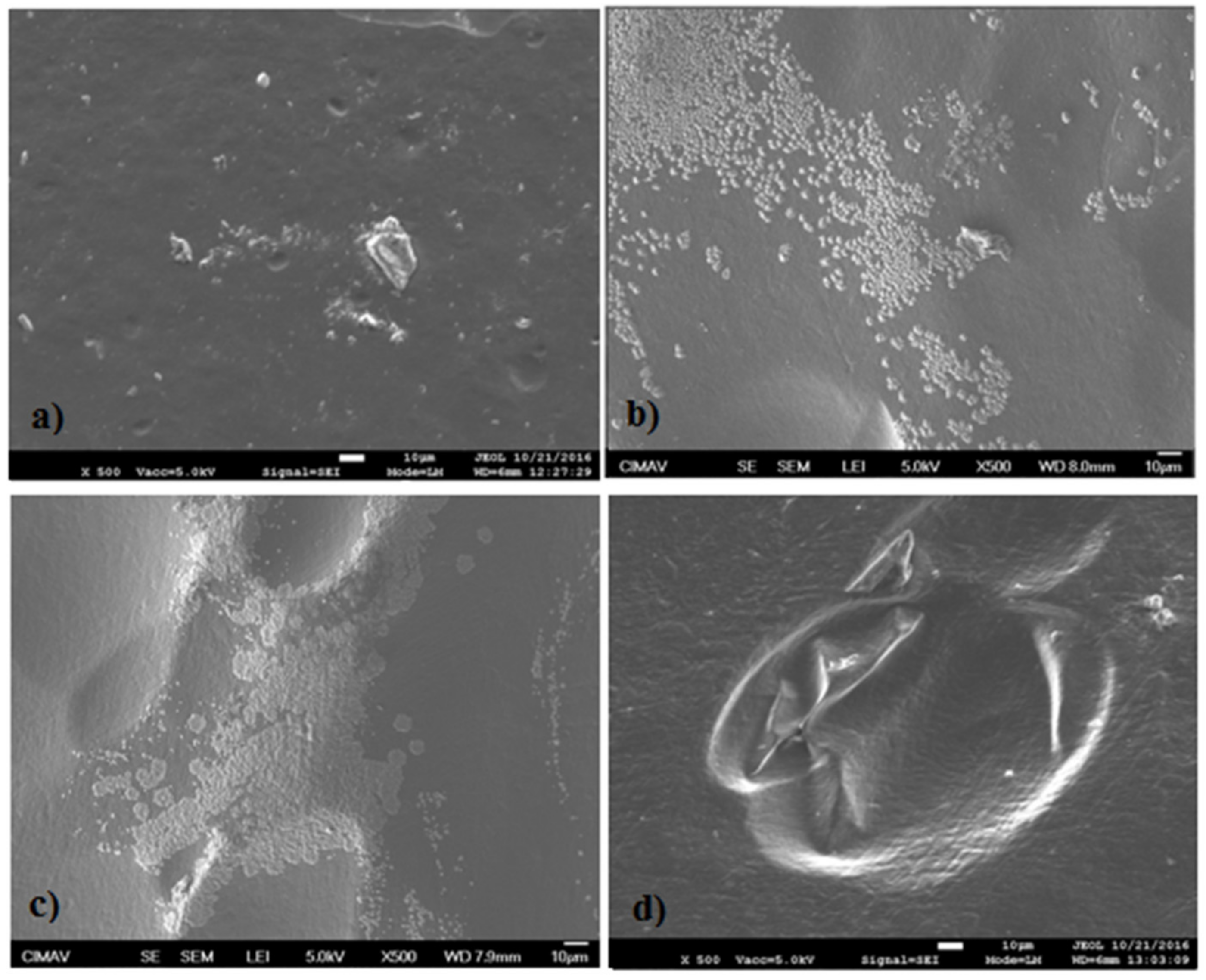
2.5. Scanning Electronic Microscopy (SEM)
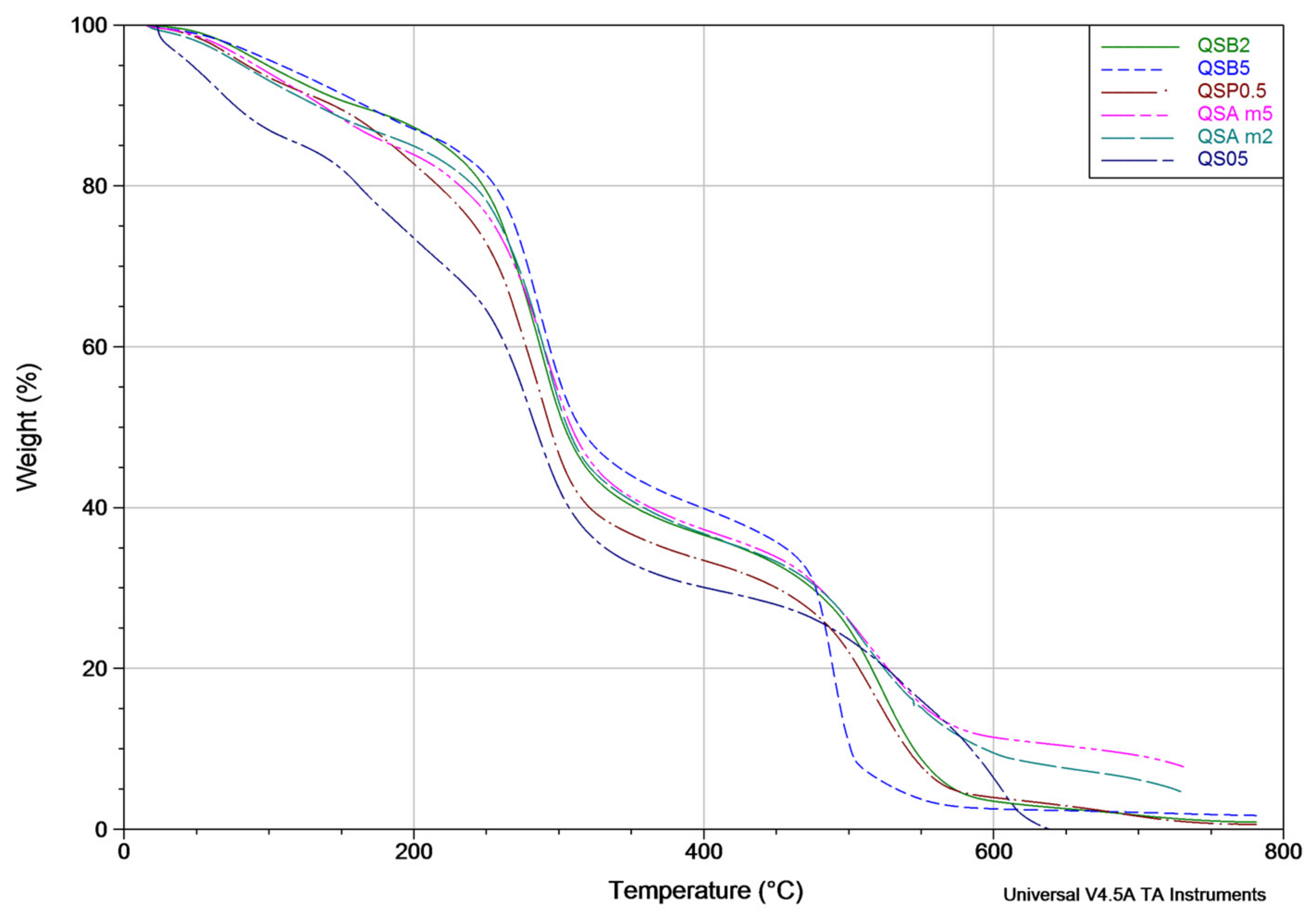
2.6. Thermogravimetric Analysis (TGA)
3. Discussion
3.1. Evaluation of Films Coloration
3.2. Apparent Density
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Determination of Antioxidant Capacity (Reducing Capacity) of the Films
3.5. Scanning Electronic Microscopy (SEM)
3.6. Thermogravimetric Analysis (TGA)
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Films
4.3. Evaluation of Films Coloration
4.4 Apparent Density
- D = sample diameter
- H = sample height
- W = sample weight.
4.5 Fourier Transform Infrared Spectroscopy (FTIR)
4.6. Determination of Antioxidant Capacity of the Films
4.6.1. Extraction
4.6.2. Qualification of Natural Antioxidants
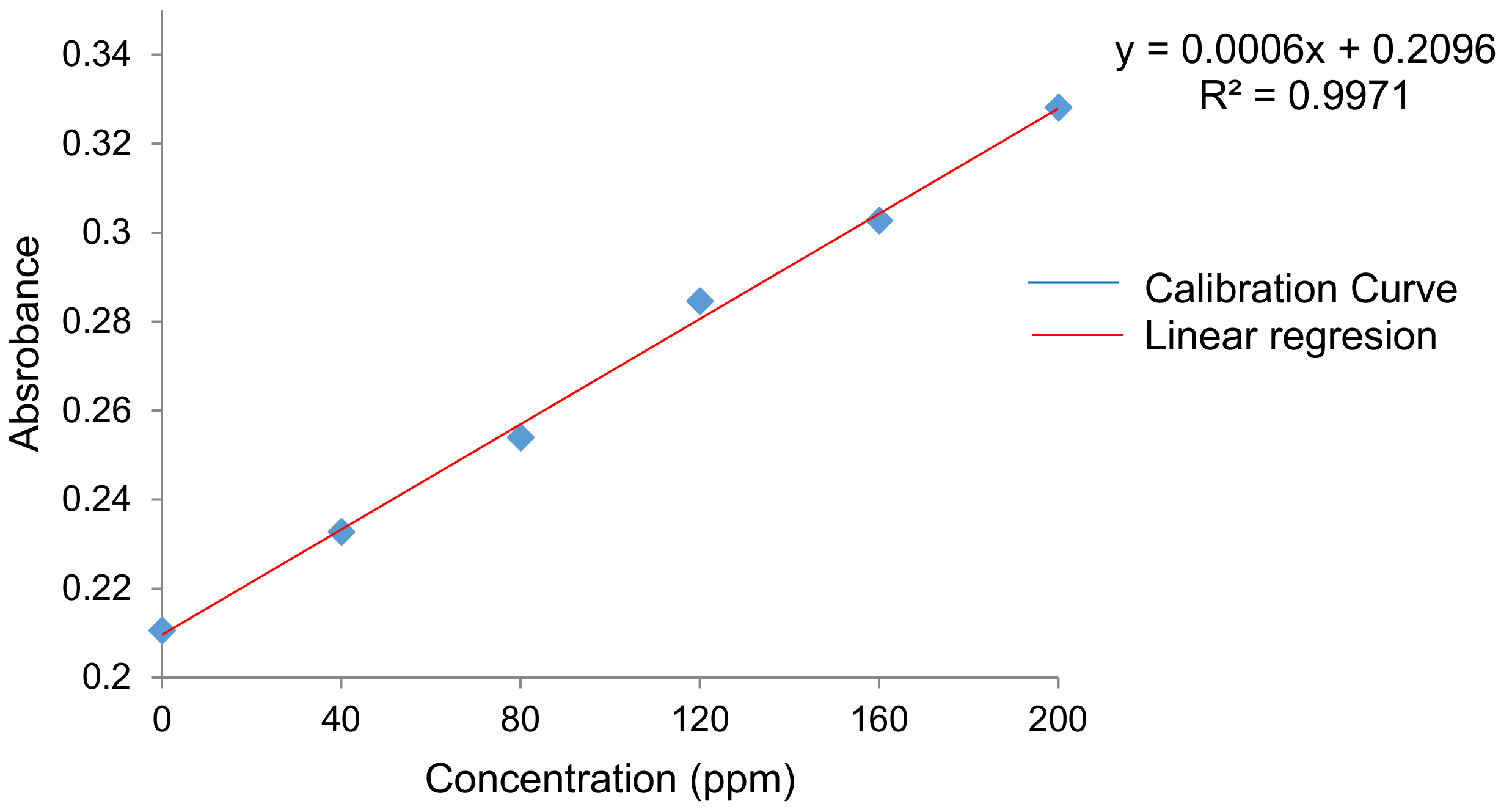
4.6.3. Antioxidant Capacity (Reducing Capacity)
4.7. Scanning Electronic Microscopy (SEM)
4.8. Thermogravimetric Analysis (TGA)
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arancibia, M.Y.; Alemán, A.; Calvo, M.M.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Antimicrobial and antioxidant chitosan solutions enriched with active shrimp (Litopenaeus vannamei) waste materials. Food Hydrocoll. 2014, 35, 710–717. [Google Scholar] [CrossRef]
- Aljawish, A.; Desobry, S.; Muniglia, L.; Klouj, A.; Jasniewski, J.; Scher, J. Characterization of films based on enzymatically modified chitosan derivatives with phenol compounds. Food Hydrocoll. 2016, 60, 551–558. [Google Scholar] [CrossRef]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch-chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, Z.; Hu, H.; Lv, J.; Wang, H.; Jiang, S. Adsorption of procyanidins onto chitosan-modified porous rice starch. LWT-Food Sci. Technol. 2017, 84, 10–17. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural Plant Pigments with Potential Application in Functional Foods. LWT-Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Sánchez-Chávez, W.; Cortez-Arredondo, J.; Solano-Cornejo, M.; Vidaurre-Ruiz, J. Cinética de degradación térmica de betacianinas, betaxantinas vitamina C en una bebida a base de jugo de remolacha (Beta vulgaris L.) y miel de abeja. Sci. Agropecu. 2015, 6, 111–118. [Google Scholar] [CrossRef]
- Aravena, G.; García, O.; Muñoz, O.; Pérez-Correa, J.R.; Parada, J. The impact of cooking and delivery modes of thymol and carvacrol on retention and bioaccessibility in starchy foods. Food Chem. 2016, 196, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A.; Addona, R. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dai, Y.; Cai, J.; Zhong, N.; Xiao, H.; McClements, D.J.; Hu, K. Resveratrol encapsulation in core-shell biopolymer nanoparticles Impact on antioxidant and anticancer activities. Food Hydrocoll. 2017, 64, 157–165. [Google Scholar] [CrossRef]
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Berizi, E.; Hosseinzadeha, S.; Shekarforousha, S.S.; Barbieri, G. Microbial, chemical, textural and sensory properties of coated rainbow trout by chitosan combined with pomegranate peel extract during frozen storage. Int. J. Biol. Macromol. 2018, 106, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Avila-Sosa, R.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Palou, E.; López-Malo, A. Chapter 47 Combinational Approaches for Antimicrobial Packaging: Chitosan and Oregano Oil. In Antimicrobial Food Packaging, 1st ed.; Barros-Velazquez, J., Ed.; Academic Press: London, UK, 2016; pp. 581–588. ISBN 978-0-12-800810-2. [Google Scholar]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [PubMed]
- Kueppers Teoria del Color. Temas Generales. Available online: http://www.ipsi.fraunhofer.de/Kueppersfarbe/es/index.html (accessed on 20 December 2017).
- Auracaria Blog, Densidad Aparente. Available online: http://araucarias.blogspot.mx/2005/09/densidad-aparente.html (accessed on 17 April 2017).
- Gambini, J.; López-Grueso, R.; Olaso-González, G.; Inglés, M.; Abdelazid, K.; El Alami, M.; Bonet-Costa, V.; Borrás, C.; Viña, J. Resveratrol: Distribución, propiedades y perspectivas. Rev. Esp. Geriatr. Gerontol. 2013, 48, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Granados Pérez, R.; Villaverde Peris, C. Microbiología Tomo 1, 3rd ed.; Thomson Paraninfo: Madrid, Spain, 2003; pp. 3–17. ISBN 8497321235. [Google Scholar]
- Carpenter, P. Microbiología, 4th ed.; Editorial Interamericana: México City, Mexico, 1979; pp. 144–145, 150–157, 218, 334–335, 376. ISBN 9682502055. [Google Scholar]
- Thatcher, F.S.; Clark, D. S Análisis Microbiológico de Los Alimentos, 1st ed.; Acribia: Zaragoza, Spain, 1973; pp. 31–33. ISBN 8420003085. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [PubMed]
- Amadio, C.; Zimmermann, M.É.; Medina, R.; Miralles, S.; Dediol, C. Aceite esencial de orégano: Un potencial aditivo alimentario. Rev. Fac. Cienc. Agrar. 2011, 43, 237–245. [Google Scholar]
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I: Crystallographic properties. Polym. Int. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Rodríguez-Gonzalez, C.; Martínez-Hernandez, A.L.; Castaño, V.M.; Kharissova, O.V.; Ruoff, R.S.; Velasco-Santos, C. Polysaccharide Nanocomposites Reinforced with Graphene Oxide and Keratin-Grafted Graphene Oxide. Ind. Eng. Chem. Res. 2012, 51, 3619–3629. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Mina, J.; Valadez-González, A.; Herrera-Franco, P.; Delvasto, S. Caracterización fisicoquímica del almidón termoplástico de yuca natural y acetilada. Univ. Nac. Colomb. Med. 2011, 78, 166–173. [Google Scholar] [CrossRef]
- Benavides Rodríguez, L.; Sibaja Ballestero, M.; Vega-Baudrit, J.; Camacho Elizondo, M.; Madrigal Carballo, S. Estudio Cinético de la degradación térmica de Quitina y Quitosano de camarón de la especie “Heterocarpus Vicarius” empleando la técnica termogravimétrica en modo dinámico. Revista Iberoamericana de Polímeros 2010, 11, 561–562. [Google Scholar]
- Yang, D.; Li, J.; Jiang, Z.; Lu, L.; Chen, X. Chitosan/TiO2 nanocomposite pervaporation membranes for ethanol dehydration. Chem. Eng. Sci. 2009, 64, 3130–3137. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck and Co.: Whitehouse Station, NJ, USA, 2006. [Google Scholar]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Wesche-Ebeling, P.A.E. Purification of strawberry polyphenol oxidase and its role in anthocyanin degradation. Diss. Abs. Int. 1984, 44, 1–180. [Google Scholar] [CrossRef]
- Lewis, C.E.; Walker, J.R.L. Effect of polysaccharides on the colour of anthocyanins. Food Chem. 1995, 54, 315–319. [Google Scholar] [CrossRef]
- Marañón-Ruiz, V.F.; Rizo de la Torre, L.D.C.; Chiu-Zarate, R. Caracterización de las propiedades ópticas de Betacianinas y Betaxantinas por espectroscopía Uv-Vis y barrido en Z. Superficies y Vacío 2011, 24, 113–120. [Google Scholar]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: Isomeric Molar Absorptivities and Stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Ocampo, R.; Ríos, L.A.; Betancur, L.A.; Ocampo, D.M. Curso Práctico de Química Orgánica, 1st ed.; Editorial Universidad de Caldas: Caldas, Colombia, 2008; ISBN 9789588319186-9588319188. [Google Scholar]
- Universidad Complutense de Madrid, Facultad de Educación—Dpto. Didáctica de las Ciencias Experimentales, Porosidad y Permeabilidad. Available online: https://pendientedemigracion.ucm.es/info/diciex/proyectos/agua/esc_sub_porosidad.html (accessed on 17 April 2017).
- Park, S.Y.; Marsh, K.S.; Rhim, J.W. Characteristics of Different Molecular Weight Chitosan Films Affected by the Type of Organic Solvents. Food Eng. Phys. Prop. 2012, 67, 194–197. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Porras, G.; Calvo, M.; Esquivel, M.; Sibaja, M.; Madrigal-Carballo, S. Quitosano n-acilado con cinamaldehido: Un potencial bioplaguicida contra agentes patógenos en el campo agrícola. Revista Iberoamericana de Polímeros 2009, 10, 198–199. [Google Scholar]
- Hernández Cocoletzi, H.; Águila Almanza, E.; Flores Agustín, O.; Viveros Nava, E.L.; Ramos Cassellis, E. Obtención y caracterización de quitosano a partir de exoesqueletos de camarón. Superficies y Vacío 2009, 22, 57–60. [Google Scholar]
- Zee, F.; Yen, C.-R.; Nishina, M. Pitaya (Dragon Fruit, Strawberry Pear). Fruits Nuts 2004, 9, 1–3. [Google Scholar]
- Tangsadthakun, C.; Kanokpanont, S.; Sanchavanakit, N.; Pichyangkura, R.; Banaprasert, T.; Tabata, Y.; Damrongsakkul, S. The influence of molecular weight of chitosan on the physical and biological properties of collagen/chitosan scaffolds. J. Biomat. Sci. Polym. Ed. 2007, 18, 147–163. [Google Scholar] [CrossRef]
- Limam, Z.; Selmi, S.; Sadok, S.; El Abed, A. Extraction and characterization of chitin and chitosan from crustacean by-products: Biological and physicochemical properties. Afr. J. Biotechnol. 2011, 10, 640–647. [Google Scholar] [CrossRef]
- Tomé, L.C.; Fernandes, S.C.M.; Sadocco, P.; Causio, J.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. Antibacterial thermoplastic starch-chitosan based materials prepared by melt-mixing. Bioresources 2012, 7, 3398–3409. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Zhang, Z.-H.; Li, L.; Yuan, M.-L.; Fan, J.; Zhao, T.-R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Salleh, E.; Muhamad, I.I.; Khairuddin, N. Structural Characterization and Physical Properties of Antimicrobial (AM) Starch Based Films. World Acad. Sci. Eng. Technol. 2009, 5, 432–440. [Google Scholar]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Microstructural imaging and characterization of the mechanical, chemical, thermal and swelling properties of starch-chitosan blend films. Biopolymers 2006, 82, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Avena-Bustillos, R.J.; Du, W.-X.; Chiou, B.-S.; Williams, T.G.; Wood, D.; McHugh, T.H.; Soares, N.F.F. Physical and Antibacterial Properties of Acai Edible Films Formulated with Thyme Essential Oil and Apple Skin Polyphenols. J. Food Sci. 2014, 79, M903–M910. [Google Scholar] [CrossRef] [PubMed]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Torres-Delgado, C.L.; Diaz-Zavala, N.P.; Velasco-Santos, C.; Salas, P.; Martinez-Hernandez, A.L. Synthesis and Characterization of chitosan-starch films reinforced with TiO2 nanoparticles. In Proceedings of the Memorias del XIX International Material Research Congress, Cancún, Mexico, 15–19 August 2010; Material Research Society: Warrendale, PA, USA; Sociedad Mexicana de Materiales A. C.: Mexico City, Mexico, 2010. [Google Scholar]
- Image Color Summarizer, RGB, HSV, LCH & Lab Image Color Statistics and Clustering—Simple and Easy. Available online: http://mkweb.bcgsc.ca/color-summarizer/ (accessed on 17 December 2017).
- Ahmed, S.; Ikram, S. Chitosan and gelatin based biodegradable packaging films with UV-light protection. J. Photochem. Photobiol. B 2016, 163, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6183–6187. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Autolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]




| Film (1) | Coloration | Average RGB Day 1 | Average RGB Day 15 | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 15 | Red | Green | Blue | Red | Green | Blue | |
| QS2 | Light yellow | Light yellow | 159 ± 11.239 | 160 ± 11.532 | 158 ± 15.099 | 137 ± 45.28 | 140 ± 45.177 | 125 ± 48.445 |
| QSA0.5 | Slightly pink | Yellow | 148 ± 11.239 | 148 ± 7.024 | 140 ± 9.165 | 153 ± 30.138 | 139 ± 28.827 | 89 ± 33.501 |
| QSA2 | Slightly pink | Light brown | 146 ± 11.15 | 144 ± 7.767 | 137 ± 7.211 | 132 ± 24.986 | 94 ± 29.137 | 25 ± 31.764 |
| QSA5 | Pink | Reddish-brown | 143 ± 18.52 | 140 ± 13.051 | 131 ± 4.725 | 122 ± 40.501 | 54 ± 45.236 | 16 ± 41.218 |
| QSAm0.5 | Slightly brown | Yellow | 150 ± 10.0167 | 150 ± 10.0167 | 139 ± 9.539 | 157 ± 76.839 | 109 ± 78.619 | 29 ± 80.894 |
| QSAm2 | Light brown | Light brown | 145 ± 31.214 | 142 ± 32.036 | 130 ± 33.645 | 103 ± 53.799 | 42 ± 34.559 | 26 ± 29.67 |
| QSAm5 | Brown | Reddish-brown | 144 ± 30.643 | 136 ± 39.803 | 120 ± 47.721 | 88 ± 60.002 | 39 ± 51.643 | 27 ± 49.729 |
| QSB0.5 | Pink | Yellow | 166 ± 14.012 | 70 ± 25.515 | 85 ± 24.758 | 135 ± 20.404 | 119 ± 29.67 | 69 ± 4.509 |
| QSB2 | Pinkish-red | Brown | 146 ± 13.748 | 33 ± 35.218 | 47 ± 32.516 | 136 ± 31.973 | 92 ± 37.634 | 26 ± 38.314 |
| QSB5 | Wine red | Reddish-brown | 99 ± 27.622 | 23 ± 30.643 | 20 ± 31.005 | 108 ± 31.973 | 52 ± 37.634 | 21 ± 38.314 |
| QSG0.5 | Slightly yellow | Light yellow | 152 ± 12.055 | 153 ± 7 | 145 ± 9.504 | 150 ± 32.959 | 136 ± 36.115 | 86 ± 46.09 |
| QSG2 | Light pink | Light brown | 157 ± 9.504 | 147 ± 9.073 | 136 ± 9.073 | 136 ± 29.137 | 92 ± 39.715 | 23 ± 42.532 |
| QSG5 | Pink | Reddish-brown | 155 ± 14.295 | 146 ± 10 | 135 ± 8.544 | 108 ± 31.214 | 37 ± 39.576 | 19 ± 43.143 |
| QSO0.5 | Slightly yellow | Light yellow | 157 ± 18.877 | 157 ± 18.77 | 146 ± 17.691 | 148 ± 22.912 | 146 ± 22.185 | 130 ± 26.083 |
| QSO2 | Light yellow | Light brown | 147 ± 25.515 | 145 ± 26.058 | 133 ± 24.515 | 128 ± 18.175 | 123 ± 19.425 | 98 ± 26.633 |
| QSO5 | Yellow | Light brown | 139 ± 23.065 | 133 ± 23.007 | 109 ± 25.514 | 131 ± 17.473 | 122 ± 13 | 86 ± 6.506 |
| QSP0.5 | Pink | Brown | 148 ± 14 | 146 ± 8.505 | 136 ± 8.082 | 150 ± 13.051 | 79 ± 19.008 | 22 ± 16.093 |
| QSP2 | Pinkish-red | Reddish-brown | 148 ± 17.039 | 145 ± 14.12 | 139 ± 13.428 | 115 ± 23.544 | 44 ± 34.597 | 28 ± 32.129 |
| QSP5 | Fuchsia-red | Dark red | 147 ± 20.008 | 146 ± 14.012 | 141 ± 10.692 | 71 ± 38.436 | 31 ± 36.846 | 29 ± 36.295 |
| QSR0.5 | Slightly yellow | Light yellow | 156 ± 19.757 | 156 ± 20.297 | 154 ± 19.218 | 135 ± 32.078 | 136 ± 35.921 | 121 ± 47.57 |
| QSR2 | Slightly brown | Light brown | 147 ± 15.275 | 148 ± 15.716 | 143 ± 15.275 | 140 ± 40.951 | 136 ± 43.108 | 116 ± 47.71 |
| QSR5 | Slightly brown | Light brown | 150 ± 15.822 | 151 ± 15.395 | 146 ± 15.947 | 134 ± 38.423 | 131 ± 40.501 | 107 ± 49.426 |
| Sample | Apparent Density (g/cm3) | Sample | Apparent Density (g/cm3) |
|---|---|---|---|
| QS2 | 0.0835 ± 0.0001 | QSG2 | 0.0713 ± 0.0002 |
| QSA0.5 | 0.1034 ± 0.00004 | QSG5 | 0.1087 ± 0.0002 |
| QSA2 | 0.0933 ± 0.00035 | QSO0.5 | 0.0757 ± 0.0002 |
| QSA5 | 0.1051 ± 0.000055 | QSO2 | 0.0677 ± 0.00007 |
| QSAm0.5 | 0.0908 ± 0.00031 | QSO5 | 0.0641 ± 0.0002 |
| QSAm2 | 0.0916 ± 0.0009 | QSP0.5 | 0.0711 ± 0.0001 |
| QSAm5 | 0.0847 ± 0.00044 | QSP2 | 0.0752 ± 0.00004 |
| QSB0.5 | 0.0907 ± 0.0005 | QSP5 | 0.0646 ± 0.0002 |
| QSB2 | 0.0754 ± 0.00039 | QSR0.5 | 0.0649 ± 0.00007 |
| QSB5 | 0.0814 ± 0.00039 | QSR2 | 0.0816 ± 0.00003 |
| QSG0.5 | 0.0984 ± 0.00026 | QSR5 | 0.0807 ± 0.00009 |
| Film | Wavelength, nm | Film | Wavelength, nm |
|---|---|---|---|
| QS2 | ------ | QSG2 | 264.32 ± 0 |
| QSA0.5 | 274.99 ± 1.48 | QSG5 | 264.89 ± 2.10 |
| QSA2 | 275.70 ± 1.23 | QSO0.5 | 269.16 ± 3.58 |
| QSA5 | 273.71 ± 1.54 | QSO2 | 262.90 ± 0.65 |
| QSAm0.5 | 262.61 ± 0.74 | QSO5 | 262.47 ± 1.92 |
| QSAm2 | 267.59 ± 0.49 | QSP0.5 | 266.74 ± 0.98 |
| QSAm5 | 273.14 ± 5.16 | QSP2 | 267.31 ± 0 |
| QSB0.5 | 261.76 ± 0.74 | QSP5 | 261.48 ± 2.46 |
| QSB2 | 267.73 ± 1.13 | QSR0.5 | 262.76 ± 0.25 |
| QSB5 | 262.33 ± 1.07 | QSR2 | 267.73 ± 0.43 |
| QSG0.5 | 265.46 ± 1.72 | QSR5 | 267.31 ± 0 |
| Film | Antioxidant Capacity, mg/mg | Film | Antioxidant Capacity, mg/mg |
|---|---|---|---|
| QS2 | ------ | QSG2 | 0.20984 ± 2.64 × 10−6 |
| QSA0.5 | 0.21001 ± 4.85 × 10−5 | QSG5 | 0.20997 ± 2.15 × 10−5 |
| QSA2 | 0.20991 ± 1.61 × 10−5 | QSO0.5 | 0.21003 ± 2.73 × 10−5 |
| QSA5 | 0.20992 ± 6.65 × 10−5 | QSO2 | 0.21020 ± 1.46 × 10−4 |
| QSAm0.5 | 0.20998 ± 6.23 × 10−5 | QSO5 | 0.21003 ± 6.92 × 10−6 |
| QSAm2 | 0.20997 ± 4.69 × 10−5 | QSP0.5 | 0.20997 ± 3.48 × 10−6 |
| QSAm5 | 0.20999 ± 6.37 × 10−5 | QSP2 | 0.21002 ± 4.06 × 10−5 |
| QSB0.5 | 0.20994 ± 9.18 × 10−5 | QSP5 | 0.21008 ± 6.67 × 10−5 |
| QSB2 | 0.21004 ± 7.27 × 10−6 | QSR0.5 | 0.20995 ± 5.63 × 10−5 |
| QSB5 | 0.20998 ± 9.18 × 10−5 | QSR2 | 0.21005 ± 5.82 × 10−6 |
| QSG0.5 | 0.21003 ± 2.73 × 10−5 | QSR5 | 0.21005 ± 5.58 × 10−5 |
| Sample | Weight Loss Percentage at 135 °C | Weight Loss Percentage at 320 °C | Weight Loss Percentage at 600 °C |
|---|---|---|---|
| QSAm2 | 10.208 ± 0.2 | 57.712 ± 0.2 | 90.52 ± 0.2 |
| QSAm5 | 9.812 ± 0.2 | 53.782 ± 0.2 | 88.571 ± 0.2 |
| QSB2 | 8.264 ± 0.2 | 55.290 ± 0.2 | 96.519 ± 0.2 |
| QSB5 | 7.212 ± 0.2 | 51.424 ± 0.2 | 97.465 ± 0.2 |
| QSO5 | 17.920 ± 0.2 | 65.787 ± 0.2 | 90.507 ± 0.2 |
| QSP0.5 | 9.216 ± 0.2 | 59.783 ± 0.2 | 96.054 ± 0.2 |
| Number | Sample (1) | Number | Sample (1) |
|---|---|---|---|
| 1 | QS2 | 12 | QSG2 |
| 2 | QSA0.5 | 13 | QSG5 |
| 3 | QSA2 | 14 | QSO0.5 |
| 4 | QSA5 | 15 | QSO2 |
| 5 | QSAm0.5 | 16 | QSO5 |
| 6 | QSAm2 | 17 | QSP0.5 |
| 7 | QSAm5 | 18 | QSP2 |
| 8 | QSB0.5 | 19 | QSP5 |
| 9 | QSB2 | 20 | QSR0.5 |
| 10 | QSB5 | 21 | QSR2 |
| 11 | QSG0.5 | 22 | QSR5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials 2018, 11, 120. https://doi.org/10.3390/ma11010120
Lozano-Navarro JI, Díaz-Zavala NP, Velasco-Santos C, Melo-Banda JA, Páramo-García U, Paraguay-Delgado F, García-Alamilla R, Martínez-Hernández AL, Zapién-Castillo S. Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials. 2018; 11(1):120. https://doi.org/10.3390/ma11010120
Chicago/Turabian StyleLozano-Navarro, Jessica I., Nancy P. Díaz-Zavala, Carlos Velasco-Santos, José A. Melo-Banda, Ulises Páramo-García, Francisco Paraguay-Delgado, Ricardo García-Alamilla, Ana L. Martínez-Hernández, and Samuel Zapién-Castillo. 2018. "Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties" Materials 11, no. 1: 120. https://doi.org/10.3390/ma11010120
APA StyleLozano-Navarro, J. I., Díaz-Zavala, N. P., Velasco-Santos, C., Melo-Banda, J. A., Páramo-García, U., Paraguay-Delgado, F., García-Alamilla, R., Martínez-Hernández, A. L., & Zapién-Castillo, S. (2018). Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials, 11(1), 120. https://doi.org/10.3390/ma11010120