A Template-Free Microwave Synthesis of One-Dimensional Cu2O Nanowires with Desired Photocatalytic Property
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation
2.3. Characterization of the Samples
2.4. Photocatalytic Activity
3. Results and Discussion
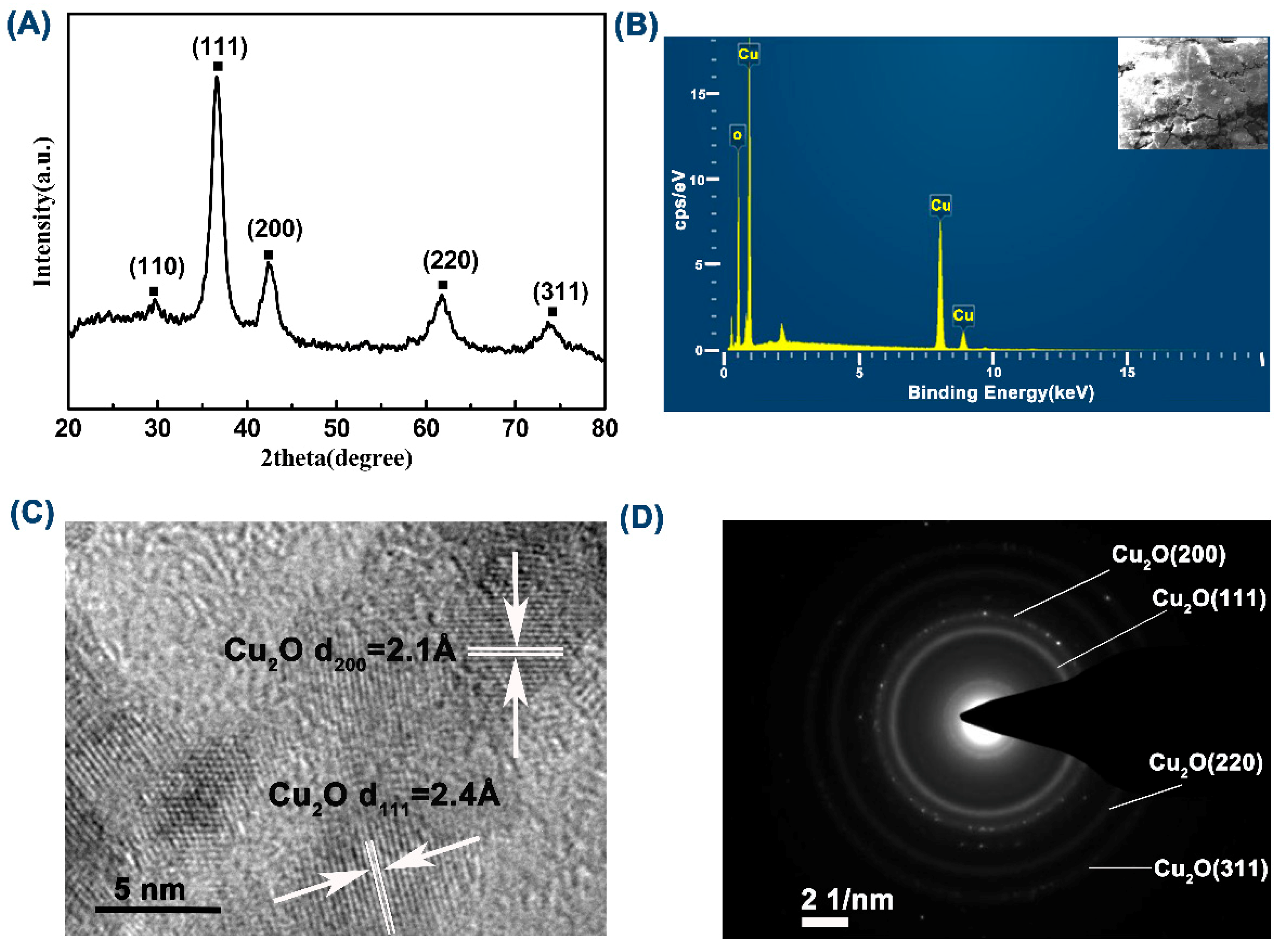
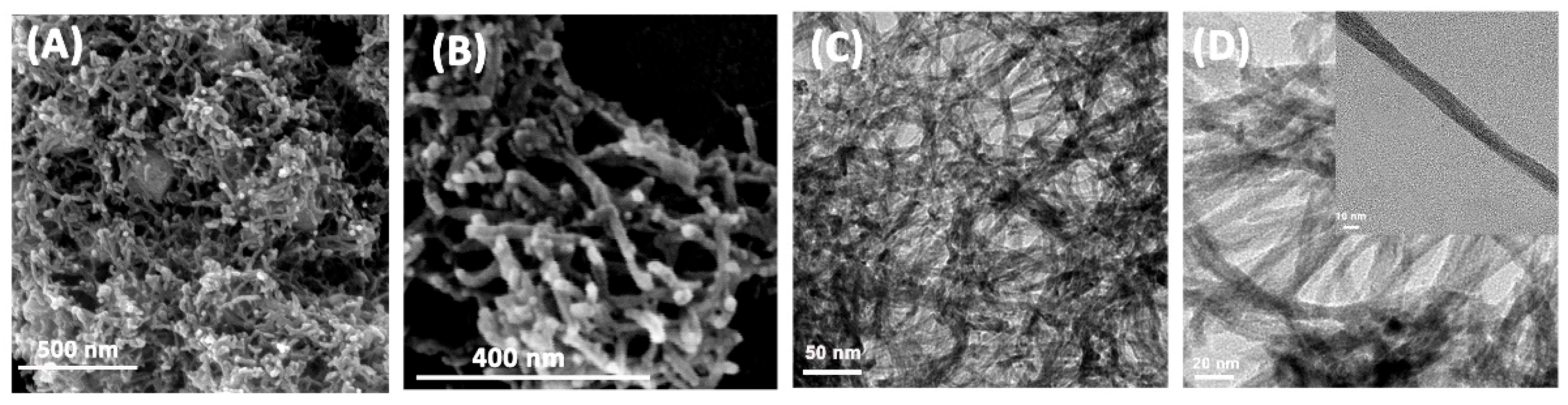
3.1. Structure and Morphology
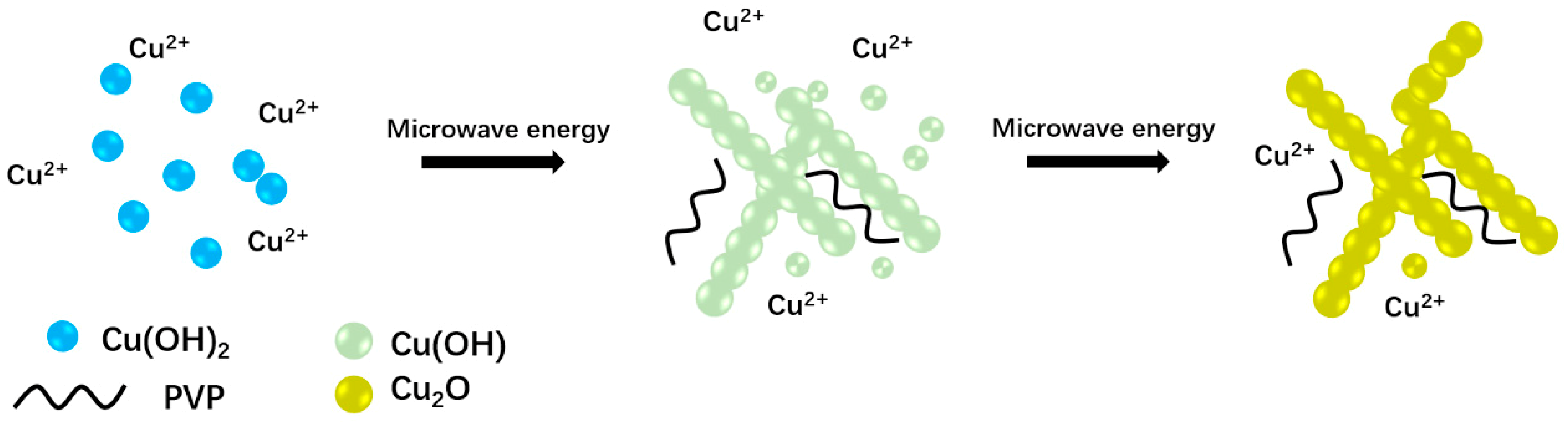
3.2. The Mechanism Analysis
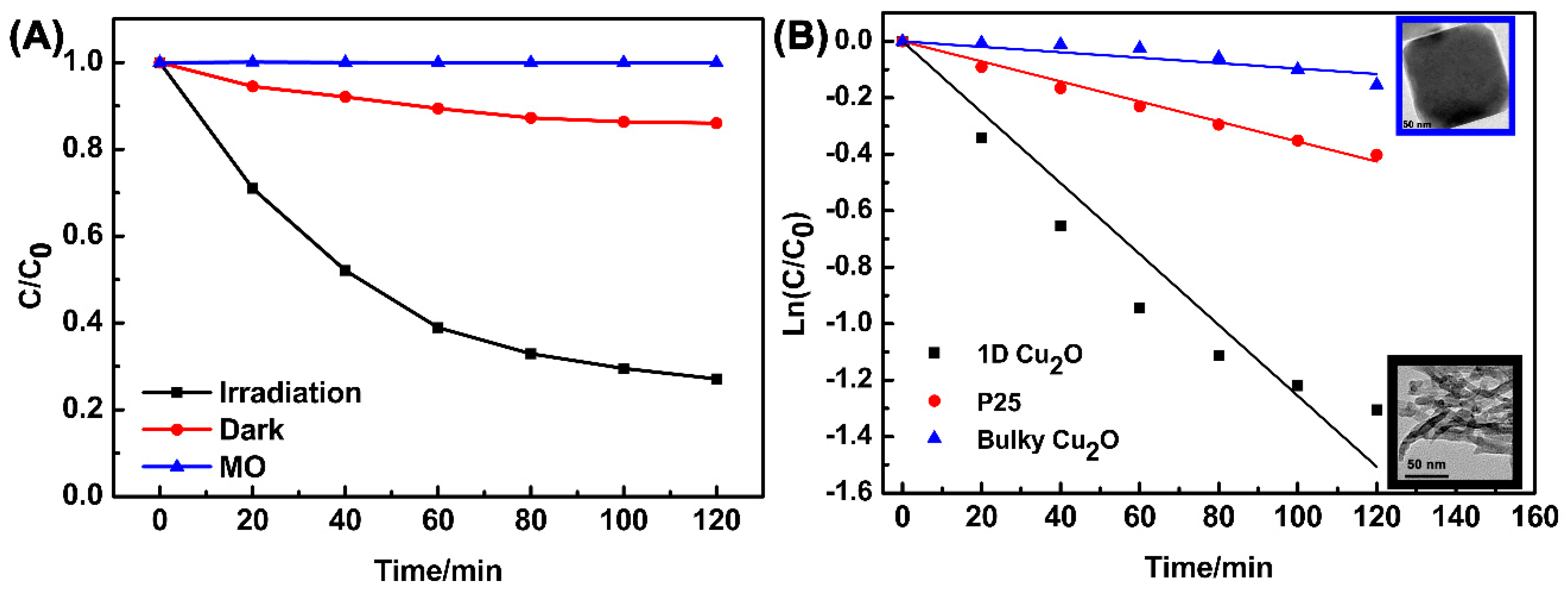
3.3. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Wang, B.; Hao, C.; Liang, Y.; Shi, H.; Ao, L.; Wang, W. Au/Cu2O Schottky contact heterostructures with enhanced photocatalytic activity in dye decomposition and photoelectrochemical water splitting under visible light irradiation. J. Alloys Compd. 2016, 684, 445–452. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, B.; Zhang, T.; Gao, D.; Xu, A.W. Shape Effects of Cu2O Polyhedral Microcrystals on Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 5073–5079. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Akhavan, O.; Bijanzad, K. Photocatalytic activity of CuO nanoparticles incorporated in mesoporous structure prepared from bis(2-aminonicotinato) copper(II) microflakes. Trans. Nonferrous Met. Soc. China 2015, 25, 3634–3642. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Zhu, W. Shape Evolution and Size-Controllable Synthesis of Cu2O Octahedra and Their Morphology-Dependent Photocatalytic Properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Naresh, G.; Chen, H.S. Facet-Dependent Electrical, Photocatalytic, and Optical Properties of Semiconductor Crystals and Their Implications for Applications. ACS Appl. Mater. Interfaces 2017, 10, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, B.; Wang, Z.; Guo, M.; Qin, X.; Zhang, X.; Wang, P.; Dai, Y. Crystal Faces of Cu2O and Their Stabilities in Photocatalytic Reactions. J. Phys. Chem. C 2009, 113, 14448–14453. [Google Scholar] [CrossRef]
- Ma, J.; Guo, S.; Guo, X.; Ge, H. Preparation, characterization and antibacterial activity of core–shell Cu2O@Ag composites. Surf. Coat. Technol. 2015, 272, 268–272. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.; Park, S.H.; Park, H.; Huh, Y.D. Morphology-dependent antibacterial activities of Cu2O. Mater. Lett. 2011, 65, 818–820. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, S.; Zhang, G.; Li, W.; Gao, C.; Shen, C.; Meng, Q. Antimicrobial polysulfone blended ultrafiltration membranes prepared with Ag/Cu2O hybrid nanowires. J. Membr. Sci. 2016, 509, 83–93. [Google Scholar] [CrossRef]
- Lamberti, A.; Destro, M.; Bianco, S.; Quaglio, M.; Chiodoni, A.; Pirria, C.F.; Gerbaldi, C. Facile fabrication of cuprous oxide nanocomposite anode films for flexible Li-ion batteries via thermal oxidation. Electrochim. Acta 2012, 86, 323–329. [Google Scholar] [CrossRef]
- Ma, S.; Liu, Q.; Lei, D.; Guo, X.; Li, S.; Li, Z. A powerful LiO2 battery based on an efficient hollow Cu2O cathode catalyst with tailored crystal plane. Electrochim. Acta 2018, 260, 31–39. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, W.; Chen, L.; Zeng, L.; Wei, M. Facile synthesis of Cu2O nanorod arrays on Cu foam as a self-supporting anode material for lithium ion batteries. J. Alloys Compd. 2017, 723, 172–178. [Google Scholar] [CrossRef]
- Şişman, O.; Kılınç, N.; Öztürk, Z.Z. H2 Sensing Properties of Cu2O Nanowires on Glass Substrate. Procedia Eng. 2015, 120, 1170–1174. [Google Scholar] [CrossRef]
- Yin, H.; Cui, Z.; Wang, L.; Nie, Q. In situ reduction of the Cu/Cu2O/carbon spheres composite for enzymaticless glucose sensors. Sens. Actuators B-Chem. 2016, 222, 1018–1023. [Google Scholar] [CrossRef]
- Wang, M.; Song, X.; Song, B.; Liu, J.; Hu, C.; Wei, D.; Wong, C.P. Precisely quantified catalyst based on in situ growth of Cu2O nanoparticles on a graphene 3D network for highly sensitive glucose sensor. Sens. Actuators B-Chem. 2017, 250, 333–341. [Google Scholar] [CrossRef]
- He, P.; Shen, X.; Gao, H. Size-controlled preparation of Cu2O octahedron nanocrystals and studies on their optical absorption. J. Colloid Interface Sci. 2005, 284, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yang, Z.; Shen, L.; Lin, H.; Wang, Y.; Pun, E.Y.B.; Chen, Y.; Ho, J.C. Design and fabrication of 1-D semiconductor nanomaterials for high-performance photovoltaics. Sci. Bull. 2016, 61, 357–367. [Google Scholar] [CrossRef]
- Zhou, T.; Zang, Z.; Wei, J.; Zheng, J.; Hao, J.; Ling, F.; Tang, X.; Fang, L.; Zhou, M. Efficient charge carrier separation and excellent visible light photoresponse in Cu2O nanowires. Nano Energy 2018, 50, 118–125. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Yang, Q.; Liang, S.; Zhang, X.; Yang, Z. Cuprous oxide (Cu2O) crystals with tailored architectures: A comprehensive review on synthesis, fundamental properties, functional modifications and applications. Prog. Mater. Sci. 2018, 96, 111–173. [Google Scholar] [CrossRef]
- Ng, S.Y.; Ngan, A.H.W. One- and two-dimensional cuprous oxide nano/micro structures fabricated on highly orientated pyrolytic graphite (HOPG) by electrodeposition. Electrochim. Acta 2013, 114, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.Z.; Wang, G.H.; Wang, X.S.; Zhan, Y.J.; Liu, Y.K.; Zheng, C.L. Synthesis and Characterization of Cu2O Nanowires by a Novel Reduction Route. Adv. Mater. 2002, 14, 67–69. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Q.; Wen, X.; Li, X.; Yang, S. Thermal oxidation of Cu2S nanowires: A template method for the fabrication of mesoscopic CuxO (x = 1,2) wires. Phys. Chem. Chem. Phys. 2002, 4, 3425–3429. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xie, Y.; Choi, C.L.; Wan, K.C.; Li, X.Y.; Yang, S. Copper-Based Nanowire Materials: Templated Syntheses, Characterizations, and Applications. Langmuir 2005, 21, 4729–4737. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Pimentel, A.; Barquinha, P.; Carvalho, P.A.; Fortunato, E.; Martins, R. Cu2O polyhedral nanowires produced by microwave irradiation. J. Phys. Chem. C 2014, 2, 6097–6103. [Google Scholar] [CrossRef]
- Nunes, D.; Calmeiro, T.R.; Nandy, S.; Pinto, J.V.; Pimentel, A.; Barquinha, P.; Carvalho, P.A.; Walmsley, J.C.; Fortunato, E.; Martins, R. Charging effects and surface potential variations of Cu-based nanowires. Thin Solid Films 2016, 601, 45–53. [Google Scholar] [CrossRef]
- Tan, Y.; Xue, X.; Peng, Q.; Zhao, H.; Wang, H.; Li, Y. Controllable Fabrication and Electrical Performance of Single Crystalline Cu2O Nanowires with High Aspect Ratios. Nano Lett. 2007, 7, 3723–3728. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Z.; Zhang, R.; Xie, Y.; Yang, J.; Wu, C. From Complex Chains to 1D Metal Oxides: A Novel Strategy to Cu2O Nanowires. J. Phys. Chem. B 2003, 107, 3697–3702. [Google Scholar] [CrossRef]
- Orel, Z.C.; Anžlovar, A.; Dražić, G.; Žigon, M. Cuprous Oxide Nanowires Prepared by an Additive-Free Polyol Process. Cryst. Growth Des. 2007, 7, 453–458. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, R.; Xiong, S.; Liu, Y.; Chai, L.; Bao, K.; Qian, Y. Well-aligned Cu2O nanowire arrays prepared by an ethylene glycol-reduced process. Mater. Chem. Phys. 2009, 114, 213–216. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Bhatte, K.D.; Bhanage, B.M. A rapid, one pot microwave assisted synthesis of nanosize cuprous oxide. Powder Technol. 2013, 235, 516–519. [Google Scholar] [CrossRef]
- Liu, F.K.; Huang, P.W.; Chang, Y.C.; Ko, C.J.; Ko, F.H.; Chu, T.C. Formation of silver nanorods by microwave heating in the presence of gold seeds. J. Cryst. Growth 2005, 273, 439–445. [Google Scholar] [CrossRef]
- Bhatte, K.D.; Tambade, P.; Fujita, S.; Arai, M.; Bhanage, B.M. Microwave-assisted additive free synthesis of nanocrystalline zinc oxide. Powder Technol. 2010, 203, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Bhatte, K.D.; Sawant, D.N.; Deshmukh, K.M.; Bhanage, B.M. Additive free microwave assisted synthesis of nanocrystalline Mg(OH)2 and MgO. Particuology 2012, 10, 384–387. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Li, B.; Xu, J.; Zhao, X.; Liu, X. Microwave-assisted synthesis of Cu2O microcrystals with systematic shape evolution from octahedral to cubic and their comparative photocatalytic activities. RSC Adv. 2014, 4, 38059–38063. [Google Scholar] [CrossRef]
- Zou, X.; Fan, H.; Tian, Y.; Zhang, M.; Yan, X. Microwave-assisted hydrothermal synthesis of Cu/Cu2O hollow spheres with enhanced photocatalytic and gas sensing activities at room temperature. Dalton Trans. 2015, 44, 7811–7821. [Google Scholar] [CrossRef] [PubMed]
- Luévano-Hipólito, E.; Torres-Martínez, L.M.; Sánchez-Martínez, D.; Alfaro Cruz, M.R. Cu2O precipitation-assisted with ultrasound and microwave radiation for photocatalytic hydrogen production. Int. J. Hydrogen Energy 2017, 42, 12997–13010. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Chen, R.; Lu, J.; Wang, Z.; Zhou, Q.; Zheng, M. Microwave Synthesis of Cu/Cu2O/SnO2 Composite with Improved Photocatalytic Ability Using SnCl4 as a Protector. J. Mater. Sci. 2018, 53, 9557–9566. [Google Scholar] [CrossRef]
- Cheng, Z.; Xu, J.; Zhong, H.; Chu, X.Z.; Song, J. Repeatable synthesis of Cu2O nanorods by a simple and novel reduction route. Mater. Lett. 2011, 65, 1871–1874. [Google Scholar] [CrossRef]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, Q.; Li, B.; Dong, L.; Fan, M.; Zhang, F. Synthesis and characterization of Cu2O–modified Bi2O3 nanospheres with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2015, 357, 1072–1079. [Google Scholar] [CrossRef]
- Zhou, Z.; Long, M.; Cai, W.; Cai, J. Synthesis and photocatalytic performance of the efficient visible light photocatalyst Ag–AgCl/BiVO4. J. Mol. Catal. A-Chem. 2012, 353–354, 22–28. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, M.; Chen, R.; Wang, Z. One-pot synthesis of an AgBr/ZnO hierarchical structure with enhanced photocatalytic capacity. RSC Adv. 2017, 7, 31230–31238. [Google Scholar] [CrossRef] [Green Version]



| Sample | Kinetic Constant (k, min−1) | R2 |
|---|---|---|
| One-dimensional Cu2O | 1.26 × 10−2 | 0.976 |
| P25 | 3.55 × 10−3 | 0.995 |
| Bulky Cu2O | 9.64 × 10−4 | 0.877 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Wang, Z.; Zhou, Q.; Lu, J.; Zheng, M. A Template-Free Microwave Synthesis of One-Dimensional Cu2O Nanowires with Desired Photocatalytic Property. Materials 2018, 11, 1843. https://doi.org/10.3390/ma11101843
Chen R, Wang Z, Zhou Q, Lu J, Zheng M. A Template-Free Microwave Synthesis of One-Dimensional Cu2O Nanowires with Desired Photocatalytic Property. Materials. 2018; 11(10):1843. https://doi.org/10.3390/ma11101843
Chicago/Turabian StyleChen, Rui, Zuoshan Wang, Qingqing Zhou, Juan Lu, and Min Zheng. 2018. "A Template-Free Microwave Synthesis of One-Dimensional Cu2O Nanowires with Desired Photocatalytic Property" Materials 11, no. 10: 1843. https://doi.org/10.3390/ma11101843




