Electrochemical Measurements of Multiwalled Carbon Nanotubes under Different Plasma Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MWCNTs
2.2. Post-Treatment of MWCNTs
2.3. Physical Characterization
2.4. Electrochemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Instrumental Analysis
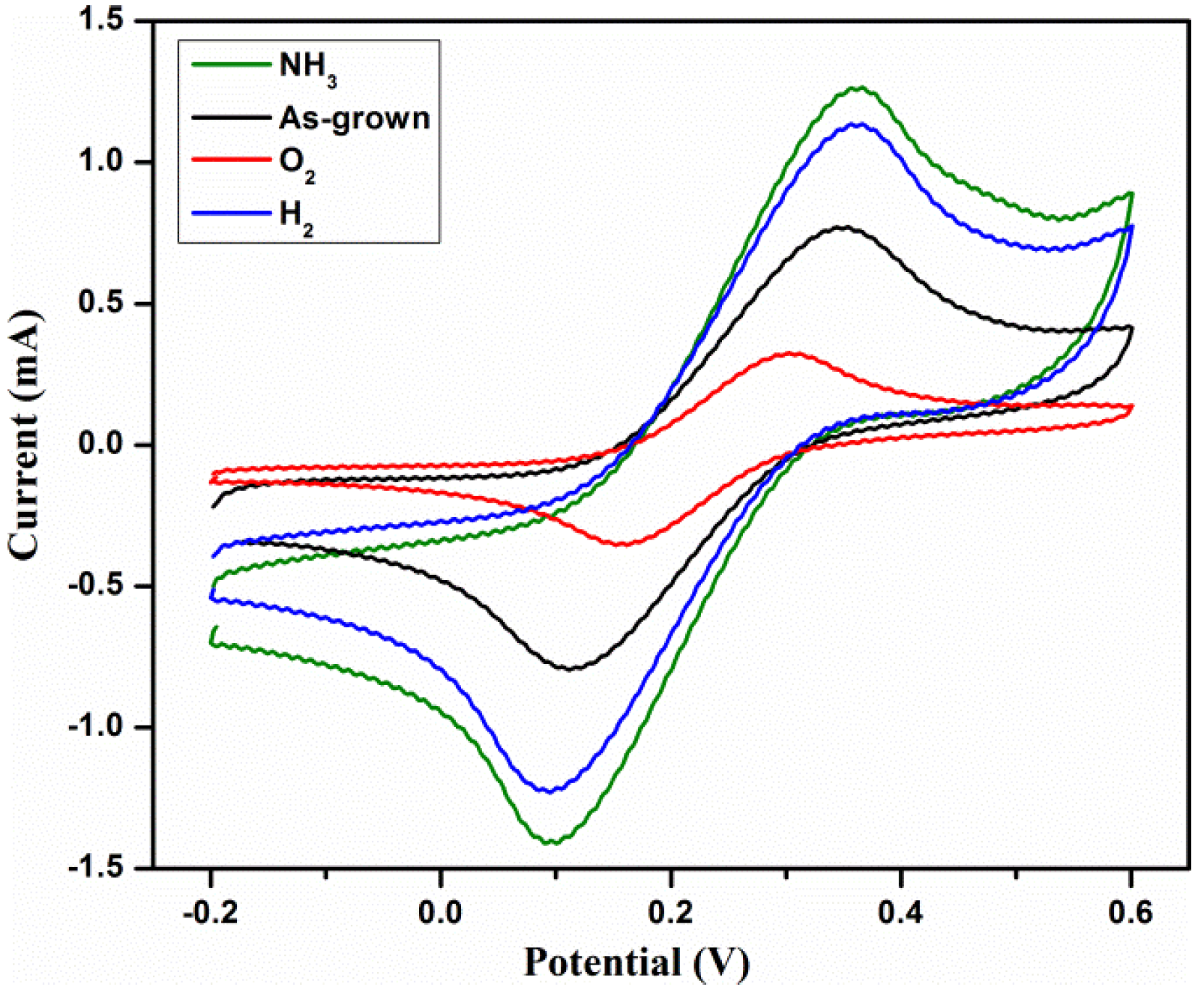
3.2. Electrochemical Characterization
3.2.1. Effect of Sensitivity
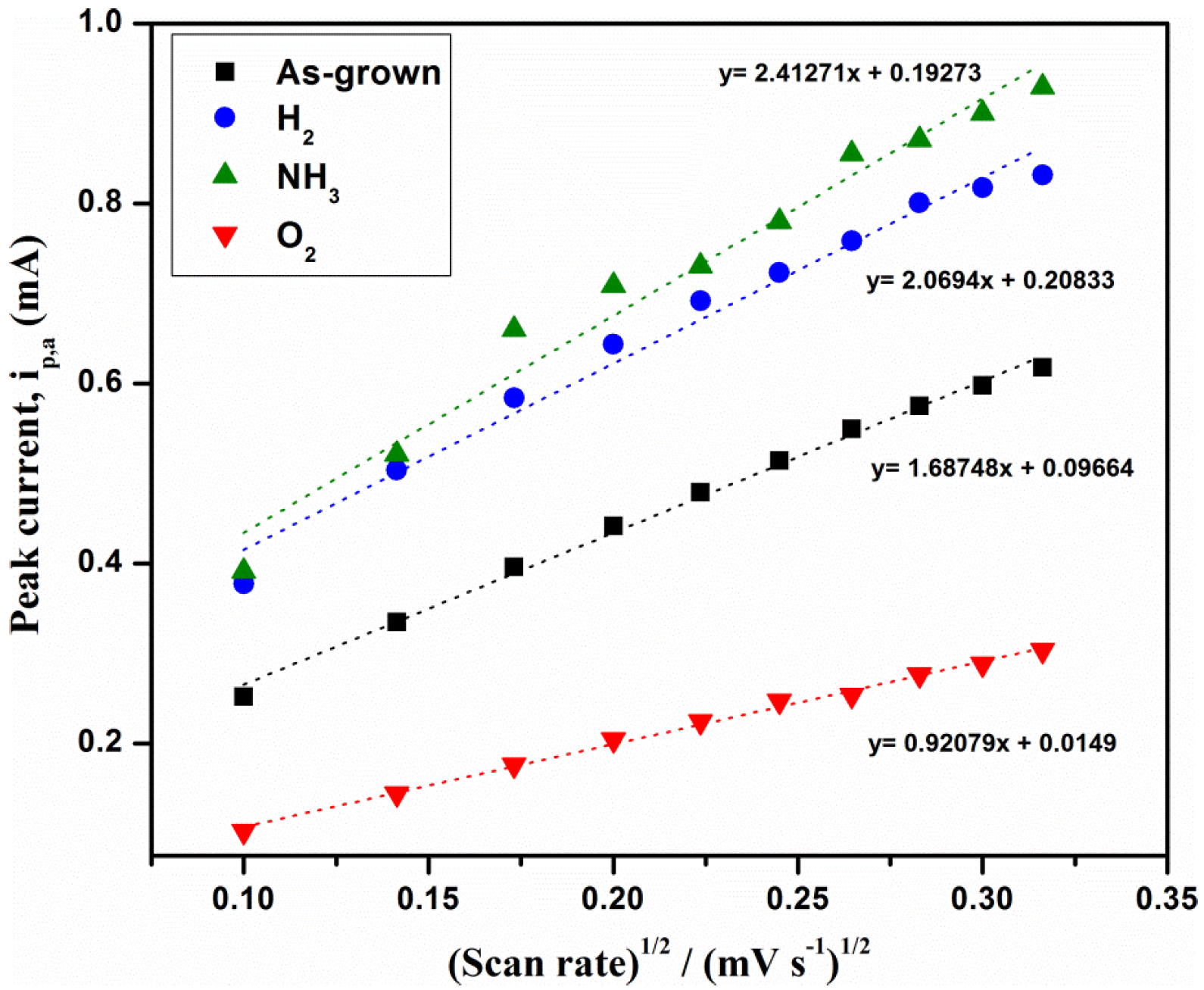
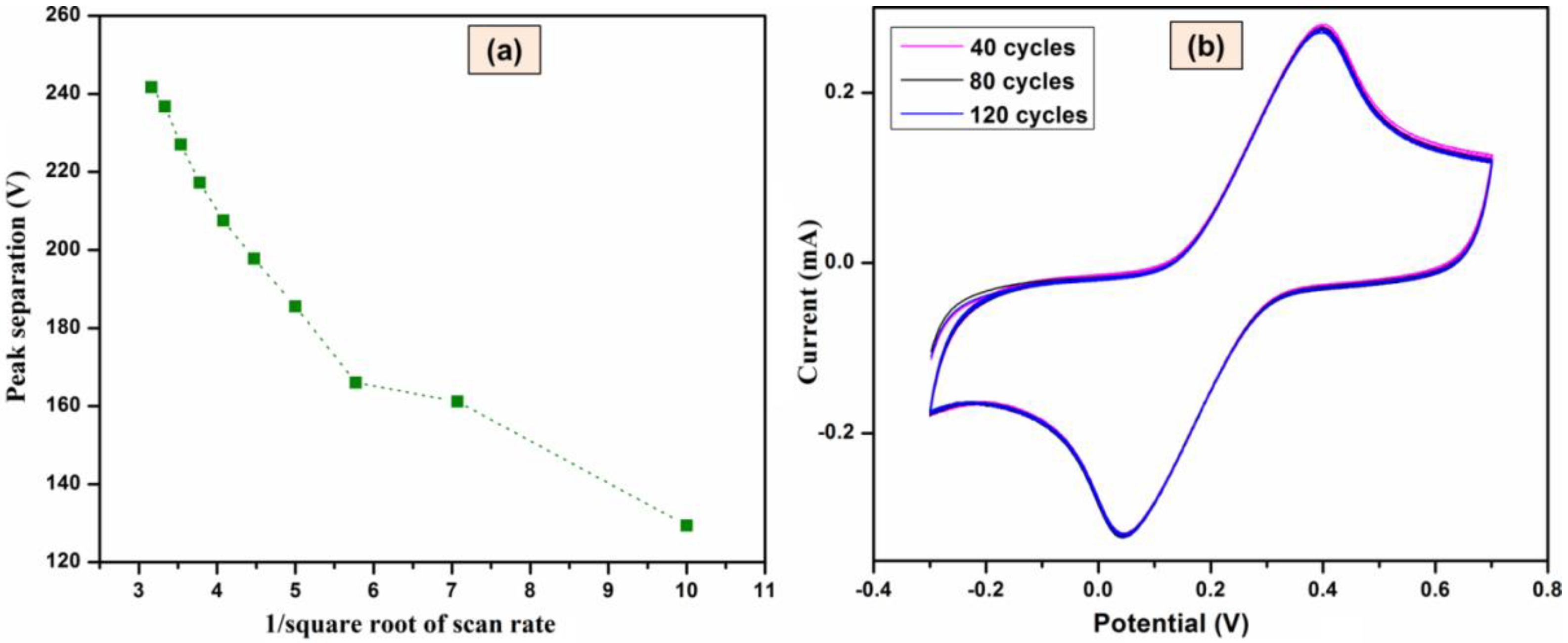
3.2.2. Effect of Scan Rate
3.2.3. NH3/MWCNTs Electrode Performances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Ijima, S. Helical microtubles of graphitic carbon. Lett. Nat. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Cole, M.T.; Mann, M.; Teo, K.B.K.; Milne, W.I. Chapter 5. Engineered carbon nanotube field emission devices. In Emerging Nanotechnologies for Manufacturing, 2nd ed.; William Andrew: Norwich, UK; New York, NY, USA, 2015; pp. 125–186. [Google Scholar]
- Wang, N.; Pandit, S.; Ye, L.; Edwards, M.; Mokkapati, V.R.S.S.; Murugesan, M.; Kuzmenko, V.; Zhao, C.; Westerlund, F.; Mijakovic, I.; et al. Efficient surface modification of carbon nanotubes for fabricating high performance CNT based hybrid nanostructures. Carbon 2017, 111, 402–410. [Google Scholar] [CrossRef]
- Meyyappan, M. Topical review: A review of plasma enhanced chemical vapour deposition of carbon nanotubes. J. Phys. D Appl. Phys. 2009, 42, 213001. [Google Scholar] [CrossRef]
- Kyzioł, K.; Koper, K.; Środa, M.; Klich, M.; Kaczmarek, Ł. Influence of gas mixture during N+ ion modification under plasma conditions on surface structure and mechanical properties of Al–Zn alloys. Surf. Coat. Technol. 2015, 278, 30–37. [Google Scholar] [CrossRef]
- Kyzioł, K.; Oczkowsk, J.; Kottfer, D.; Klich, M.; Kaczmarek, Ł.; Kyzioł, A.; Grzesik, Z. Physicochemical and biological activity analysis of low-density polyethylene substrate modified by multi-layer coatings based on DLC structures, obtained using RF CVD method. Coatings 2018, 8, 135. [Google Scholar] [CrossRef]
- Dementev, N.; Osswald, S.; Gogotsi, Y.; Borguet, E. Purification of carbon nanotubes by dynamic oxidation in air. J. Mater. Chem. 2009, 19, 7904–7908. [Google Scholar] [CrossRef]
- Simmons, J.M.; Nichols, B.M.; Baker, S.E.; Marcus, M.S.; Castellini, O.M.; Lee, E.; Hamers, R.J.; Eriksson, M.A. Effect of ozone oxidation on single-walled carbon nanotubes. J. Phys. Chem. B 2006, 110, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Moreno, H.; Bertran, E. RF-PECVD growth and nitrogen plasma functionalization of CNTs on copper foil for electrochemical applications. Diam. Relat. Mater. 2014, 49, 55–61. [Google Scholar] [CrossRef]
- Yook, J.Y.; Jun, J.; Kwak, S. Amino functionalization of carbon nanotube surfaces with NH3 plasma treatment. Appl. Surf. Sci. 2010, 256, 6941–6944. [Google Scholar] [CrossRef]
- Mathur, A.; Roy, S.S.; Hazra, K.S.; Wadhwa, S.; Ray, S.C.; Mitra, S.K.; Misra, D.S.; McLaughlin, J.A. Oxygen plasma assisted end-opening and field emission enhancement in vertically aligned multiwall carbon nanotubes. Mater. Chem. Phys. 2012, 134, 425–429. [Google Scholar] [CrossRef]
- Park, E.J.; Jin, J.H.; Kim, J.H.; Min, N.K. Surface activation of plasma-patterned carbon nanotube based DNA sensing electrodes. Microchim. Acta 2011, 174, 231–238. [Google Scholar] [CrossRef]
- Merenda, A.; des Ligneris, E.; Sears, K.; Chaffraix, T.; Magniez, K.; Cornu, D.; Schütz, J.A.; Dumée, L.F. Assessing the temporal stability of surface functional groups introduced by plasma treatments on the outer shells of carbon nanotubes. Sci. Rep. 2016, 6, 31565. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, R.; Chen, X.; Wang, L.; Liu, J.H.; Huang, X.J. A cation trap for anodic stripping voltammetry: NH3-plasma treated carbon nanotubes for adsorption and detection of metal ions. Anal. Chim. Acta 2012, 755, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of ozone treatment on surface properties of activated carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Ruelle, B.; Felten, A.; Ghijsen, J.; Drube, W.; Johnson, R.L.; Liang, D.; Erni, R.; Van Tendeloo, G.; Dubois, P.; Hecq, M. Functionalization of MWCNTs with atomic nitrogen: Electronic structure. Micron 2009, 40, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, P.; Zhou, O. Effects of NH3 plasma pretreatment on the growth of carbon nanotubes. Diam. Relat. Mater. 2006, 15, 361–364. [Google Scholar] [CrossRef]
- Yoo, K.P.; Kwon, K.H.; Min, N.K.; Lee, M.J.; Lee, C.J. Effects of O2 plasma treatment on NH3 sensing characteristics of multiwall carbon nanotube/polyaniline composite films. Sens. Actuators B Chem. 2009, 143, 333–340. [Google Scholar] [CrossRef]
- Chen, C.; Liang, B.; Lu, D.; Ogino, A.; Wang, X.; Nagatsu, M. Amino group introduction onto multiwall carbon nanotubes by NH3/Ar plasma treatment. Carbon 2010, 48, 939–948. [Google Scholar] [CrossRef]
- Mishra, P.; Islam, S.S. Surface modification of MWCNTs by O2 plasma treatment and its exposure time dependent analysis by SEM, TEM and vibrational spectroscopy. Superlattices Microstruct. 2013, 64, 399–407. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Jover, R.; Bertran, E. Nitrogen plasma functionalization of carbon nanotubes for supercapacitor applications. J. Mater. Sci. 2013, 48, 7620–7628. [Google Scholar] [CrossRef]
- Haniff, M.A.S.M.; Lee, H.W.; Bien, D.C.S.; Azid, I.A. Horizontally networked carbon nanotubes grown on Au–Fe catalyst nanoparticles. J. Nanoparticle Res. 2014, 16, 2568. [Google Scholar] [CrossRef]
- Cho, W.; Schulz, M.; Shanov, V. Growth and characterization of vertically aligned centimeter long CNT arrays. Carbon 2014, 72, 264–273. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Taurino, I.; Carrara, S.; Giorcelli, M.; Tagliaferro, A.; De Micheli, G. Comparison of two different carbon nanotube-based surfaces with respect to potassium ferricyanide electrochemistry. Surf. Sci. 2012, 606, 156–160. [Google Scholar] [CrossRef] [Green Version]
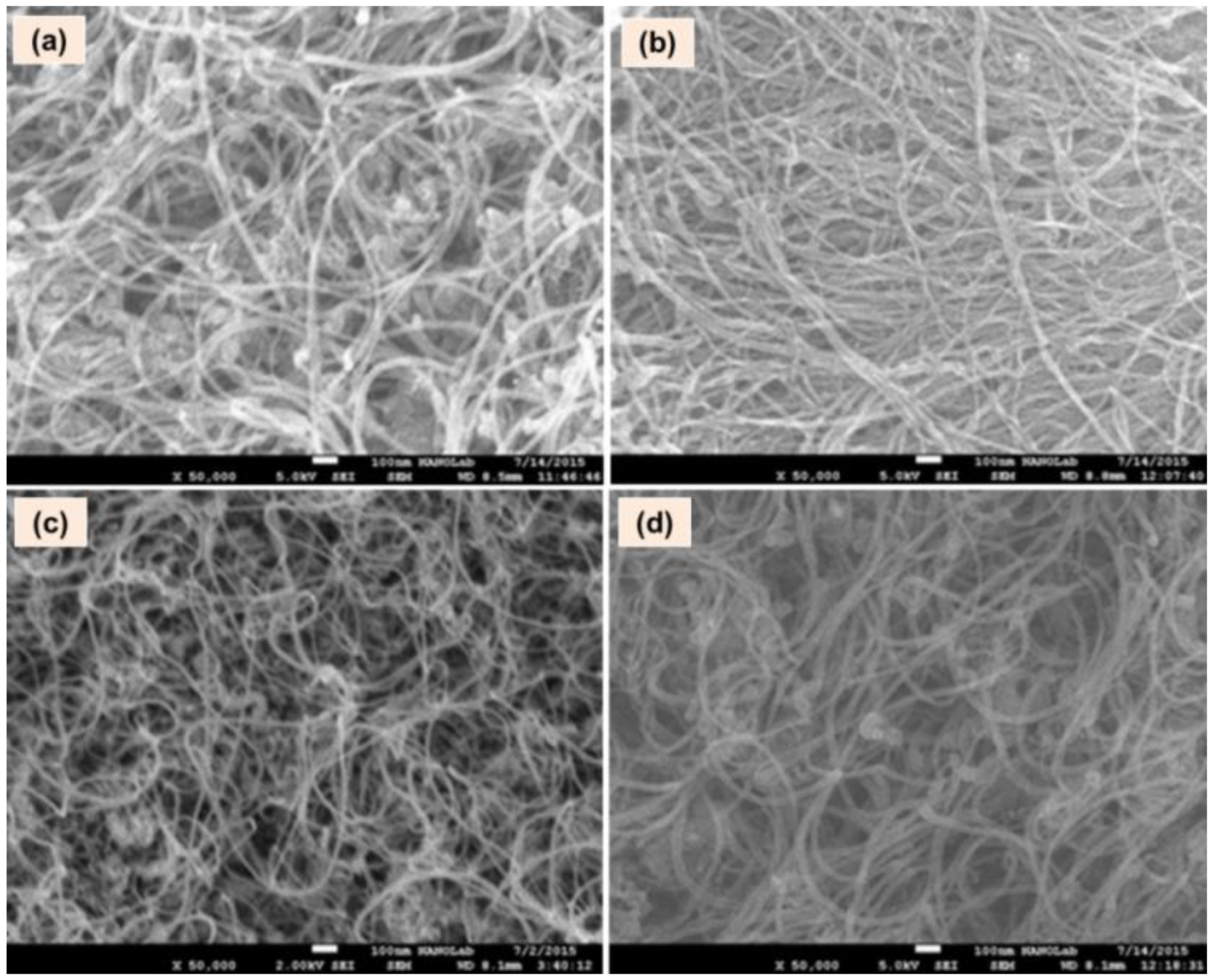
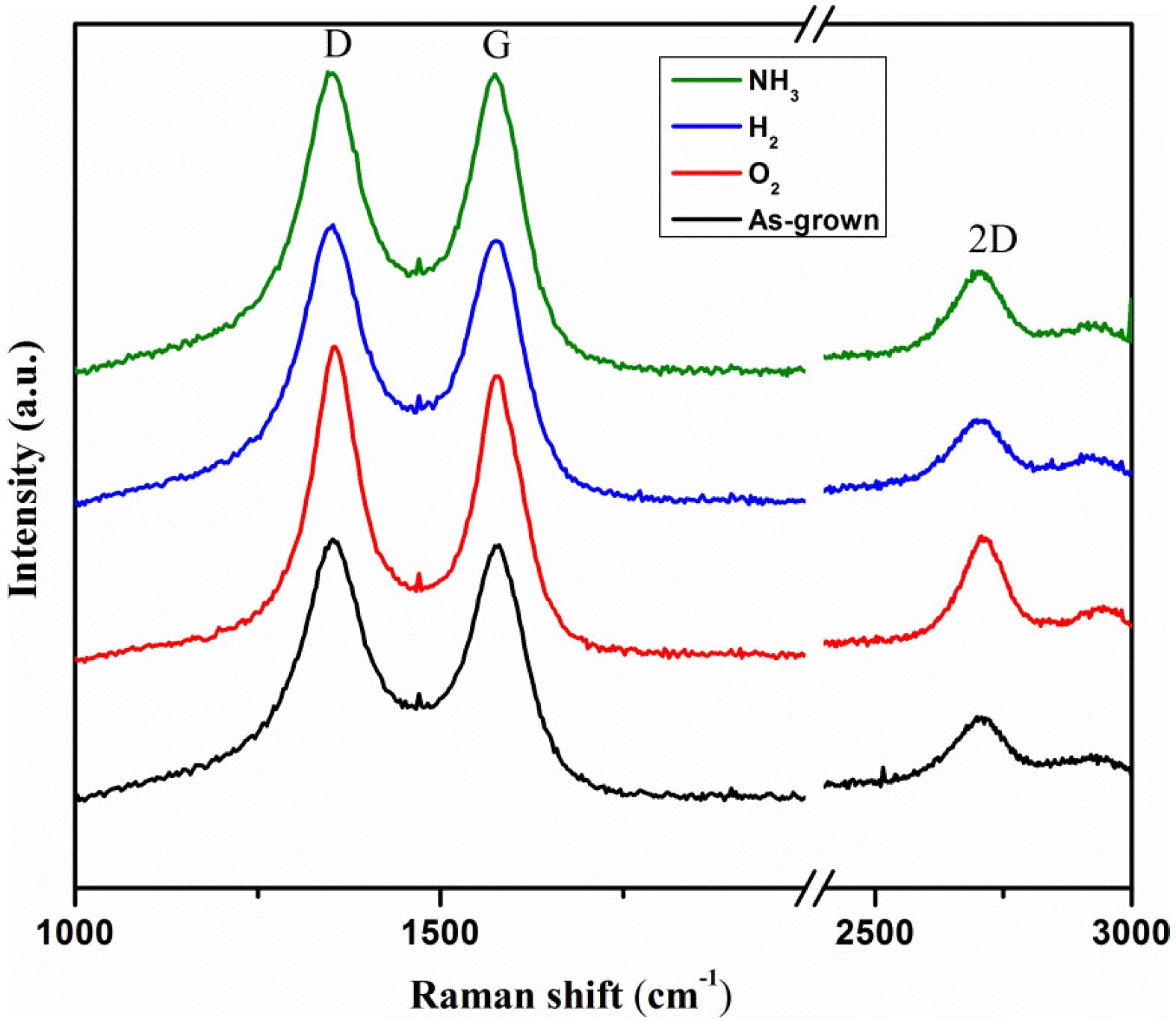


| Type of MWCNTs | ID/IG Ratio | RSD (%) | I2D/IG | RSD (%) | I2D/ID | RSD (%) |
|---|---|---|---|---|---|---|
| As-grown MWCNTs | 0.998 | 2.545 | 0.225 | 2.545 | 0.125 | 4.421 |
| NH3/MWCNTs | 1.034 | 1.654 | 0.211 | 1.654 | 0.141 | 2.544 |
| H2/MWCNTs | 1.017 | 3.934 | 0.198 | 3.934 | 0.138 | 3.534 |
| O2/MWCNTs | 1.089 | 2.911 | 0.165 | 2.911 | 0.088 | 3.310 |
| MWCNTs Type | ipa/ipc | Epa (mV) | Epc (mV) | ∆P (mV) |
|---|---|---|---|---|
| As grown MWCNTs | 1.003 | 340 | 122 | 218 |
| NH3/MWCNTs | 1.014 | 347 | 105 | 242 |
| H2/MWCNTs | 0.974 | 349 | 100 | 249 |
| O2/MWCNTs | 1.028 | 298 | 159 | 139 |
| MWCNTs Type | ipa | RSD (%) | ipc | RSD (%) | Correlation Coefficient (R) | Effective Surface Area (cm²) |
|---|---|---|---|---|---|---|
| As-grown MWCNTs | 0.335 | 3.612 | −0.334 | 4.212 | 0.99561 | 0.32 |
| NH3/MWCNTs | 0.522 | 2.333 | −0.536 | 4.213 | 0.99516 | 0.50 |
| H2/MWCNTs | 0.504 | 2.301 | −0.517 | 3.312 | 0.97808 | 0.48 |
| O2/MWCNTs | 0.144 | 2.701 | −0.140 | 1.521 | 0.9702 | 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Rahim, Z.; Yusof, N.A.; Mohammad Haniff, M.A.S.; Mohammad, F.; Syono, M.I.; Daud, N. Electrochemical Measurements of Multiwalled Carbon Nanotubes under Different Plasma Treatments. Materials 2018, 11, 1902. https://doi.org/10.3390/ma11101902
Abdul Rahim Z, Yusof NA, Mohammad Haniff MAS, Mohammad F, Syono MI, Daud N. Electrochemical Measurements of Multiwalled Carbon Nanotubes under Different Plasma Treatments. Materials. 2018; 11(10):1902. https://doi.org/10.3390/ma11101902
Chicago/Turabian StyleAbdul Rahim, Zulaiha, Nor Azah Yusof, Muhammad Aniq Shazni Mohammad Haniff, Faruq Mohammad, Mohd Ismahadi Syono, and Nurulhaidah Daud. 2018. "Electrochemical Measurements of Multiwalled Carbon Nanotubes under Different Plasma Treatments" Materials 11, no. 10: 1902. https://doi.org/10.3390/ma11101902





