Boosted Visible-Light Photodegradation of Methylene Blue by V and Co Co-Doped TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalysts
2.3. Characterization of Photocatalysts
2.4. Photocatalytic Performance of Photocatalysts
3. Results and Discussion
3.1. Morphology and Structure
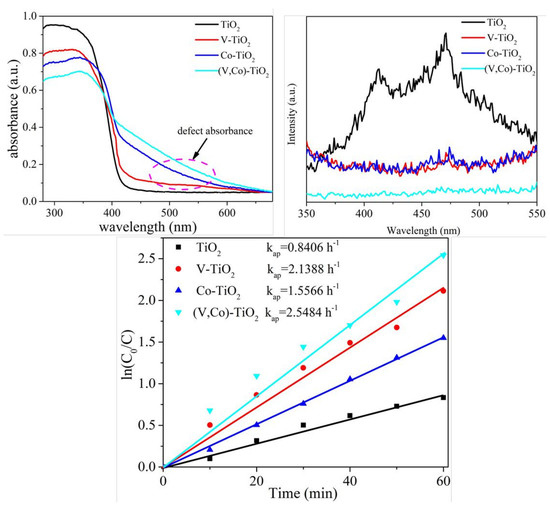
3.2. Optical Property
3.3. Photocatalytic Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibiet, M.; Elimame, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Khataee, A.R.; Vatanpour, V.; Ghadim, A.R.A. Decolorization of C.I. Acid Blue 9 solution by UV/Nano-TiO2, fenton, fenton-like, electro-fenton and electrocoagulation processes: A comparative study. J. Hazard. Mater. 2002, 161, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Peng, J.M.; Zheng, Y.; He, X.; Hou, Y.D.; Wu, L.; Fu, X.Z. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites. Appl. Catal. B Environ. 2018, 221, 223–234. [Google Scholar] [CrossRef]
- Chen, H.H.; Nanayakkara, C.E.; Grassian, V.H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef] [PubMed]
- Zyoud, A.; Zaatar, N.; Saadeddin, I.; Helal, M.H.; Campet, G.; Hakim, M.; Park, D.; Hilal, H.S. Alternative natural dyes in water purification: Anthocyaninas TiO2-sensitizer in methyl orange photo-degradation. Solid State Sci. 2011, 13, 1268–1275. [Google Scholar] [CrossRef]
- Lin, X.X.; Rong, F.; Fu, D.G.; Yuan, C.W. Enhanced photocatalytic activity of fluorine doped TiO2 by loaded with Ag for degradation of organic pollutants. Powder Technol. 2012, 219, 173–178. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, A.D.; Afrin, T.; Sun, L.; Wang, X.G. Self-cleaning wool: Effect of noble metals and silica on visible-light-induced functionalities of nano TiO2 colloid. J. Text. Inst. 2015, 106, 1348–1361. [Google Scholar] [CrossRef]
- Chu, H.P.; Lei, W.Y.; Liu, X.J.; Li, J.L.; Zheng, W.; Zhu, G.; Li, C.; Pan, L.K.; Sun, C.Q. Synergetic effect of TiO2 as co-catalyst for enhanced visible light photocatalytic reduction of Cr(VI) on MoSe2. Appl. Catal. A Gen. 2016, 521, 19–25. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, A.D.; Seyedin, S.; Wang, J.F.; Razal, J.M.; Sun, L.; Wang, X.G. Tunable photocatalytic selectivity of TiO2/SiO2 nanocomposites: Effect of silica and isolation approach. Colloid Surf. A 2018, 522, 130–141. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.W.; Xie, W.J.; Sun, L.; Chen, Y.; Lei, W.W. Porous BN/TiO2 hybrid nanosheets as highly efficient visible-light-driven photocatalysts. Appl. Catal. B Environ. 2017, 207, 72–78. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Mul, G.; Vossoughi, M. E. coli inactivation by visible light irradiation using a Fe-Cd/TiO2 photocatalyst: Statistical analysis and optimization of operating parameters. Appl. Catal. B Environ. 2015, 168–169, 441–447. [Google Scholar] [CrossRef]
- Janisch, R.; Gopal, P.; Spaldin, N.A. Transition metal-doped TiO2 and ZnO-present status of the field. J. Phys. Condens. Matter 2005, 17, R657–R689. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Zhou, C.; Jin, Q.T. Enhanced photocatalytic activity of La and N co-doped TiO2/diatomite composite. Powder Technol. 2017, 322, 296–300. [Google Scholar] [CrossRef]
- Quan, X.J.; Zhao, Q.H.; Tan, H.Q.; Sang, X.M.; Wang, F.Q.; Dai, Y. Comparative study of lanthanide oxide doped titanium dioxide photocatalysts prepared by coprecipitation and sol-gel process. Mater. Chem. Phys. 2009, 114, 90–98. [Google Scholar] [CrossRef]
- Zhou, W.F.; Liu, Q.J.; Zhu, Z.Q.; Zhang, J. Preparation and properties of vanadium-doped TiO2 photocatalysts. J. Phys. D Appl. Phys. 2010, 43, 035301. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Qi, K.H.; Fei, B.; Xin, J.H. Visible light-active iron-doped anatase nanocrystallites and their self-cleaning property. Thin Solid Films 2011, 519, 2438–2444. [Google Scholar] [CrossRef]
- Bingham, S.; Daoud, W.A. Recent advances in making nano-sized TiO2 visible-light active through rare-earth metal doping. J. Mater. Chem. 2011, 21, 2041–2050. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl. Catal. B Environ. 2015, 170–171, 153–161. [Google Scholar] [CrossRef]
- Lin, W.C.; Lin, Y.J. Effect of Vanandium (IV)-Doping the Visible Light-Induced Catalytic of Titanium Dioxide Catalysts for Methylene Blue Degradation. Environ. Eng. Sci. 2012, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Fard, E.H.; Saadati, S.; Rabbani, M. Degradation of methylene blue via Co-TiO2 nano powders modified by meso-tetra (carboxyphenyl) porphyrin. J. Sol-Gel Sci. Technol. 2012, 62, 351–357. [Google Scholar] [CrossRef]
- Chen, W.F.; Koshy, P.; Sorrell, C.C. Effect of intervalence charge transfer on photocatalytic performance of cobalt- and vanadium-codoped TiO2 thin films. Int. J. Hydrogen Energy 2015, 40, 16215–16229. [Google Scholar] [CrossRef]
- Amirsalehi, M.; Askari, M. Infulence of vanadium, cobalt-codoping on electrochemical performance of titanium dioxide bronze nanobelts used as lithium ion battery anodes. J. Mater. Sci. Mater. Electr. 2018, 29, 13068–13076. [Google Scholar] [CrossRef]
- Das, K.; Sharma, S.N.; Kumar, M.; De, S.K. Morphology dependent luminescence properties of Co doped TiO2 nanostructures. J. Phys. Chem. C 2009, 113, 14783–14792. [Google Scholar] [CrossRef]
- Du, P.; Bueno-López, A.; Verbaas, M.; Almeida, A.R.; Makkee, M.; Moulijn, J.A.; Mul, G. The effect of surface OH-population on the photocatalytic activity of rare earth-doped P25-TiO2 in methylene blue degradation. J. Catal. 2008, 260, 75–80. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.J.; Zhang, X.W.; Wang, L. Photoisomerization of norbornadiene to quadricyclane using transition metal doped TiO2. Ind. Eng. Chem. Res. 2012, 49, 8526–8531. [Google Scholar] [CrossRef]
- Niu, J.F.; Yao, B.H.; Chen, Y.Q.; Peng, C.; Yu, X.J.; Zhang, J.; Bai, G.H. Enhanced photocatalytic activity of nitrogen doped TiO2 photocatalysts sensitized by metallo Co, Ni-porphyrins. Appl. Surf. Sci. 2013, 271, 39–44. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Wang, W.G.; Jaroniec, M. Nitrogen self-doped nanosized TiO2 sheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. Commun. 2011, 47, 6906–6908. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Hwang, M.J.; Ryu, K.S. High adsorption capacity of V-doped TiO2 for decolorization of methylene blue. Appl. Surf. Sci. 2012, 258, 7299–7305. [Google Scholar] [CrossRef]
- Lu, D.Z.; Chai, W.Q.; Yang, M.C.; Fang, P.F.; Wu, W.H.; Zhao, B.; Xiong, R.Y.; Wang, H.M. Visible light induced photocatalytic removal of Cr(VI) over TiO2-based nanosheets loaded with surface-enriched CoOx nanoparticles and its synergism with phenol oxidation. Appl. Catal. B Environ. 2016, 190, 44–65. [Google Scholar] [CrossRef]
- Gao, L.J.; Li, Y.G.; Ren, J.B.; Wang, S.F.; Wang, R.N.; Fu, G.S.; Hu, Y. Passivation of defect states in anatase TiO2 hollow spheres with Mg doping: Realizing efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2017, 202, 127–133. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Korotin, M.A.; Zaanen, J.; Andersen, O.K. Spin bags, polarons, and impurity potentials in La2−xSrxCuO4 from first principles. Phys. Rev. Lett. 1992, 68, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.L.; Zhao, Z.Y.; Liu, Q.J. Synergistic effects of nonmetal co-doping with sulfur in anatase TiO2: A DFT + U study. Phys. Chem. Chem. Phys. 2015, 17, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.L.; Zhao, Z.Y.; Liu, Q.J. Analysis of sulfur modification mechanism for anatase and rutile TiO2 by different doping modes based on GGA + U calculations. RSC Adv. 2014, 4, 32100–32107. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.L.; Chen, F.; Anpo, M.; He, D.N. Preparation, photocatalytic activity, and mechanism of nano-TiO2 Co-doped with nitrogen and Iron (III). J. Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Liu, Q.L.; Zhao, Z.Y.; Liu, Q.J. Impact of sulfur-, tantalum-, or co-doping on the electronic structure of anatase titanium dioxide: A systematic density functional theory investigation. Mater. Sci. Semicon. Proc. 2015, 33, 94–102. [Google Scholar] [CrossRef]
- Ma, X.J.; Zhou, W.R.; Chen, Y. Structure and photocatalytic properties of Mn-doped TiO2 loaded on wood-based activated carbon fiber composites. Materials 2017, 10, 631. [Google Scholar]
- Shang, F.M.; Chen, S.Y.; Liang, J.; Liu, C.S. The Photocatalytic properties and mechanistic study of ZnO, Ag multiphase Co-composited TiO2 nanotube arrays film prepared by one-step anodization method. J. Electrochem. Soc. 2018, 165, D258–D265. [Google Scholar] [CrossRef]
- Wang, F.L.; Feng, Y.P.; Chen, P.; Wang, Y.F.; Su, Y.H.; Zhang, Q.X.; Zeng, Y.Q.; Xie, Z.J.; Liu, H.J.; Lv, W.Y.; et al. Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B Environ. 2018, 227, 114–122. [Google Scholar] [CrossRef]










| Samples with Different Doping Element | TiO2 | V–TiO2 | Co–TiO2 | (V,Co)–TiO2 |
|---|---|---|---|---|
| Anatase content (%) | 64.07 | 78.62 | 47.07 | 70.05 |
| Rutile content (%) | 35.93 | 21.38 | 52.93 | 29.95 |
| Sample | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| V–TiO2 | 35.270 | 0.056 | 7.4 |
| Co–TiO2 | 22.812 | 0.033 | 6.5 |
| (V,Co)–TiO2 | 40.865 | 0.051 | 5.5 |
| TiO2 | 61.314 | 0.093 | 6.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, T.; Zhao, J.; Chen, M.; Shen, K.; Zhang, D.; Zhang, J.; Zhang, G.; Liu, Q. Boosted Visible-Light Photodegradation of Methylene Blue by V and Co Co-Doped TiO2. Materials 2018, 11, 1946. https://doi.org/10.3390/ma11101946
Lv T, Zhao J, Chen M, Shen K, Zhang D, Zhang J, Zhang G, Liu Q. Boosted Visible-Light Photodegradation of Methylene Blue by V and Co Co-Doped TiO2. Materials. 2018; 11(10):1946. https://doi.org/10.3390/ma11101946
Chicago/Turabian StyleLv, Tianping, Jianhong Zhao, Mingpeng Chen, Kaiyuan Shen, Dongming Zhang, Jin Zhang, Genlin Zhang, and Qingju Liu. 2018. "Boosted Visible-Light Photodegradation of Methylene Blue by V and Co Co-Doped TiO2" Materials 11, no. 10: 1946. https://doi.org/10.3390/ma11101946