Synthesis of Silica Microspheres—Inspired by the Formation of Ice Crystals—With High Homogeneous Particle Sizes and Their Applications in Photonic Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SMs
2.2. Surface Modification of SMs
2.2.1. Aminated Modification of SMs
2.2.2. Chemical Modification of SMs by the Carbonylation Method
2.3. Preparation of Close-Packed PCs
2.4. Preparation of Non-Dense Packed PCs
2.5. Identification of Ethanol Using the Inverse Opal Photonic Crystal Films
2.6. Characterization of SMs and PCs
3. Results and Discussion
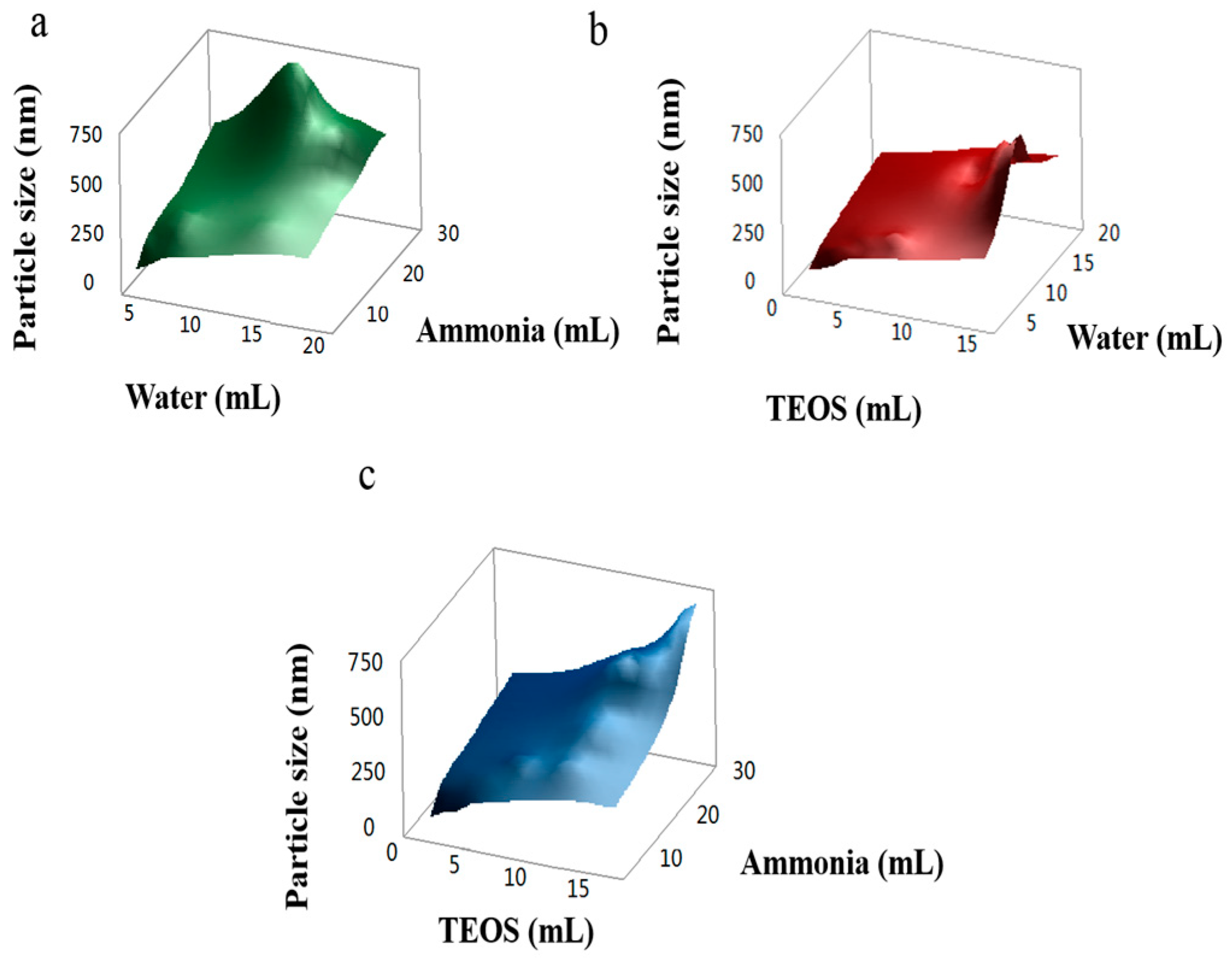
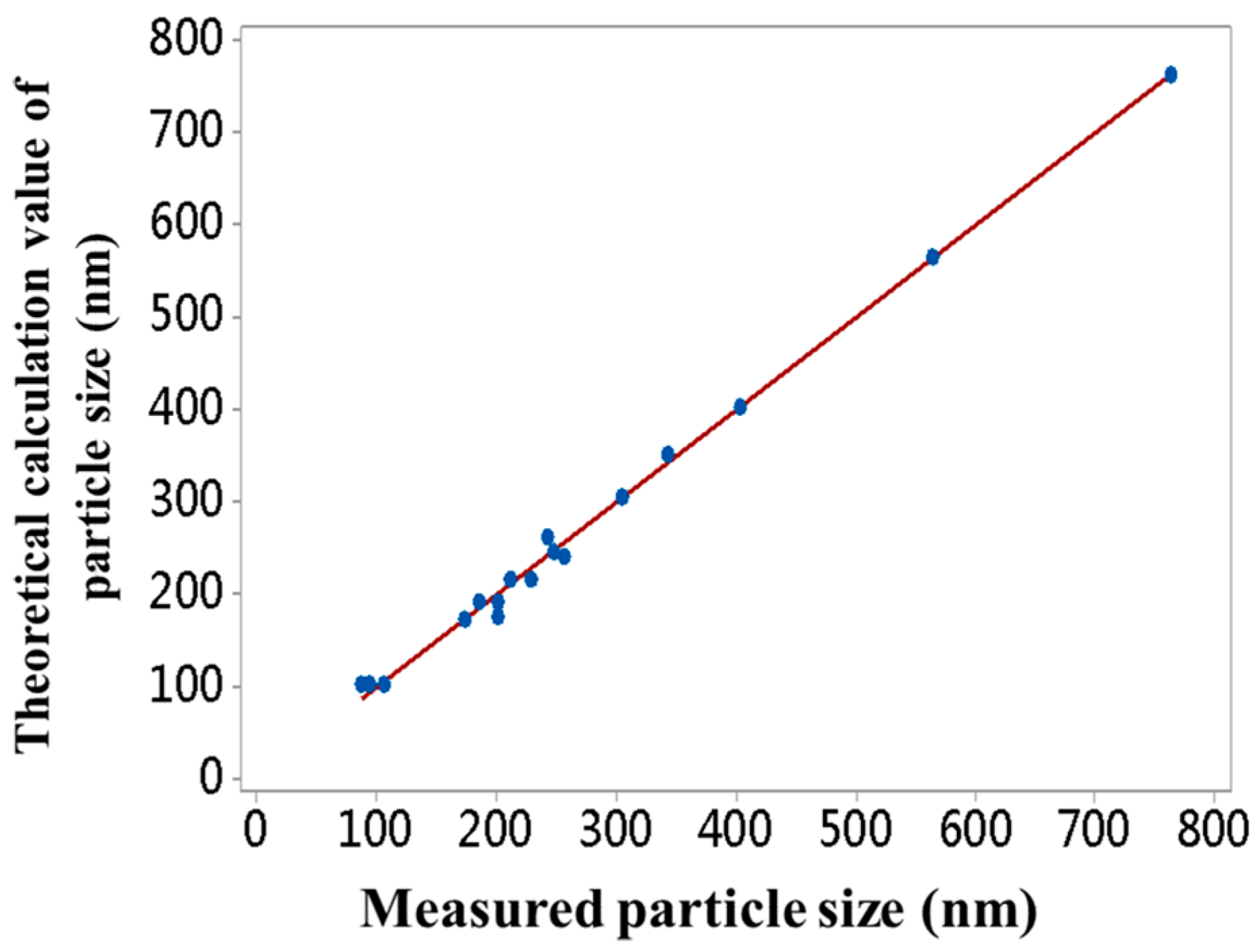
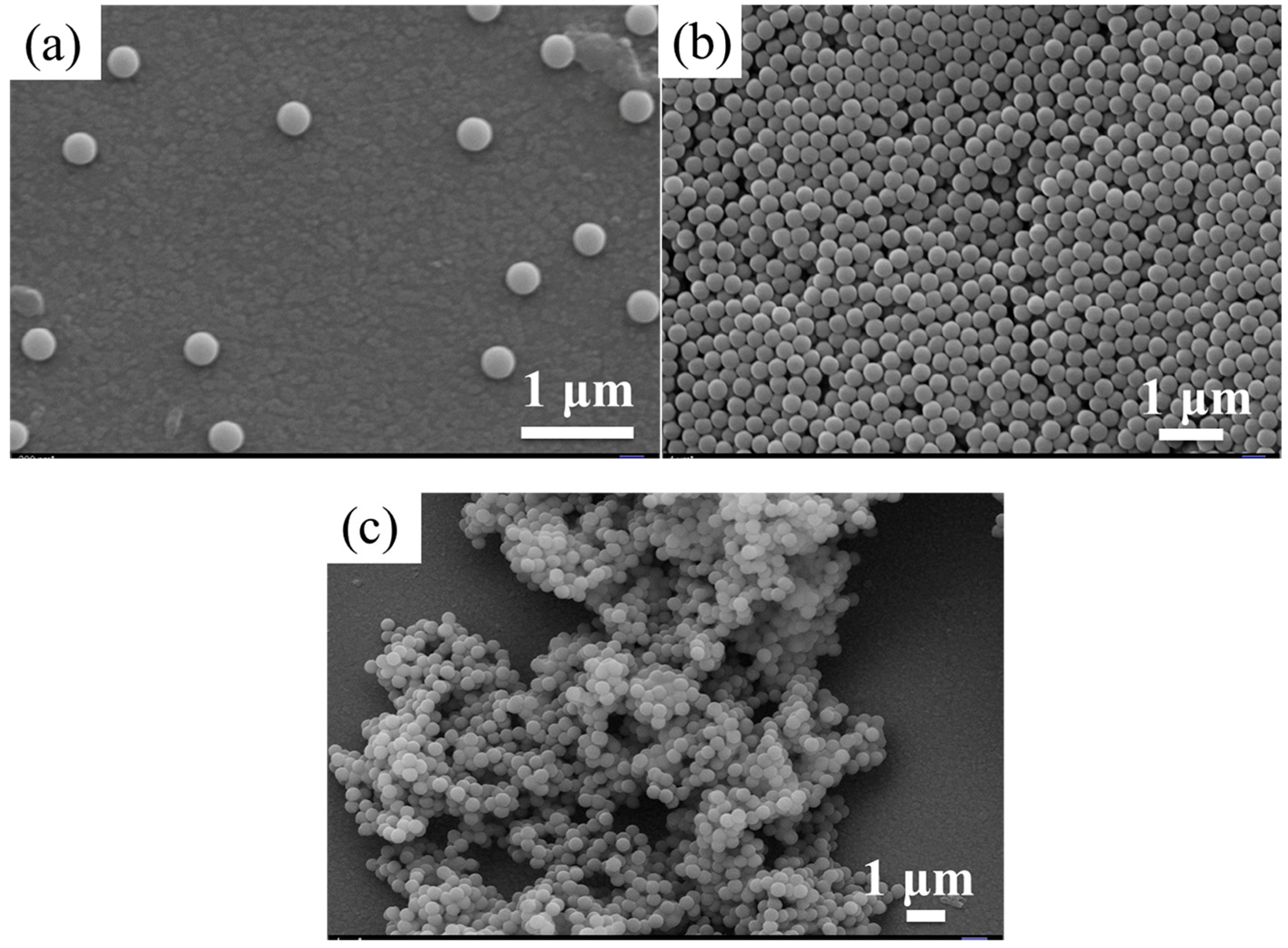
3.1. Synthesis and Characterization of Monodisperse SMs with Uniform Particle Sizes
3.2. Surface Modification of SMs
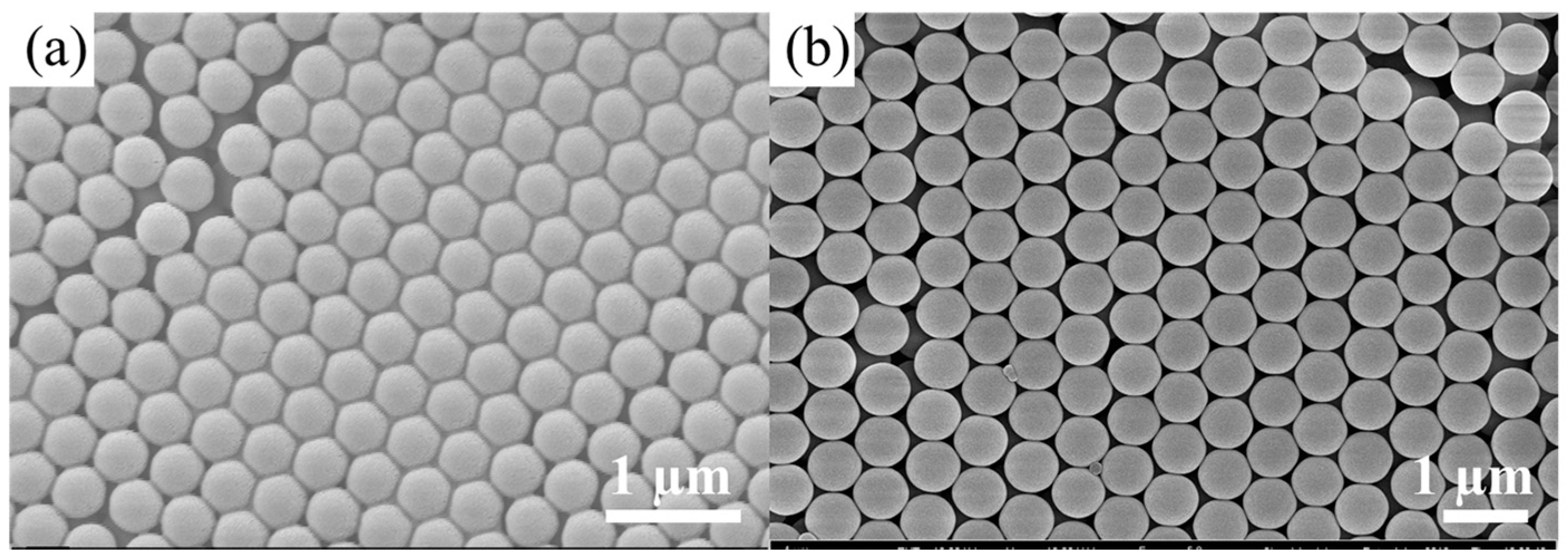
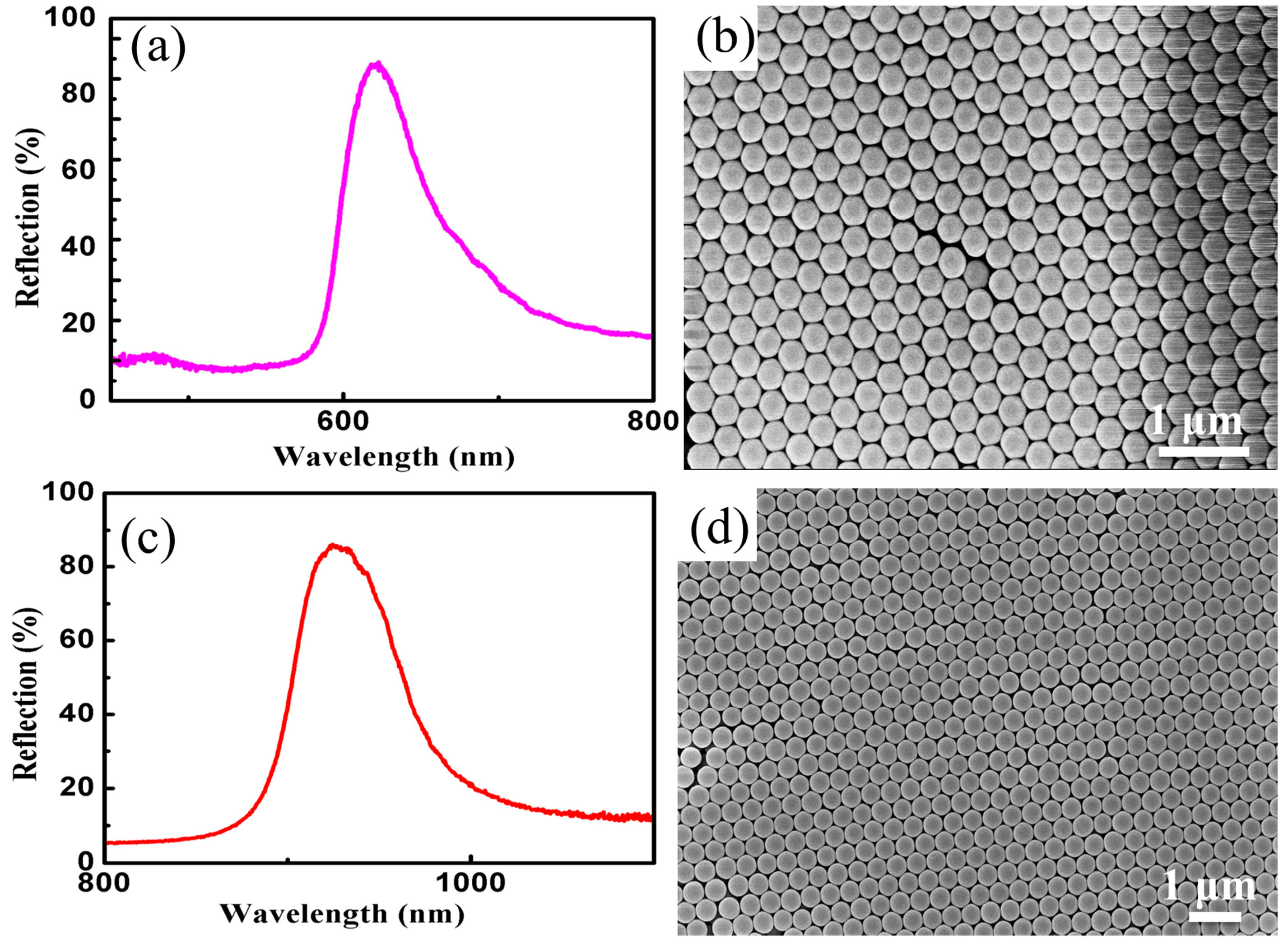
3.3. Fabrication of Close-Packed Opal PCs
3.4. Preparation of Non-Close-Packed Opal PCs and Inverse Opal PCs
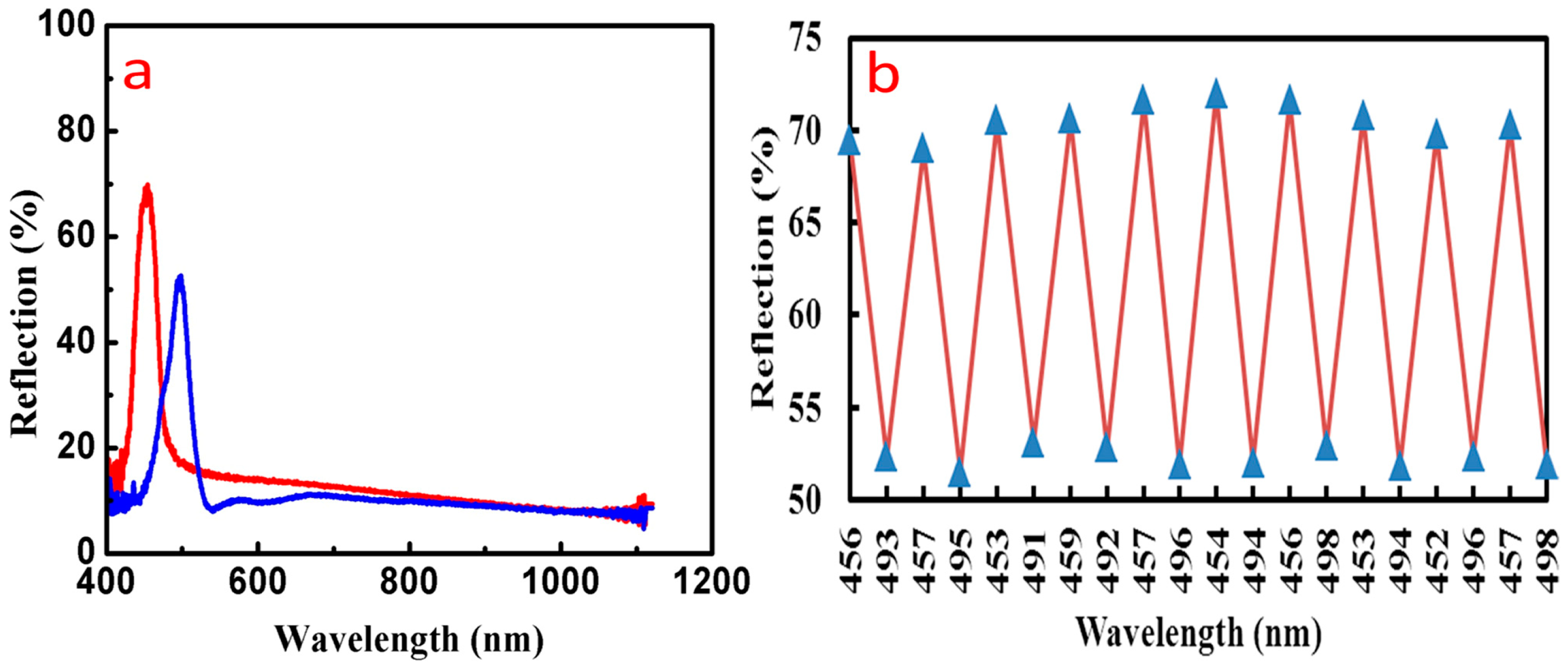
3.5. Detection of Ethanol Using Inverse Opal Structure PCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Noda, S.; Tomoda, K.; Yamamoto, N.; Chutinan, A. Full three-dimensional photonic bandgap crystals at near-infrared wavelengths. Science 2000, 289, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Guo, H.; Fu, L.; Kaiser, S.; Schweizer, H.; Giessen, H. Three-dimensional photonic metamaterials at optical frequencies. Nat. Mater. 2008, 7, 31–37. [Google Scholar] [CrossRef]
- Ondič, L.; Varga, M.; Pelant, I.; Valenta, J.; Kromka, A.; Elliman, R.G. Silicon nanocrystal-based photonic crystal slabs with broadband and efficient directional light emission. Sci. Rep. 2017, 7, 5763. [Google Scholar] [CrossRef] [PubMed]
- Frei, W.R.; Tortorelli, D.A.; Johnson, H.T. Topology optimization of a photonic crystal waveguide termination to maximize directional emission. Appl. Phys. Lett. 2005, 86, 111114. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Sun, X.; Mao, J. Harmonic suppression with photonic bandgap and defected ground structure for a microstrip patch antenna. IEEE Microw. Wirel. Compon. Lett. 2005, 15, 55–56. [Google Scholar]
- Shelby, R.A.; Smith, D.R.; Schultz, S. Experimental Verification of a Negative Index of Refraction. Science 2001, 292, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.R.; Pendry, J.B.; Wiltshire, M.C.K. Metamaterials and Negative Refractive Index. Science 2004, 305, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, W.; Panoiu, N.C.; Malloy, K.J.; Osgood, R.M.; Brueck, S.R. Experimental demonstration of near-infrared negative-index metamaterials. Phys. Rev. Lett. 2005, 95, 137404. [Google Scholar] [CrossRef]
- Yu, X.; Fan, S. Bends and splitters for self-collimated beams in photonic crystals. Appl. Phys. Lett. 2003, 83, 3251–3253. [Google Scholar] [CrossRef]
- Matthews, A.F. Experimental demonstration of self-collimation beaming and splitting in photonic crystals at microwave frequencies. Opt. Commun. 2009, 282, 1789–1792. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, H.; Kawashima, T.; Tomita, A.; Notomi, M.; Tamamura, T.; Sato, T.; Kawakami, S. Superprism phenomena in photonic crystals: toward microscale lightwave circuits. Phys. Rev. B 1998, 58, 10096–10099. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wu, J.Y.; Horng, H.E.; Hong, C.Y.; Yang, H.C. Direct observations for the superprism effect in photonic crystals utilizing negative refraction. J. Appl. Phys. 2008, 103, 053110. [Google Scholar] [CrossRef]
- He, G.; Pfeiffer, M.; Leo, K.; Hofmann, M. High-efficiency and low-voltage p-i-n electrophosphorescent organic light-emitting diodes with double-emission layers. Appl. Phys. Lett. 2004, 85, 3911–3913. [Google Scholar] [CrossRef]
- Cregan, R.F.; Mangan, B.J.; Knight, J.C.; Birks, T.A.; Russell, P.S.; Roberts, P.J.; Allan, D.C. Single-Mode Photonic Band Gap Guidance of Light in Air. Science 1999, 285, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed self-assembly of nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Li, J.; Liu, L.; Van, S.C.; Song, K.; Clays, K. Instantaneous, simple, and reversible revealing of invisible patterns encrypted in robust hollow sphere colloidal photonic crystals. Adv. Mater. 2018, 30, 1707246. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Mujumdar, S.; Polshettiwar, V. Self-assembled photonic crystals of monodisperse dendritic fibrous nanosilica for lasing: Role of fiber density. ACS Appl. Mater. Interfaces 2018, 10, 23392–23398. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C.G.; Navaraj, W.T.; Liu, F.; Shakthivel, D.; Dahiya, R. Large-area self-assembly of silica microspheres/nanospheres by temperature-assisted dip-coating. ACS Appl. Mater. Interfaces 2018, 10, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Kohoutek, T.; Parchine, M.; Bardosova, M.; Fudouzi, H.; Pemble, M. Large-area flexible colloidal photonic crystal film stickers for light trapping applications. Opt. Mater. Express 2018, 8, 960. [Google Scholar] [CrossRef]
- Parchine, M.; Kohoutek, T.; Bardosova, M.; Pemble, M.E. Large area colloidal photonic crystals for light trapping in flexible organic photovoltaic modules applied using a roll-to-roll Langmuir-Blodgett method. Sol. Energy Mater. Sol. Cells 2018, 185, 158–165. [Google Scholar] [CrossRef]
- Hua, C.; Xu, H.; Zhang, P.; Chen, X.; Lu, Y.; Gan, Y.; Li, Y. Process optimization and optical properties of colloidal self-assembly via refrigerated centrifugation. Colloid Polym. Sci. 2017, 295, 1655–1662. [Google Scholar] [CrossRef]
- Pan, L.; Xu, H.; Sun, Y.; Zhao, J.; Li, Y. Preparation of Three-Dimensional Photonic Crystals of Zirconia by Electrodeposition in a Colloidal Crystals Template. Crystals 2016, 6, 76. [Google Scholar] [CrossRef]
- Blaaderen, A.V.; Vrij, A. Synthesis and Characterization of Monodisperse Colloidal Organo-silica Spheres. J. Colloid Interface Sci. 1993, 156, 1–18. [Google Scholar] [CrossRef] [Green Version]
- And, A.M.J.; Schmedake, T.A. A Novel Approach to Monodisperse, Luminescent Silica Spheres. Chem. Mater. 2006, 18, 3173–3175. [Google Scholar]
- Lu, Z.; Dai, J.; Song, X.; Wang, G.; Yang, W. Facile synthesis of Fe3O4/SiO2 composite nanoparticles from primary silica particles. Colloid Surface. A 2008, 317, 450–456. [Google Scholar] [CrossRef]
- Ni, P.; Dong, P.; Cheng, B.; Li, X.; Zhang, D. Synthetic SiO2 opals. Adv. Mater. 2001, 13, 437–441. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Matsoukas, T.; Gulari, E. Dynamics of growth of silica particles from ammonia-catalyzed hydrolysis of tetra-ethyl-orthosilicate. J. Colloid Interface Sci. 1988, 124, 252–261. [Google Scholar] [CrossRef]
- Matsoukas, T.; Gulari, E. Monomer-addition growth with a slow initiation step: A growth model for silica particles from alkoxides. J. Colloid Interface Sci. 1989, 132, 13–21. [Google Scholar] [CrossRef]
- Bogush, G.H.; Tracy, M.A.; Iv, C.F.Z. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non-Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Bogush, G.H.; Iv, C.F.Z. Studies of the kinetics of the precipitation of uniform silica particles through the hydrolysis and condensation of silicon alkoxides. J. Colloid Interface Sci. 1991, 142, 1–18. [Google Scholar] [CrossRef]
- Chen, S.L.; Dong, P.; Yang, G.H.; Yang, J.J. Characteristic Aspects of Formation of New Particles during the Growth of Monosize Silica Seeds. J. Colloid Interface Sci. 1996, 180, 237–241. [Google Scholar] [CrossRef]
- Chen, S.L.; Dong, P.; Yang, G.H. The Size Dependence of Growth Rate of Monodisperse Silica Particles from Tetraalkoxysilane. J. Colloid Interface Sci. 1997, 189, 268–272. [Google Scholar] [CrossRef]
- Kim, K.D.; Kim, H.T. Formation of Silica Nanoparticles by Hydrolysis of TEOS Using a Mixed Semi-Batch/Batch Method. J. Sol-Gel Sci. Technol. 2002, 25, 183–189. [Google Scholar] [CrossRef]
- Krauss, T.F.; Rue, R.M.D.L. Photonic crystals in the optical regime—Past, present and future. Rue. Prog. Quant. Electron. 1999, 23, 51–96. [Google Scholar] [CrossRef]
- Meseguer, F.; Blanco, A.; MíGuez, H.; GarcíA-SantamaríA, F.; Ibisate, M.; López, C. Synthesis of inverse opals. Colloid Surface A 2002, 202, 281–290. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bogush, G.H.; Iv, C.F.Z. Uniform silica particle precipitation: An aggregative growth model. J. Colloid Interface Sci. 1991, 142, 19–34. [Google Scholar] [CrossRef]
- Lamer, V.K.; Dinegar, R.H. In Theory, Production and mechanism of formation of monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Thanh, N.T.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Pietryga, J.M.; Park, Y.S.; Lim, J.; Fidler, A.F.; Wan, K.B.; Brovelli, S.; Klimov, V.I. Spectroscopic and device aspects of nanocrystal quantum dots. Chem. Rev. 2016, 116, 10513–10622. [Google Scholar] [CrossRef] [PubMed]
- Zerda, T.W.; Bradley, M.; Jonas, J. Raman study of the sol to gel transformation under normal and high pressure. Mater. Lett. 1985, 3, 124–126. [Google Scholar] [CrossRef]
- Artaki, I.; Bradley, M.; Zerda, T.W.; Jonas, J. NMR and Raman study of the hydrolysis reaction in sol-gel processes. J. Phys. Chem. 1985, 89, 4399–4404. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Xu, H.; Hua, C.; Zhao, J.; Li, Y.; Song, Y. Synthesis of Silica Microspheres—Inspired by the Formation of Ice Crystals—With High Homogeneous Particle Sizes and Their Applications in Photonic Crystals. Materials 2018, 11, 2017. https://doi.org/10.3390/ma11102017
Chen X, Xu H, Hua C, Zhao J, Li Y, Song Y. Synthesis of Silica Microspheres—Inspired by the Formation of Ice Crystals—With High Homogeneous Particle Sizes and Their Applications in Photonic Crystals. Materials. 2018; 11(10):2017. https://doi.org/10.3390/ma11102017
Chicago/Turabian StyleChen, Xiaoyi, Hongbo Xu, Chunxia Hua, Jiupeng Zhao, Yao Li, and Ying Song. 2018. "Synthesis of Silica Microspheres—Inspired by the Formation of Ice Crystals—With High Homogeneous Particle Sizes and Their Applications in Photonic Crystals" Materials 11, no. 10: 2017. https://doi.org/10.3390/ma11102017
APA StyleChen, X., Xu, H., Hua, C., Zhao, J., Li, Y., & Song, Y. (2018). Synthesis of Silica Microspheres—Inspired by the Formation of Ice Crystals—With High Homogeneous Particle Sizes and Their Applications in Photonic Crystals. Materials, 11(10), 2017. https://doi.org/10.3390/ma11102017





