Formation Mechanism and Cohesive Energy Analysis of Metal-Coated Graphene Nanocomposites Using In-Situ Co-Reduction Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Fabrication of Graphene Oxide (GO)
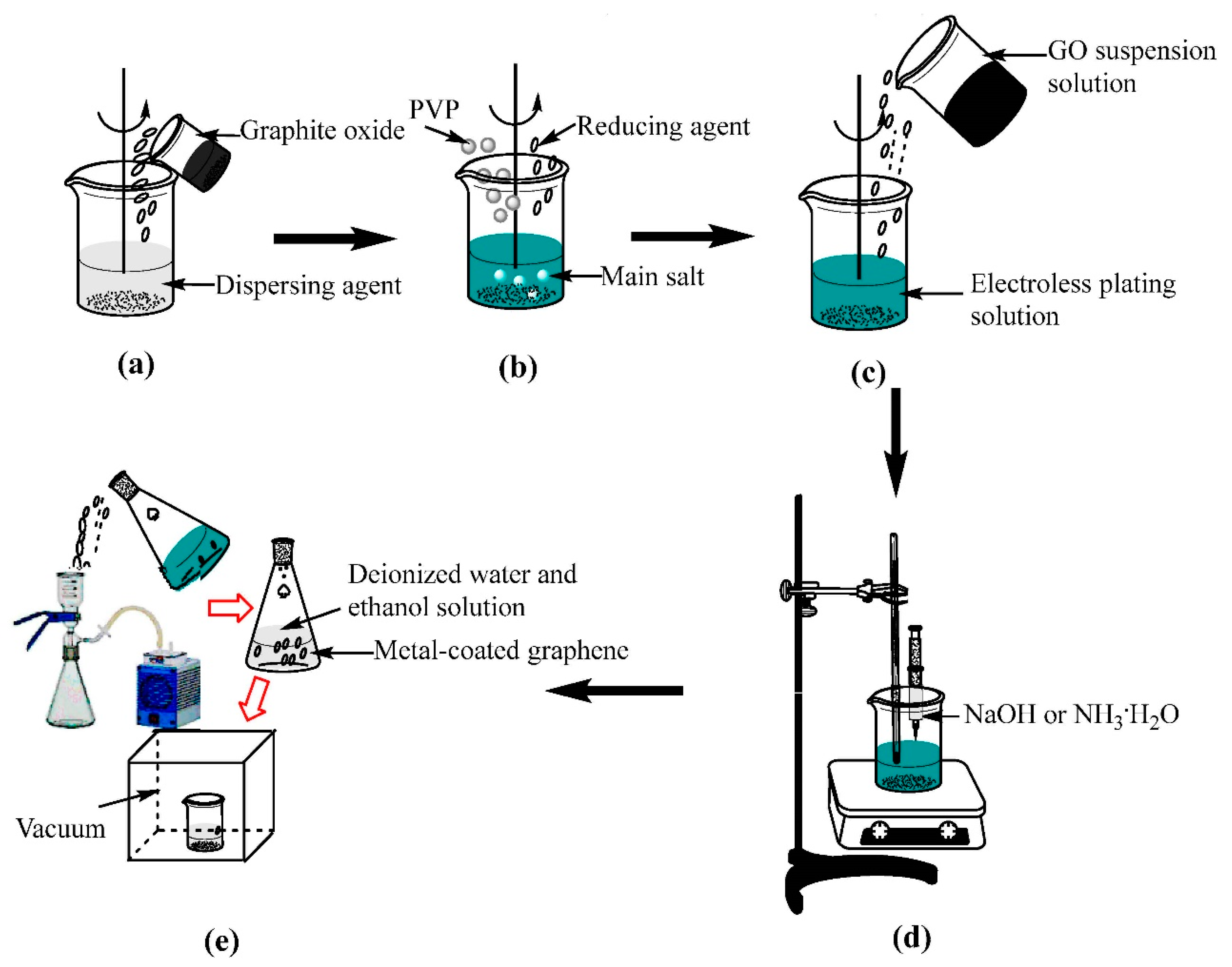
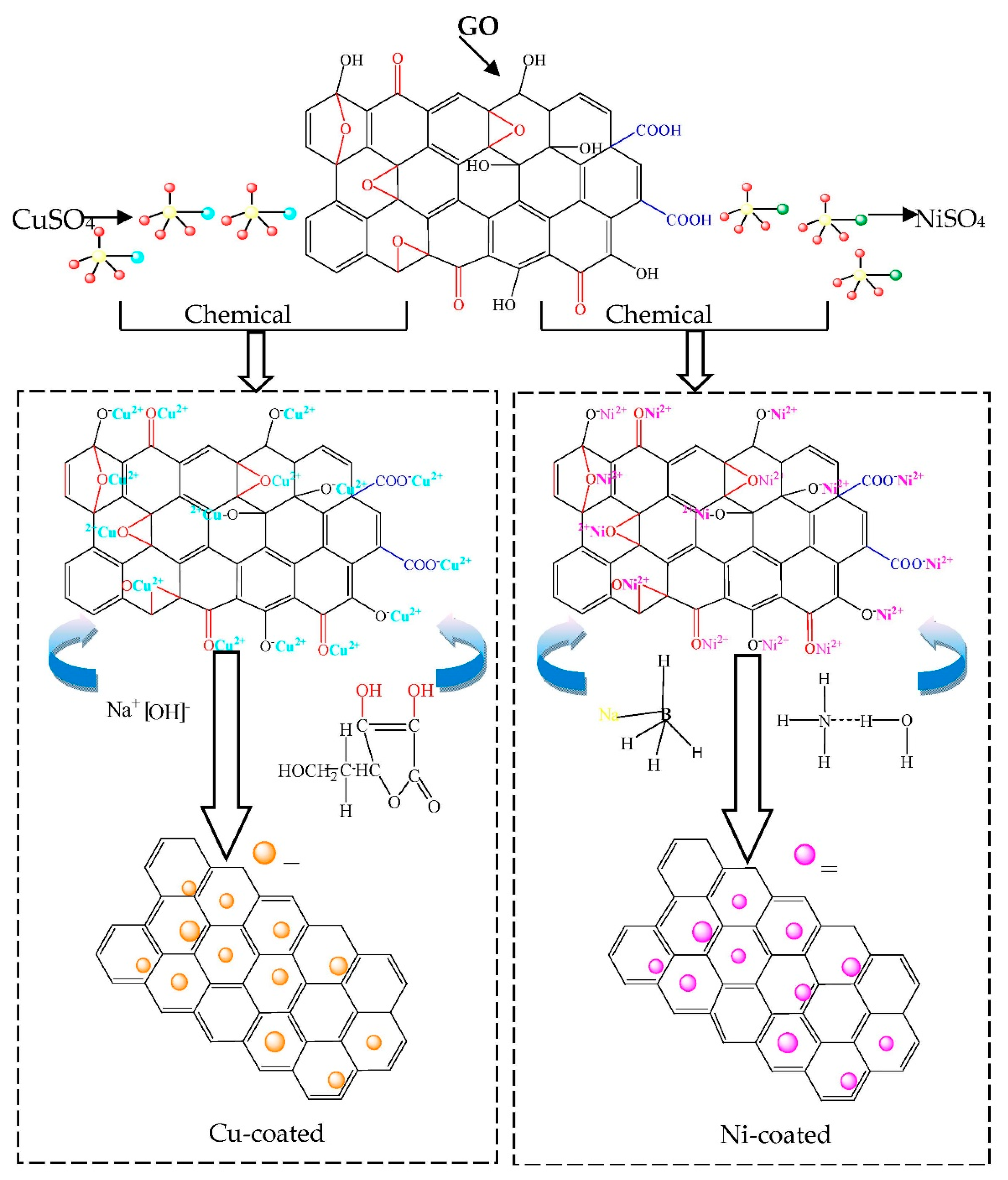
2.3. Fabrication of Metal-Coated Graphene (Metal@Graphene) Nanocomposites Powders
- (a)
- 20 mg of GO was dispersed into 100 mL dispersing agent solution in an ultrasound bath for 3 h to obtain a homogeneous GO suspension solution (0.2 mg/mL) as shown in Figure 1a. The dispersing agent in the Cu plating and Ni plating are N-Methyl pyrrolidone and DI mixture solution;
- (b)
- The salts (e.g., copper sulfate was used for copper plating and nickel sulfate for nickel plating), reducing agents (e.g., ascorbic acid in the copper plating and sodium borohydride in the nickel plating) and surfactant agents were mixed together to form an electroless plating solution (Figure 1b);
- (c)
- Then the GO suspension solution was added into the electroless plating solution (Figure 1c);
- (d)
- The mixed solution was intensively stirred using a magnetic stirrer to disperse the GO suspension solution. The copper plating process was done at 90 °C for 3 h and the nickel plating was done at 30 °C for 30 min. Simultaneously the NaOH solution or ammonia solution was dripped into the mixed solution to adjust the pH value to between 9 and 10 (Figure 1d). The pH value in the bath was continuously measured using a pH meter and was maintained by adjusting the added NaOH solution or ammonia solution using a peristaltic pump. The temperature during the electroless plating process was controlled using a fixed-temperature water bath.
- (e)
- The precipitates were separated from the solution after the chemical reactions were completed (Figure 1e). DI water and ethanol solution were used to wash the precipitates for several times and dried at 60 °C for 8 h using a vacuum freeze-drying method.
2.4. Characterization
2.5. Modeling and Calculation Details
3. Results and Discussions
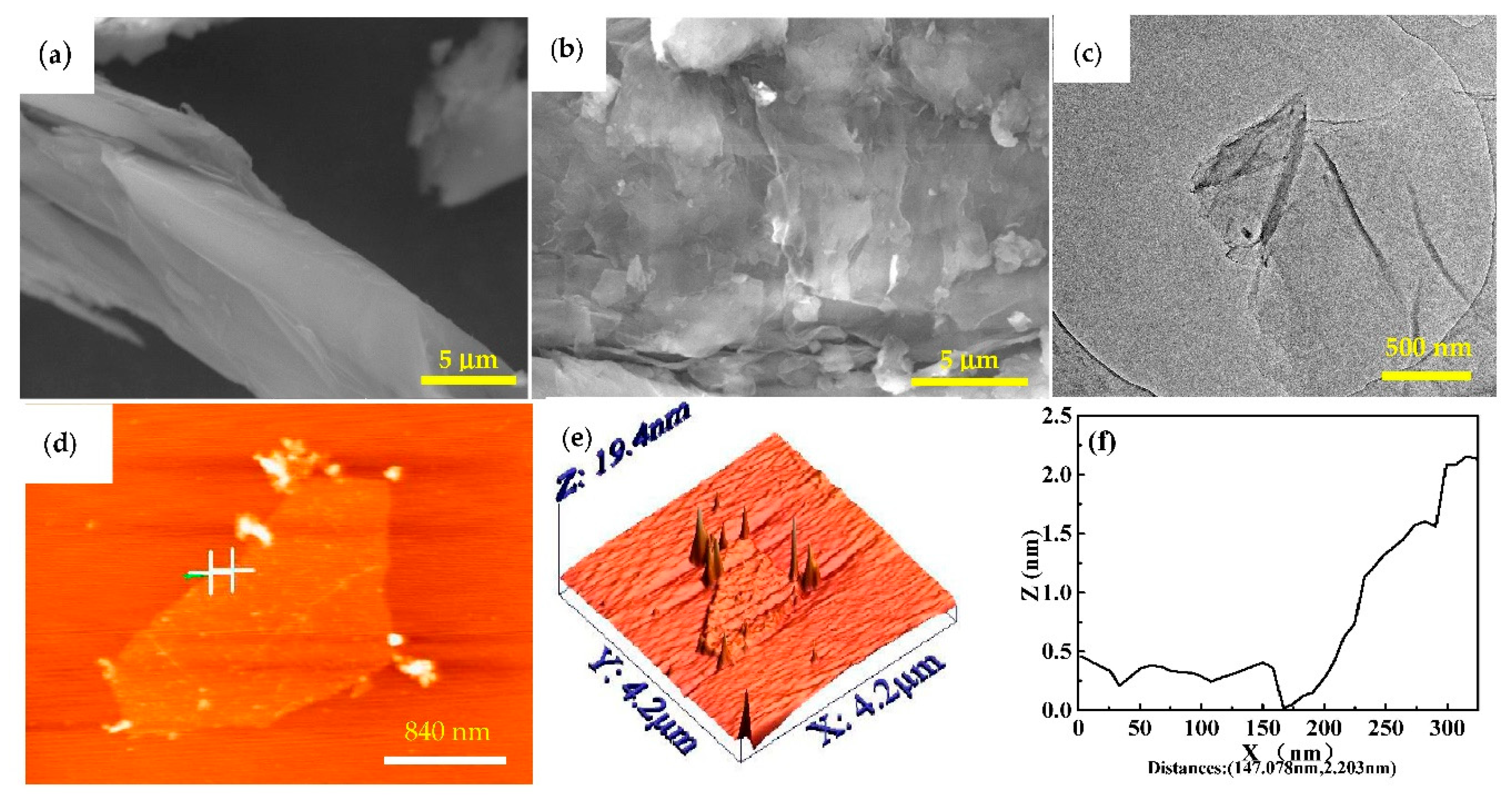
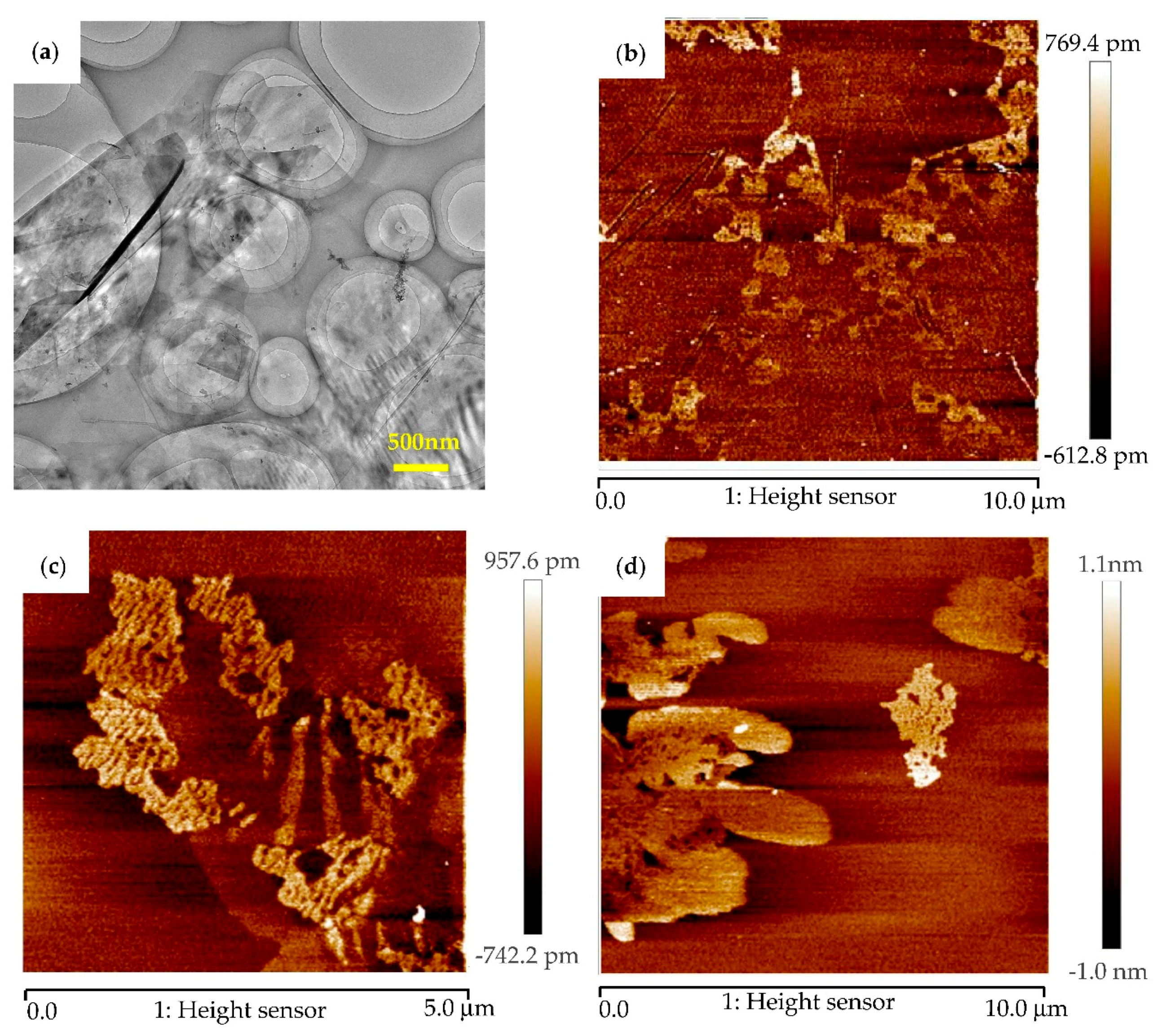
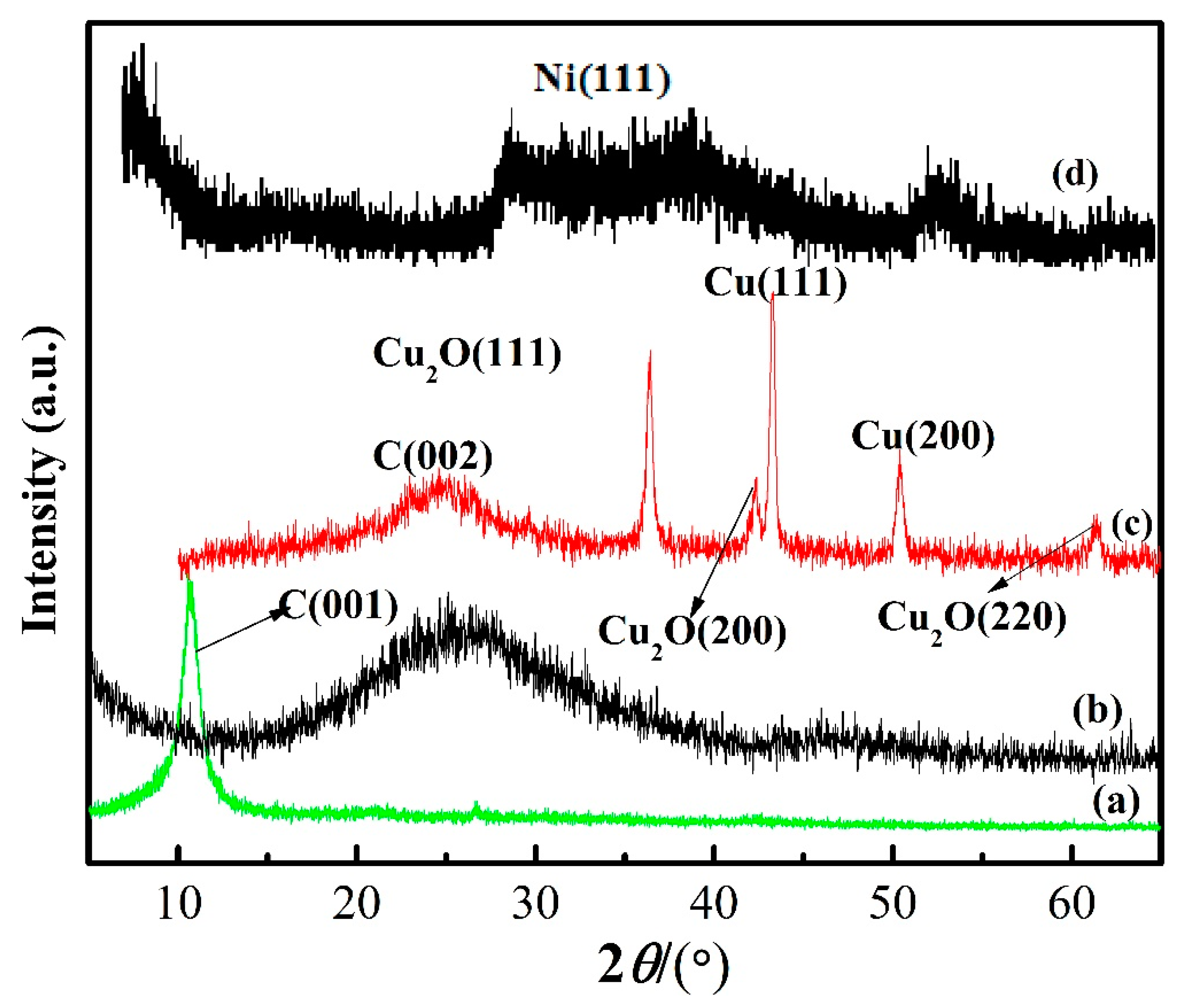
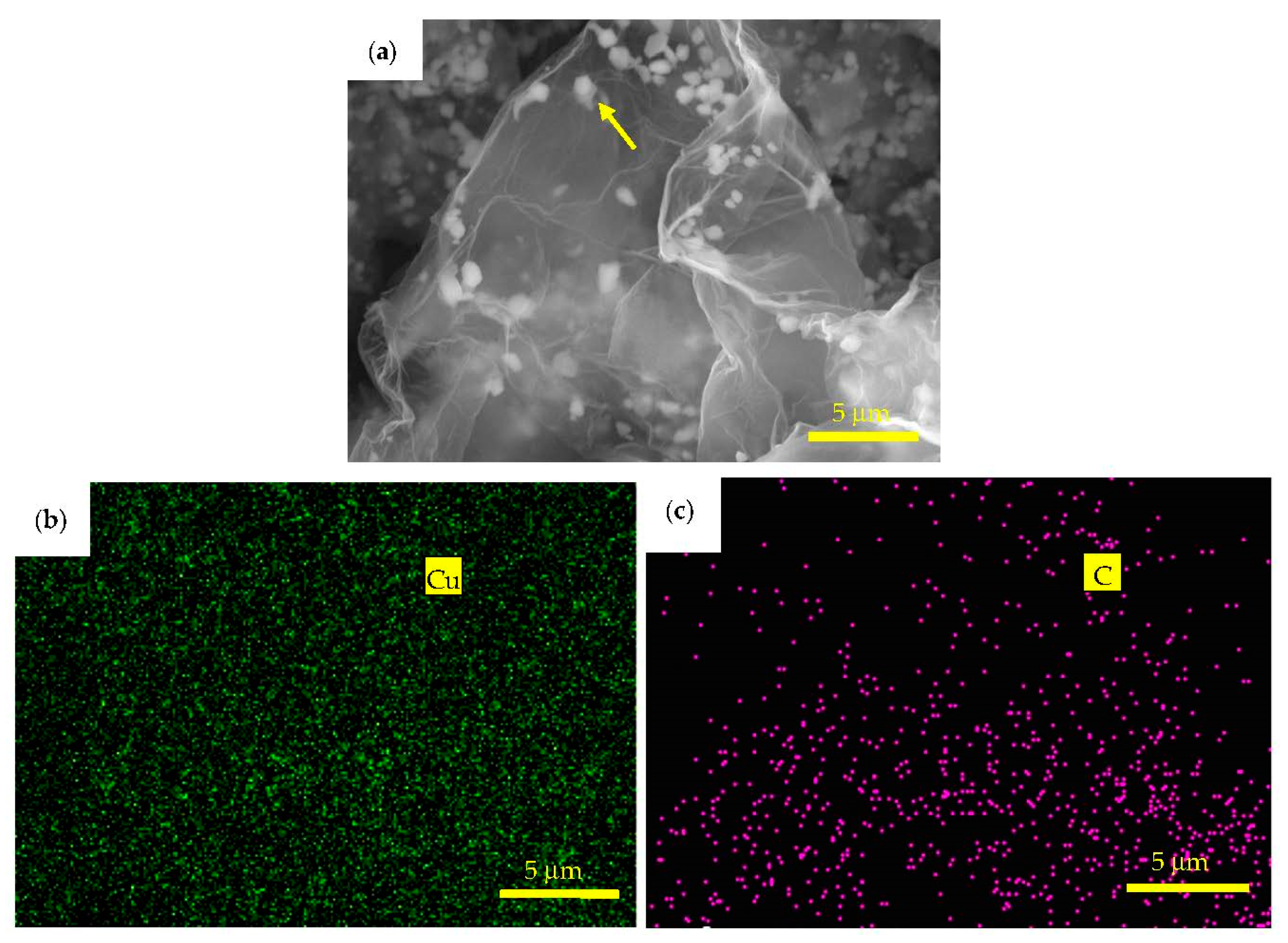
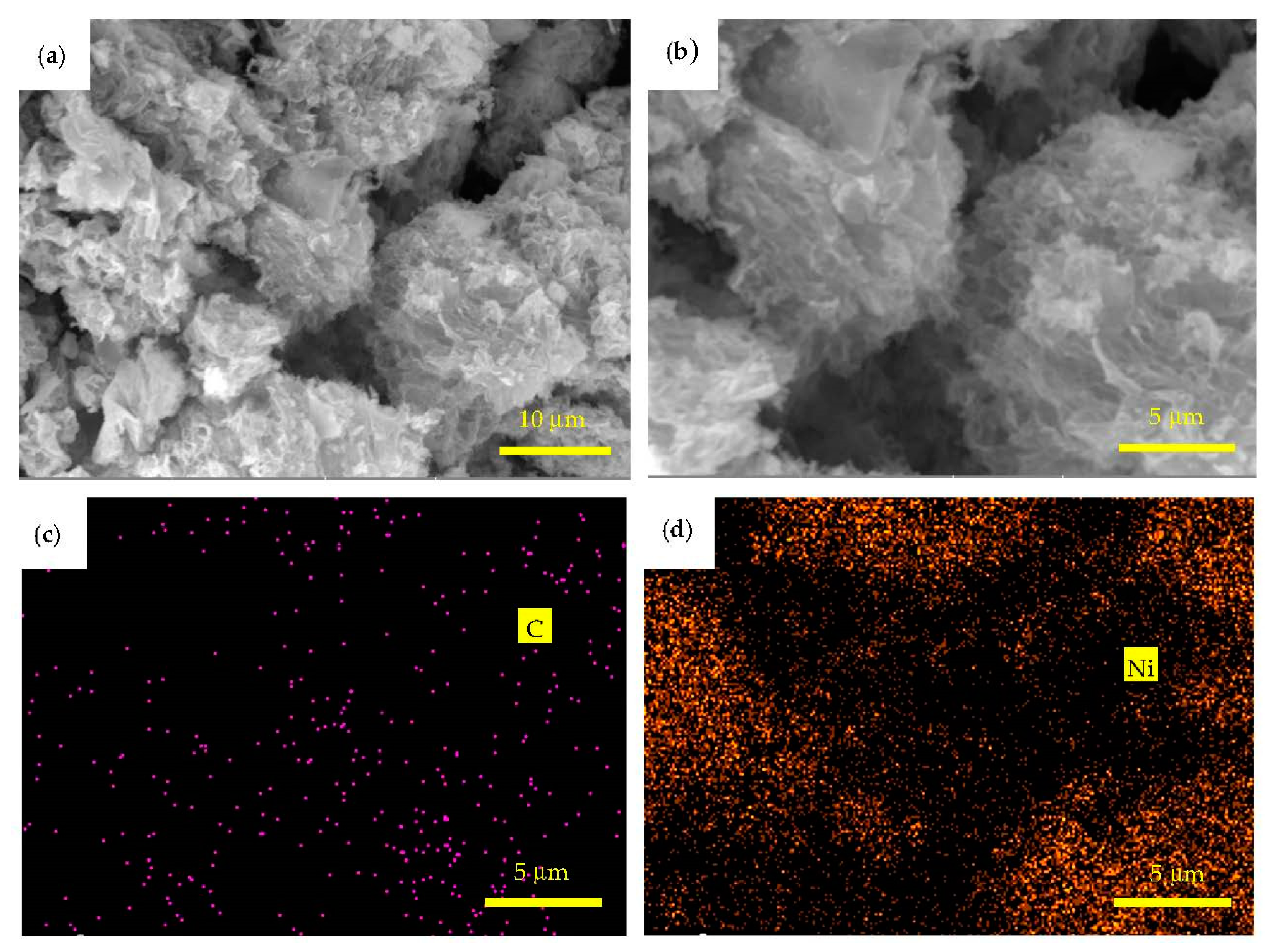
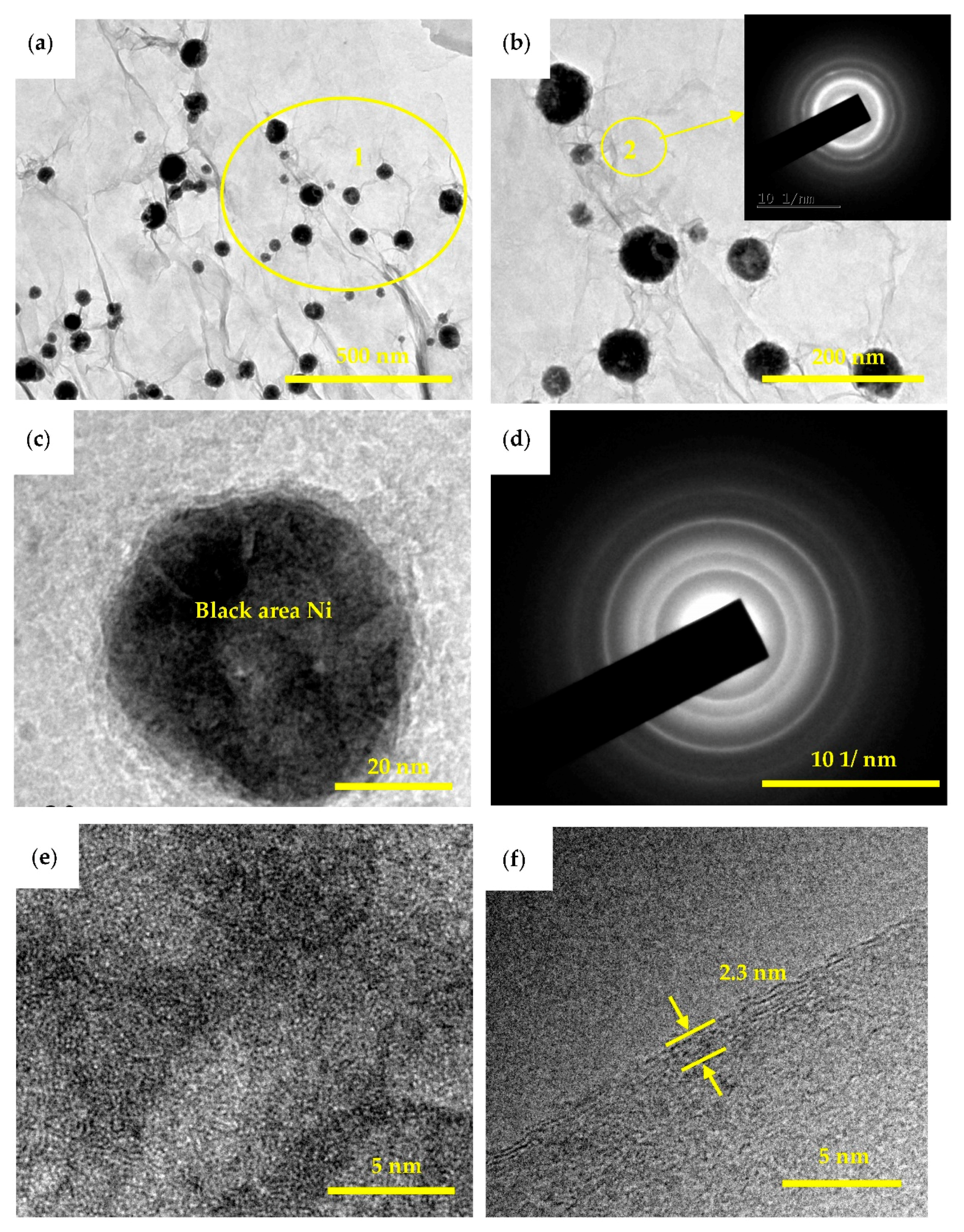
3.1. Microstructure Characterization and Morphology Analysis
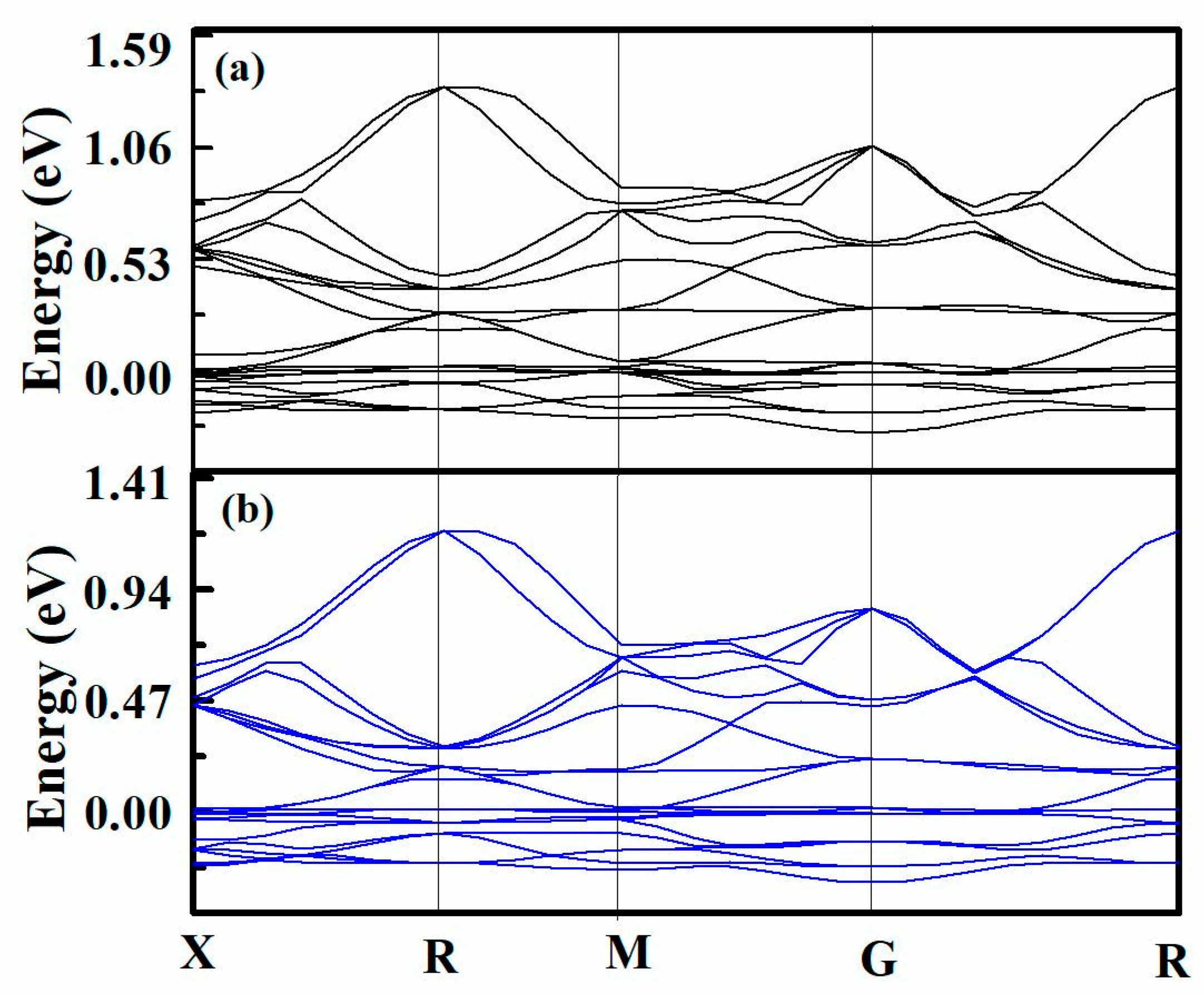
3.2. Cohesive Interfacial Energy Analysis between Graphene and Metal Using First-Principle Calculations
3.3. Formation Mechanisms of Metal@Graphene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, C.J.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.D.; Hieu, N.N.; Ilyasov, V.V.; Phuc, H.V.; Hoi, B.D.; Feddig, E.; Thuanh, N.V.; Chuong, V.N. First principles study on the electronic properties and Schottky barrier of Graphene/InSe heterostructure. Superlattices Microstruct. 2018, 122, 570–576. [Google Scholar] [CrossRef]
- Khalil, K.; Vafai, K. Analysis of the anomalies in graphene thermal properties. Int. J. Heat Mass Transf. 2017, 104, 328–336. [Google Scholar]
- Mohan, V.B.; Souri, H.; Jayaraman, K.; Bhattacharyya, D. Mechanical properties of thin films of graphene materials: A study on their structural quality and functionalities. Curr. Appl. Phys. 2018, 18, 879–885. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.L.; Chen, W.G. Development on preparation, characteristics and application of graphene. Carbon Tech. 2016, 35, 1–7. [Google Scholar]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Porg. Mater. Sci. 2018, 90, 75–127. [Google Scholar] [CrossRef]
- Dong, L.L.; Xiao, B.; Liu, Y.; Li, Y.L.; Fu, Y.Q.; Zhao, Y.Q.; Zhang, Y.S. Sintering effect on microstructural evolution and mechanical properties of spark plasma sintered Ti matrix composites reinforced by reduced graphene oxides. Ceram. Int. 2018, 44, 17835–17844. [Google Scholar] [CrossRef]
- Dong, L.L.; Chen, W.G.; Deng, N. Investigation on arc erosion behaviors and mechanism of W70Cu30 electrical contact materials adding graphene. J. Alloy. Compd. 2017, 696, 923–930. [Google Scholar] [CrossRef]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust Covalently Cross-linked Polybenzimidazole/Graphene Oxide Membranes for High-Flux Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 16140–16147. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, W.; Kawasaki, A. Highly Conductive Few-Layer Graphene/Al2O3 Nanocomposites with Tunable Charge Carrier Type. Adv. Funct. Mater. 2012, 22, 3882–3889. [Google Scholar] [CrossRef]
- Du, T.; Zhang, H.D.; Fan, T.X. Recent Progress on Graphene/Metal Composites. Mater. Rev. 2015, 29, 121–129. [Google Scholar]
- Chen, W.G.; Dong, L.L.; Wang, J.J.; Zuo, Y.; Ren, S.X.; Fu, Y.Q. Synergistic enhancing effect for mechanical and electrical properties of tungsten copper composites using spark plasma infiltrating sintering of copper-coated graphene. Sci. Rep. 2017, 7, 17836–17844. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Jiang, W.; Sun, D.P.; Wang, Y.P.; Li, F.S. One-pot synthesis of urchinlike Ni nanoparticles/RGO composites with extraordinary electromagnetic absorption properties. Appl. Surf. Sci. 2014, 314, 523–529. [Google Scholar] [CrossRef]
- Gotoh, K.; Kinumoto, T.; Fujii, E.; Yamamoto, A.; Hashimoto, H.; Ohkubo, T.; Itadani, A.; Kuroda, Y.; Ishida, H. Exfoliated graphene sheets decorated with metal/metal oxide nanoparticles: Simple preparation from cation exchanged graphite oxide. Carbon 2011, 49, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.K.; Sahu, R.K.; Rajinikanth, V.; Bapari, H.; Ghosh, M.; Paul, P. Preparation and characterization of graphene and Ni-decorated graphene using flower petals as the precursor material. Carbon 2012, 50, 4123–4129. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Carnevali, V.; Jugovac, M.; Patera, L.L.; Sala, A.; Panighel, M.; Cepek, C.; Soldano, G.; Mariscal, M.M.; Peressi, M.; et al. Graphene on nickel (100) micrograins: Modulating the interface interaction by extended moiré superstructures. Carbon 2018, 130, 441–447. [Google Scholar] [CrossRef]
- Eriksson, J.; Puglisi, D.; Kang, Y.H.; Yakimova, R.; Spetz, A.L. Adjusting the electronic properties and gas reactivity of epitaxial graphene by thin surface metallization. Phys. B Condens. Matter. 2014, 439, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.M.A.; Abdelsayed, A.; Khder, V.E.R.S.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Bao, T.J.; Xiang, L.; Su, Y.Q.; Duan, Y.X. Preparation of Ni-reduced graphene oxide nanocomposites by Pd-activated electroless deposition and their magnetic properties. Appl. Surf. Sci. 2012, 258, 8603–8608. [Google Scholar] [CrossRef]
- Kuang, D.; Xu, L.Y.; Liu, L.; Hu, W.B.; Wu, Y.T. Graphene–nickel composites. Appl. Surf. Sci. 2013, 273, 484–490. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Q.M.; Che, R.C.; Du, G.H.; Xu, B.S. One-step chemical vapor synthesis of Ni/graphene nanocomposites with excellent electromagnetic and electrocatalytic properties. Synth. Met. 2012, 162, 968–973. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-film particles of graphite oxide: High-yield synthesis and flexibility of the particles. Carbon 2004, 42, 2929–2937. [Google Scholar] [CrossRef]
- Fodi, T.; Didaskalou, C.; Kupai, J.; Balogh, G.T.; Huszthy, P.; Szekely, G. Nanofiltration-Enabled In Situ Solvent and Reagent Recycle for Sustainable Continuous-Flow Synthesis. ChemSusChem 2017, 10, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Buehler, M.J. Interface structure and mechanics between graphene and metal substrates: A first-principles study. J. Phys. Condens. Matter 2010, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.T.; Liu, G.P.; Wang, J.J.; Guo, X.L.; Wu, S.X.; Yu, J. First-Principles Calculations of the Adsorption of Au, Ag and Cu Atoms on Defected Graphene. Acta Phys. Chim. Sin. 2014, 30, 1230–1238. [Google Scholar]
- Sun, X.Y.; Wu, R.N.; Xia, R.; Chu, X.H.; Xu, Y.J. Effects of stone-wales and vacancy defects in atomic-scale friction on defective graphite. Appl. Phys. Lett. 2014, 104, 183109. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Huang, Y.; Ma, Y.F.; Wan, X.G.; Chen, Y.S. Controlled synthesis of few-layered graphene sheets on a large scale using chemical exfoliation. Carbon 2010, 48, 2367–2371. [Google Scholar] [CrossRef]
- Terrones, M.; Martín, O.; González, M.; Pozuelo, J.; Serrano, B.; Cabanelas, J.; Vega-Díaz, C.S.M.; Baselga, J. Interphases in Graphene Polymer-based Nanocomposite: Achievements and Challenges. Adv. Mater. 2011, 23, 5302–5310. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Fan, X.B.; Peng, W.C.; Li, Y.; Li, X.Y.; Wang, S.L.; Zhang, G.L.; Zhang, F.B. Deoxygenation of Exfoliated Graphite Oxide under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Dong, L.L.; Chen, W.G.; Zheng, C.H.; Deng, N. Microstructure and properties characterization of tungsten-copper composite materials doped with graphene. J. Alloy. Compd. 2017, 695, 1637–1646. [Google Scholar] [CrossRef]
- Dong, L.L.; Chen, W.G.; Deng, N.; Zheng, C.H. A novel fabrication of graphene by chemical reaction with a green reductant. Chem. Eng. J. 2016, 306, 754–762. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.; Jen, Y.R.K. Synthesis of graphene/vitamin C template-controlled polyaniline nanotubes composite for high performance supercapacitor electrode. Polymer 2014, 55, 798–805. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T. Probing layer number and stacking order of few-layer graphene by raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hontoria-Lucas, C.; López-Peinado, A.J.; López-González, J.d.D.; Rojas-Cervantes, M.L.; Martín-Aranda, R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Chen, W.F.; Yan, L.F.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Tian, T.; Tian, N.; Wang, T.; Zhang, B.; Zhang, D.D.; Liu, D.M. In situ chemical reduction method for graphene-copper. J. Chin. Electr. Microsc Soc. 2016, 35, 404–408. [Google Scholar]
- Gao, J.Q.; Wu, Y.T.; Liu, L.; Shen, B.; Hu, W.B. Crystallization temperature of amorphous electroless nickel–phosphorus alloys. Mater Lett. 2005, 59, 1665–1669. [Google Scholar]
- Hu, Q.H.; Wang, X.T.; Chen, H.; Wang, Z.F. Synthesis of Ni/graphene sheets by an electroless Ni-plating method. New Carbon Mater. 2012, 27, 35–41. [Google Scholar] [CrossRef]
- Fang, J.J.; Li, S.F.; Zha, W.K.; Cong, H.Y.; Chen, J.F.; Chen, Z.Z. Microwave Absorbing Properties of Nickel-coated Graphene. J. Inorg. Mater. 2011, 26, 467–471. [Google Scholar] [CrossRef]
- Jiang, R.G.; Zhou, X.F.; Liu, Z.P. Electroless Ni-plated graphene for tensile strength enhancement of copper. Mater. Sci. Eng. A 2017, 679, 323–328. [Google Scholar] [CrossRef]
- Ma, E.M.; Luo, S.F.; Li, P.X. A transmission electron microscopy study on the crystallization of amorphous Ni-P electroless deposited coatings. Thin Solid Films. 1998, 166, 273–280. [Google Scholar]
- Sribalaji, M.; Arunkumar, P.; Babu, K.S.; Keshri, A.K. Crystallization mechanism and corrosion property of electroless nickel phosphorus coating during intermediate temperature oxidation. Appl. Surf. Sci. 2015, 355, 112–120. [Google Scholar] [CrossRef]
- Ning, Z.R.; Chen, Z.; Du, X.J.; Ran, R.X.; Dong, W.P.; Chen, C. Nickel Dimers Adsorbed on Graphene: First-Principle Study. J. Supercond. Nov. Magn. 2013, 26, 3515–3522. [Google Scholar] [CrossRef]
- Laref, A.; Ahmed, A.; Bin-Omran, S.; Luo, S.J. First-principle analysis of the electronic and optical properties of boron and nitrogen doped carbon mono-layer graphene. Carbon 2015, 81, 179–192. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Yang, X.M.; Wang, R.R.; Li, M.X. Enhancement of the mechanical properties of graphene–copper composites with graphene–nickel hybrids. Mater. Sci. Eng. A 2014, 599, 247–254. [Google Scholar] [CrossRef]




| Plating Solution | Chemical | Function | Concentration |
|---|---|---|---|
| Copper | CuSO4·5H2O | Source Cu | 50 g·L−1 |
| PVP | surfactant agent | 0.4 mg·mL−1 | |
| Ascorbic | Reducing agent | 2 g·L−1 | |
| NaOH | Adjust the pH value | 50 g·L−1 | |
| Nickel | NiSO4·6H2O | Source Ni | 1.3 g·L−1 |
| PVP | surfactant agent | 0.4 mg·mL−1 | |
| NaBH4 | Reducing agent | 8 g·L−1 | |
| NH3·H2O | Adjust the pH value | 25–28 wt% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Chen, W.; Wang, J.; Dong, L.; Zhao, Q.; Fu, Y. Formation Mechanism and Cohesive Energy Analysis of Metal-Coated Graphene Nanocomposites Using In-Situ Co-Reduction Method. Materials 2018, 11, 2071. https://doi.org/10.3390/ma11112071
Xue Y, Chen W, Wang J, Dong L, Zhao Q, Fu Y. Formation Mechanism and Cohesive Energy Analysis of Metal-Coated Graphene Nanocomposites Using In-Situ Co-Reduction Method. Materials. 2018; 11(11):2071. https://doi.org/10.3390/ma11112071
Chicago/Turabian StyleXue, Yuanlin, Wenge Chen, Jiaojiao Wang, Longlong Dong, Qian Zhao, and Yongqing Fu. 2018. "Formation Mechanism and Cohesive Energy Analysis of Metal-Coated Graphene Nanocomposites Using In-Situ Co-Reduction Method" Materials 11, no. 11: 2071. https://doi.org/10.3390/ma11112071





