Electrochemical Behavior of Al(III) and Formation of Different Phases Al-Ni Alloys Deposits from LiCl-KCl-AlCl3 Molten Salt
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
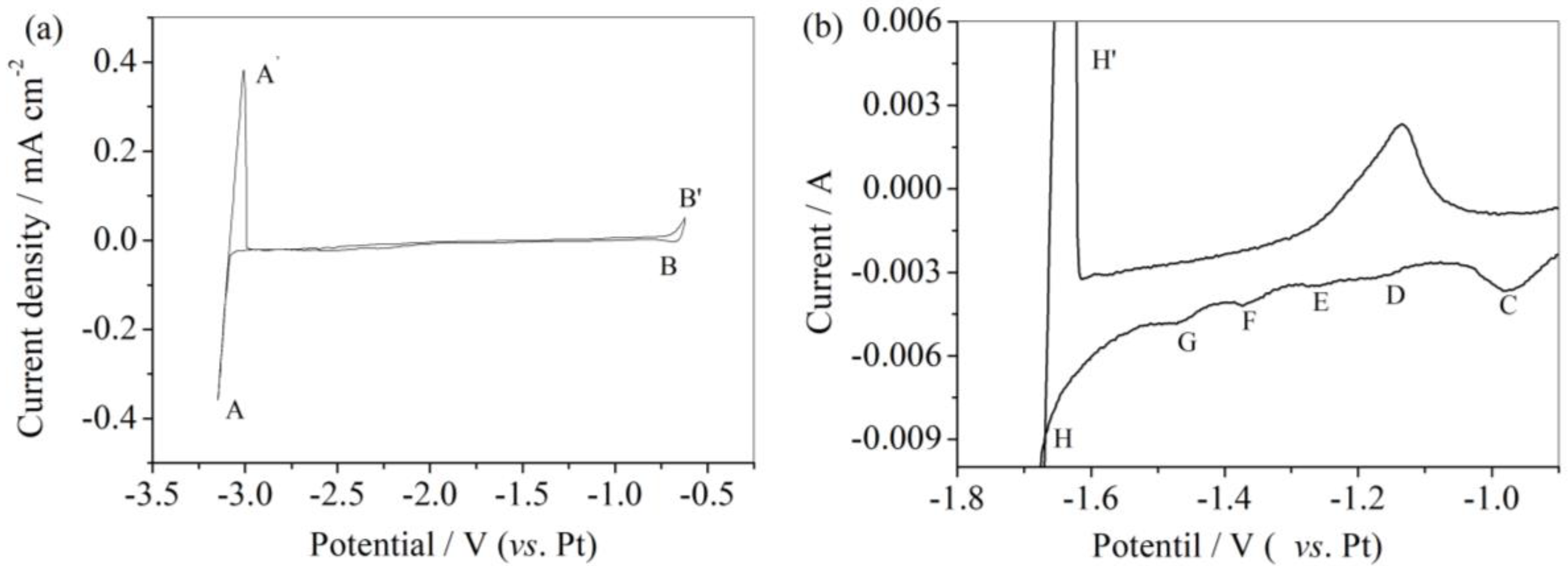
3.1. Cyclic Voltammetry
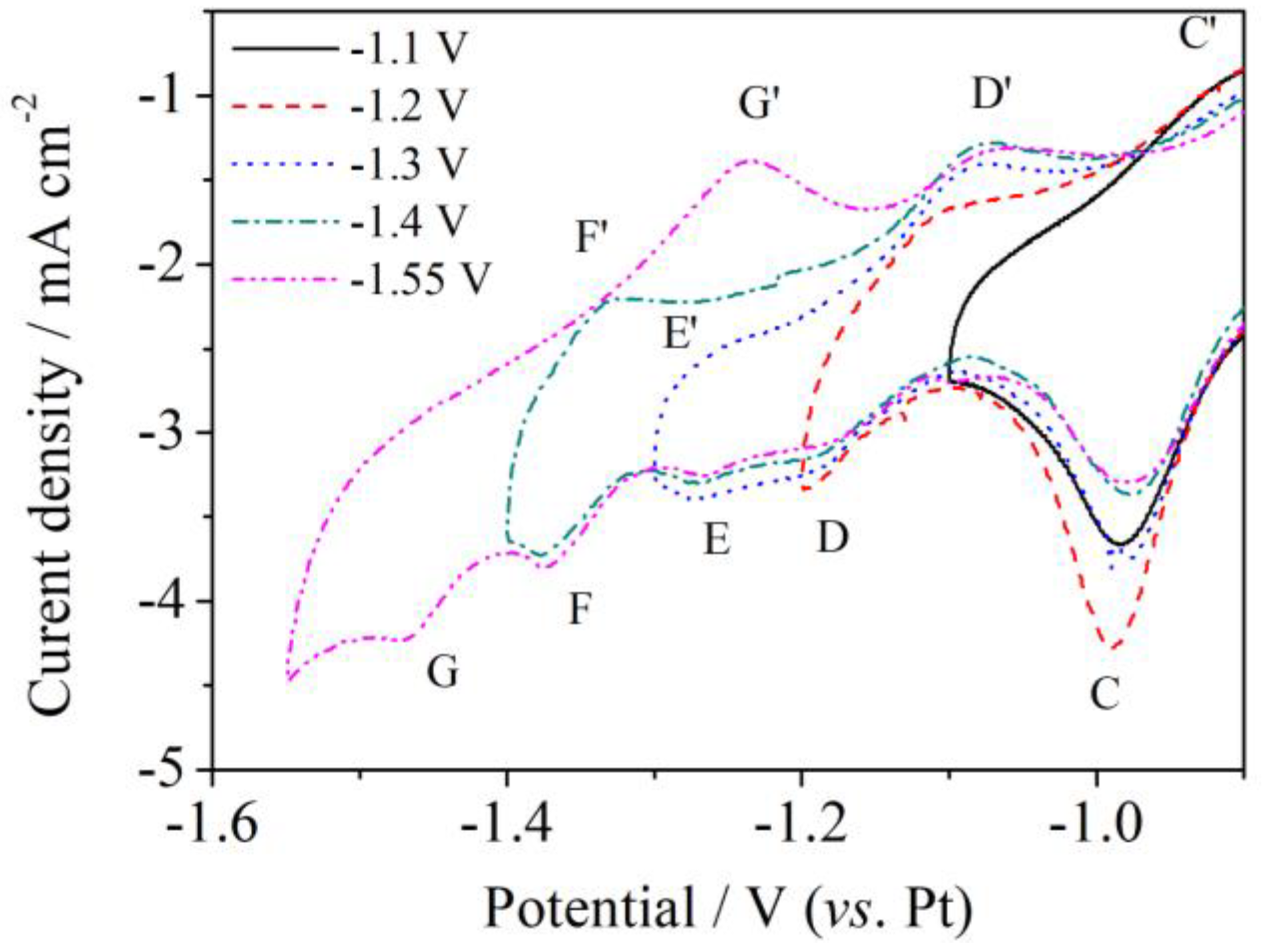
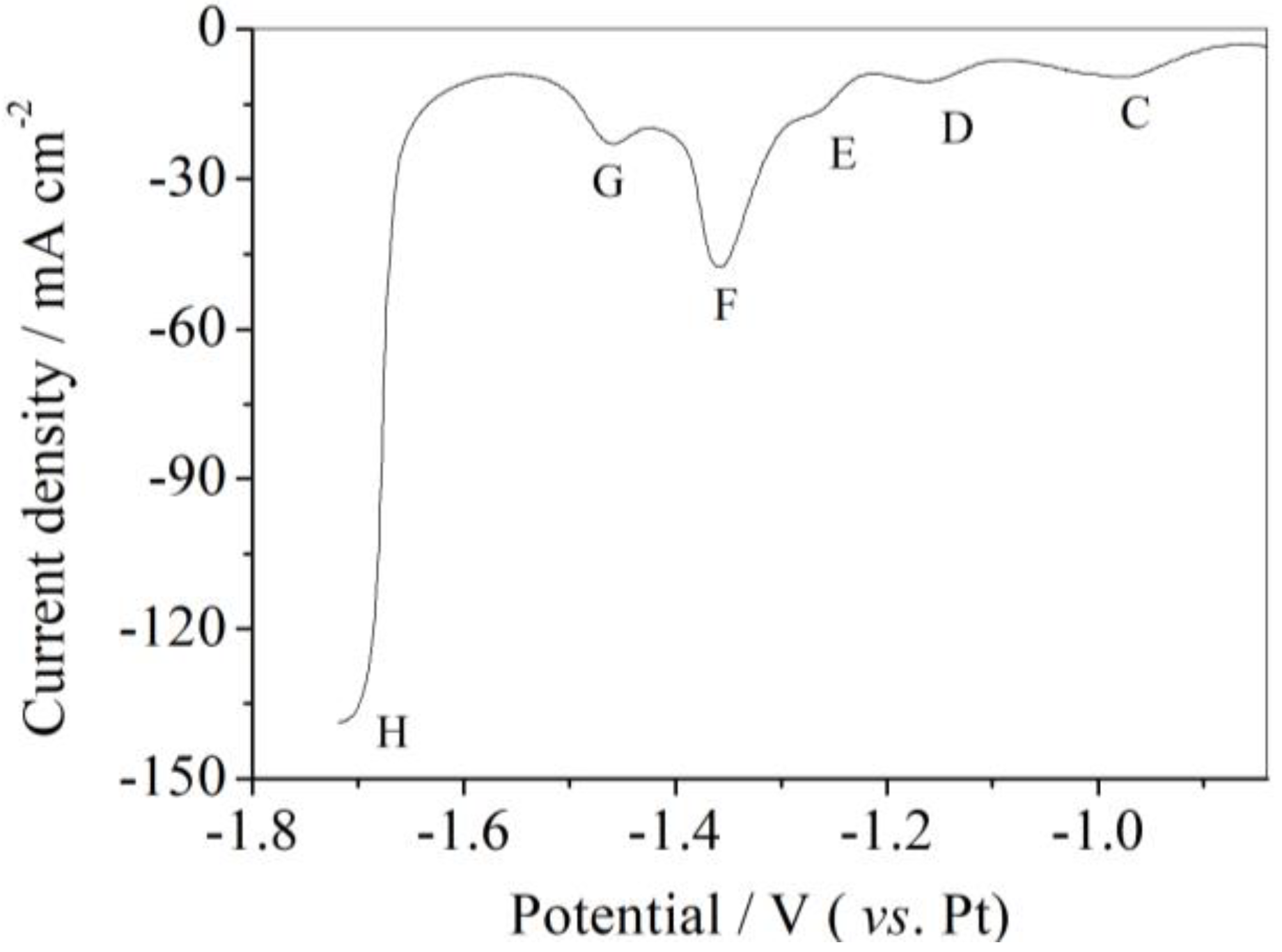
3.2. Square Wave Voltammetry
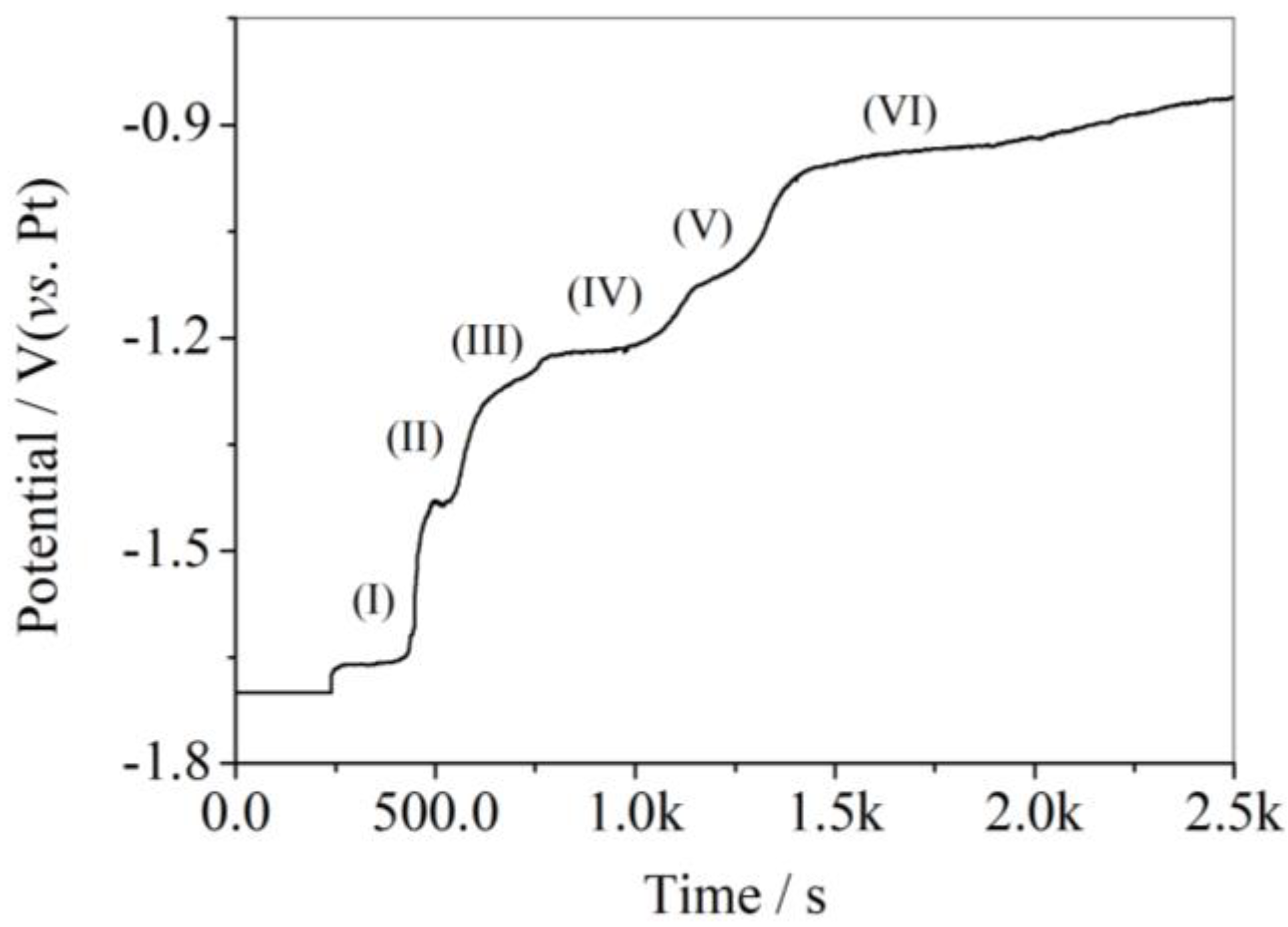
3.3. Open Circuit Chronopotentiometry
3.4. Potentiostatic Electrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matsuura, K.; Kitamutra, T.; Kudoh, M. Microstructure and mechanical properties of NiAl intermetallic compound synthesized by reactive sintering under pressure. J. Mater. Process. Tech. 1997, 63, 298–302. [Google Scholar] [CrossRef]
- Ozdemir, O.; Zeytin, S.; Bindal, C. A study on NiAl produced by pressure-assisted combustion synthesis. Vacuum 2010, 84, 430–437. [Google Scholar] [CrossRef]
- Inoue, A. Amorphous, nanoquasicrystalline and nanocrystalline alloys in Al-based systems. Prog. Mater. Sci. 1998, 43, 365–520. [Google Scholar] [CrossRef]
- Dwivedi, S.P. Effect of process parameters on tensile strength of friction stir welding A356/C355 aluminium alloys joint. J. Mech. Sci. Tech. 2014, 28, 285–291. [Google Scholar] [CrossRef]
- Sikka, V.K.; Deevi, S.C.; Viswanathan, S.; Swindeman, R.W.; Santella, M.L. Advances in processing of Ni3Al-based intermetallics and applications. Intermetallics 2000, 8, 1329–1337. [Google Scholar] [CrossRef]
- Chan, K.S. Theoretical analysis of grain size effects on tensile ductility. Scr. Metall. Mater. 1990, 24, 1725–1730. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Akiyama, E.J.; Watanabe, Y.; Katada, Y.; Tsuzaki, K. Effect of Al/Al3Ni microstructure on the corrosion behaviour of Al–5.4wt% Ni alloy fabricated by equal-channel angular pressing. Corr. Sci. 2007, 49, 2962–2972. [Google Scholar] [CrossRef]
- Osório, W.R.; Peixoto, L.C.; Canté, M.V.; Garcia, A. Electrochemical corrosion characterization of Al–Ni alloys in a dilute sodium chloride solution. Electrochim. Acta 2010, 55, 4078–4085. [Google Scholar] [CrossRef]
- Izumi, T.; Nishimoto, T.; Narita, T. Superior long-term oxidation resistance of Ni–Al coated TiAl alloys. Intermetallics 2005, 13, 727–732. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Prabhakar, K.V. Wrought aluminum-nickel alloys for high strength-high conductivity applications. Metall. Trans. A 1975, 6, 1003–1008. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.C.M.; Liu, C.T. Combustion reaction in multilayered nickel and aluminum foils. Mater. Sci. Eng. A 1997, 239–240, 532–539. [Google Scholar] [CrossRef]
- Minay, E.J.; Rawlings, R.D.; McShane, H.B. Hot extrusion reaction synthesis of nickel, titanium and iron aluminides. J. Mater. Process. Tech. 2004, 153–154, 630–636. [Google Scholar] [CrossRef]
- Poli, G.; Sola, R.; Veronesi, P. Microwave-assisted combustion synthesis of NiAl intermetallics in a single mode applicator: Modeling and optimization. Mater. Sci. Eng. A 2006, 441, 149–156. [Google Scholar] [CrossRef]
- Ali, M.R.; Nishikata, A.; Tsuru, T. Electrodeposition of Al–Ni intermetallic compounds from aluminum chloride-N-(n-butyl)pyridinium chloride room temperature molten salt. J. Electroanal. Chem. 2001, 513, 111–118. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.G.; Gobal, F.; Danaee, I. Effect of potential on the early stage of nucleation and growth during aluminum electrocrystallization from molten salt (AlCl3–NaCl–KCl). J. Electroanal. Chem. 2006, 588, 190–196. [Google Scholar] [CrossRef]
- Ueda, M.; Kigawa, H.; Ohtsuka, T. Co-deposition of Al-Cr-Ni alloys using constant potential and potential pulse techniques in AlCl3-NaCl-KCl molten salt. Electrochim. Acta 2007, 52, 2515–2519. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Wei, C.C.; Kong, W.P.; Xu, B.H.; Jia, X.Y.; Diao, C.L.; Li, S.J. The electrochemical formation of Al-Cu alloys in a LiCl-KCl-AlCl3 molten salt. Electrochim. Acta 2016, 196, 162–198. [Google Scholar] [CrossRef]
- Nohira, T.; Yasuda, K.; Ito, Y. Pinpoint and bulk electrochemical reduction of insulating silicon dioxide to silicon. Nature Mater. 2003, 2, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, S.J.; Li, W.; Wang, Y.M.; Diao, C.L.; Jia, X.Y.; Pei, Q.Q.; Wang, S.H.; Zhang, D.W.; Zhang, W.F. Preparation of a SiCf/Al composite precursor by electrochemical deposition in a LiCl-KCl-AlCl3 molten salt. J. Alloys Compd. 2017, 712, 666–671. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y.D.; Zhang, M.L.; Tang, H.; Ji, D.B.; Han, W.; Xue, Y.; Zhang, Z.J. Electrochemical formation of Al-Tm intermetallics in eutectic LiCl-KCl melt containing Tm and Al ions. J. Nucl. Mater. 2014, 452, 197–204. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, M.L.; Han, W.; Hou, Z.Y.; De, Y.Y. Electrodeposition of Li and electrochemical formation of Mg-Li alloys from the eutectic LiCl-KCl. J. Alloys Compd. 2008, 464, 174–178. [Google Scholar] [CrossRef]
- Nohira, T.; Kambara, H.; Amezawa, K.; Ito, Y. Electrochemical formation and phase control of Pr-Ni alloys in a molten LiCl-KCl-PrCl3 system. J. Electrochem. Soc. 2005, 152, C183–C189. [Google Scholar] [CrossRef]
- Paek, S.; Kim, S.H.; Yoon, D.; Kim, T.J.; Ahn, D.H.; Lee, H. Electrochemical study on various types of anode materials in LiCl–KCl eutectic salt used in the electro-winning process. J. Radioanal. Nucl. Chem. 2013, 295, 439–444. [Google Scholar] [CrossRef]
- Dauphin-Ducharme, P.; Arroyo-Currás, N.; Kurnik, M.; Ortega, G.; Li, H.; Plaxco, K.W. Simulation-based approach to determining electron transfer rates using square-wave voltammetry. Langmuir 2017, 33, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Han, W.; Zhang, M.L.; Li, M.; Yang, X.G.; Sun, Y. Electrochemical behavior of magnesium (II) on Ni electrode in LiCl-KCl eutectic. Chem. Res. Chin. Univ. 2018, 34, 107–112. [Google Scholar] [CrossRef]
- Yoon, D.; Phongikaroon, S.; Zhang, J. Electrochemical and thermodynamic properties of CeCl3 on liquid cadmium cathode (LCC) in LiCl-KCl eutectic salt. J. Electrochem. Soc. 2016, 163, E97–E103. [Google Scholar] [CrossRef]
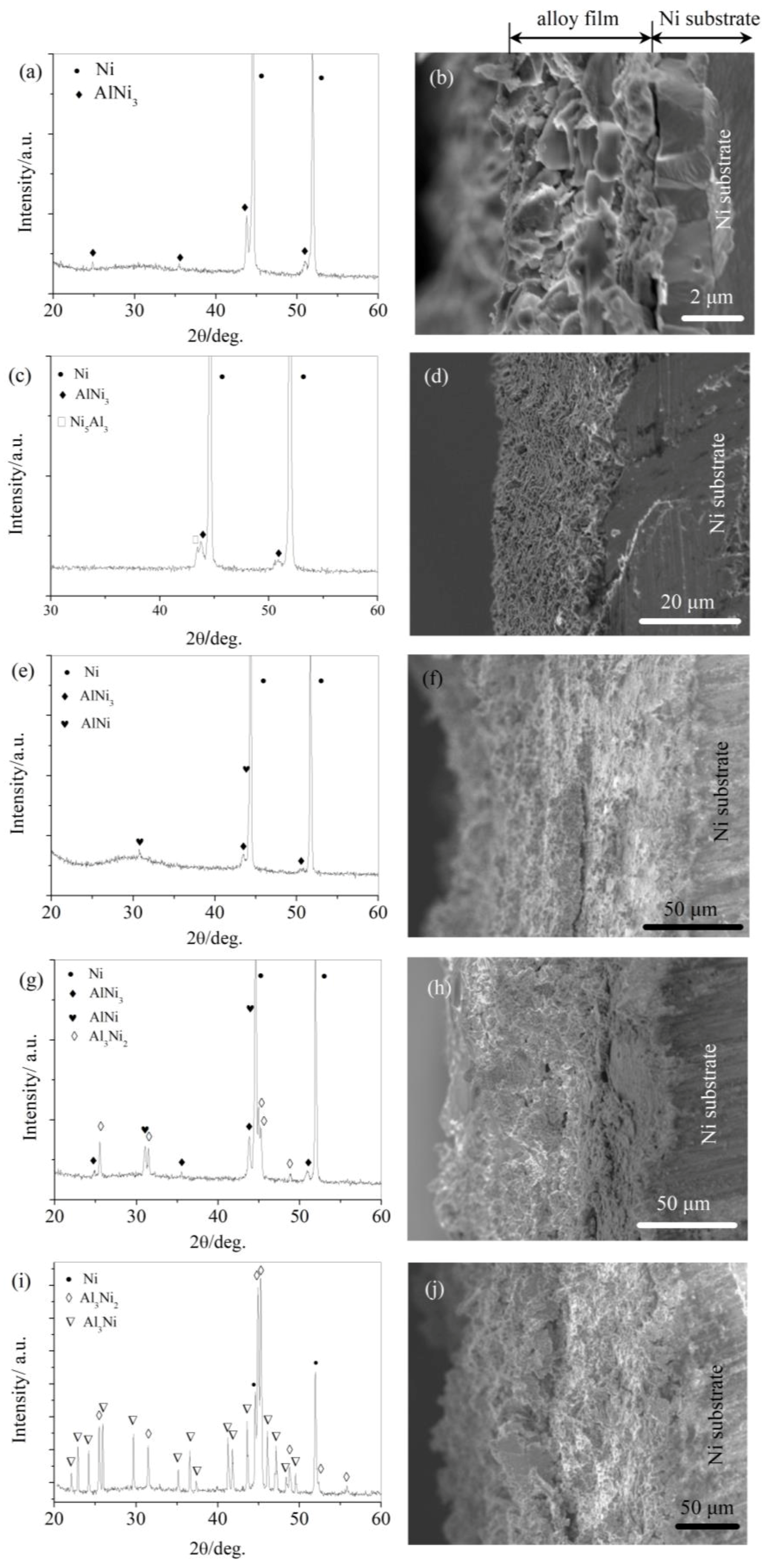

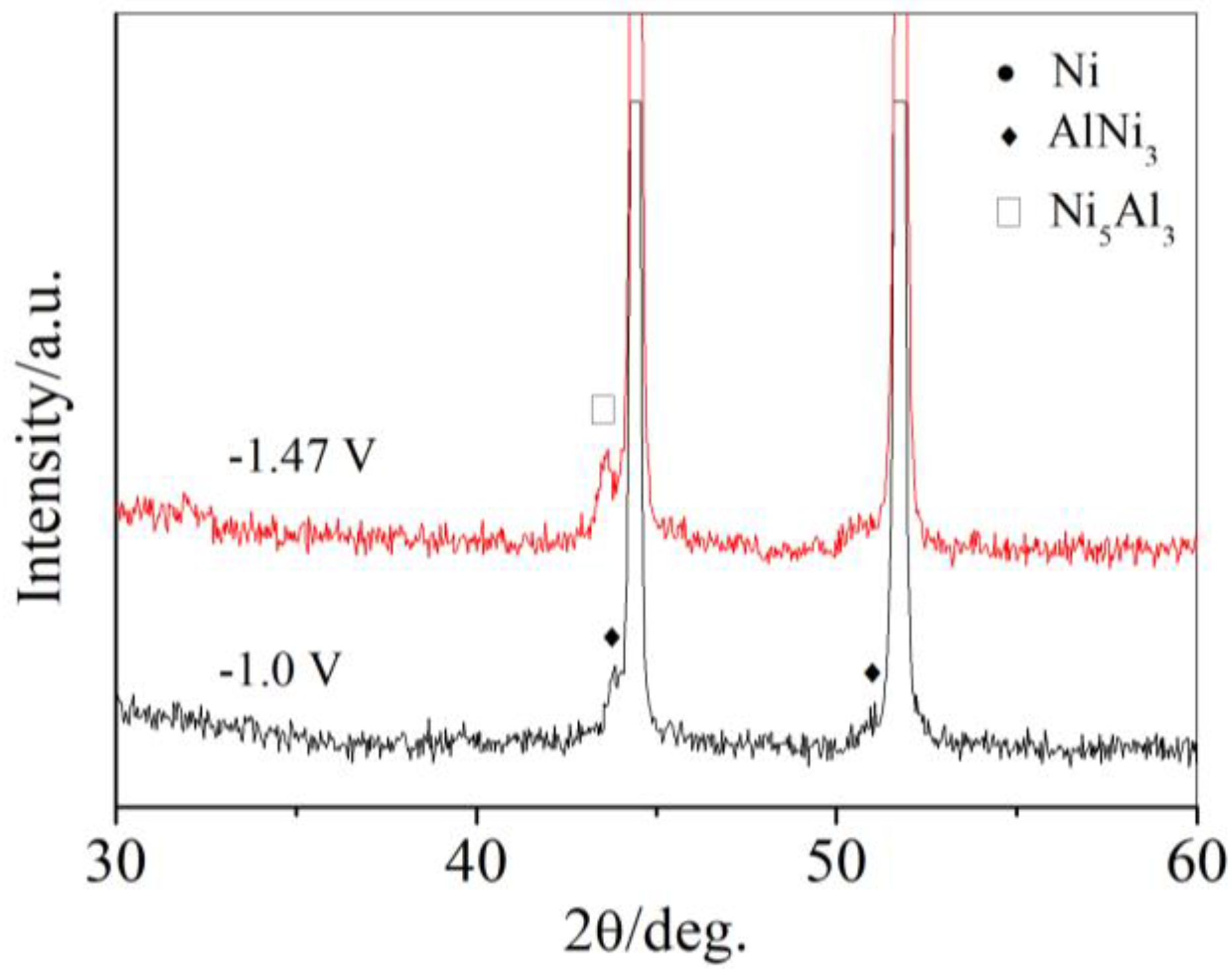
| Potential/V | Products | Al Content/at.% | Ni Content/at.% |
|---|---|---|---|
| −1.0 V | AlNi3 | 24.99 | 75.01 |
| −1.17 V | AlNi3 + Ni5Al3 | 37.64 | 62.36 |
| −1.3 V | AlNi3 + AlNi | 51.55 | 48.55 |
| −1.37 V | AlNi3 + AlNi + Al3Ni2 | 57.70 | 42.30 |
| −1.47 V | Al3Ni2 + Al3Ni | 74.22 | 25.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Chen, Z.; Bai, Y.; Pei, Q.; Li, W.; Diao, C.; Li, X.; Li, S.; Dong, S. Electrochemical Behavior of Al(III) and Formation of Different Phases Al-Ni Alloys Deposits from LiCl-KCl-AlCl3 Molten Salt. Materials 2018, 11, 2113. https://doi.org/10.3390/ma11112113
Peng Y, Chen Z, Bai Y, Pei Q, Li W, Diao C, Li X, Li S, Dong S. Electrochemical Behavior of Al(III) and Formation of Different Phases Al-Ni Alloys Deposits from LiCl-KCl-AlCl3 Molten Salt. Materials. 2018; 11(11):2113. https://doi.org/10.3390/ma11112113
Chicago/Turabian StylePeng, Yaru, Zeng Chen, Ying Bai, Qingqing Pei, Wei Li, Chunli Diao, Xijin Li, Shengjun Li, and Shaokang Dong. 2018. "Electrochemical Behavior of Al(III) and Formation of Different Phases Al-Ni Alloys Deposits from LiCl-KCl-AlCl3 Molten Salt" Materials 11, no. 11: 2113. https://doi.org/10.3390/ma11112113




