Synthesis of a Novel and Salt Sensitive Superabsorbent Hydrogel Using Soybean Dregs by UV-Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. SD-PAA and Poly(acrylic acid) (PAA) Preparation
2.3. Characterization
2.4. Water Absorption Capacity
3. Results and Discussion
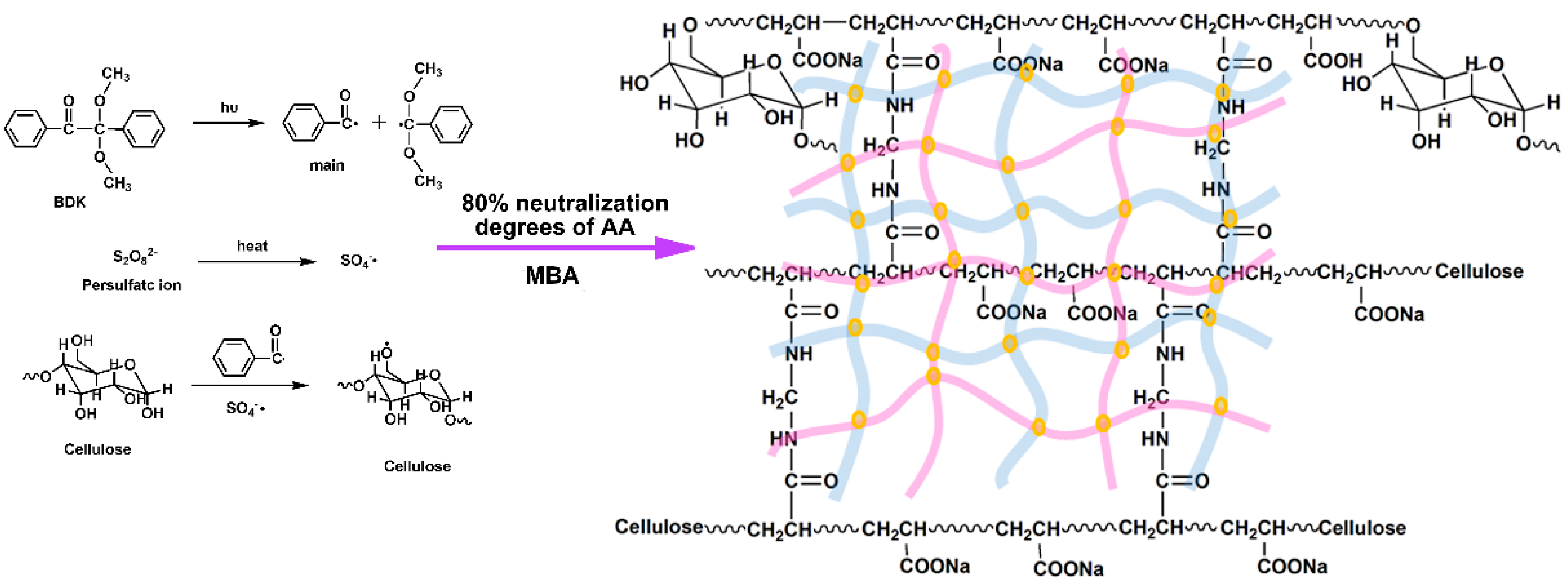
3.1. Mechanism of SD-PAA
3.2. Characterization
3.2.1. Elemental Analysis
3.2.2. SEM Analysis
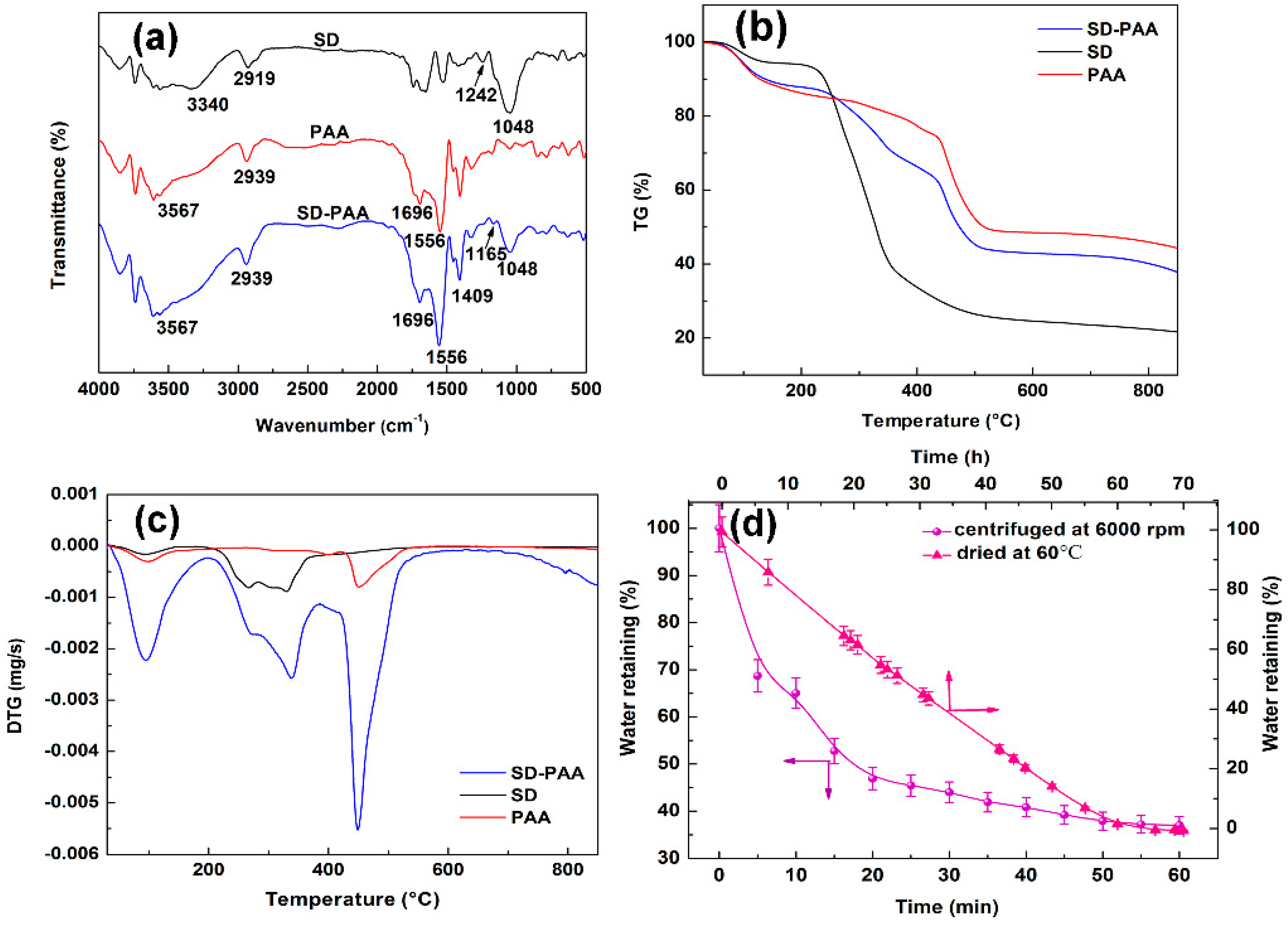
3.2.3. FITR Analysis
3.2.4. TGA Analysis
3.3. Water Retention
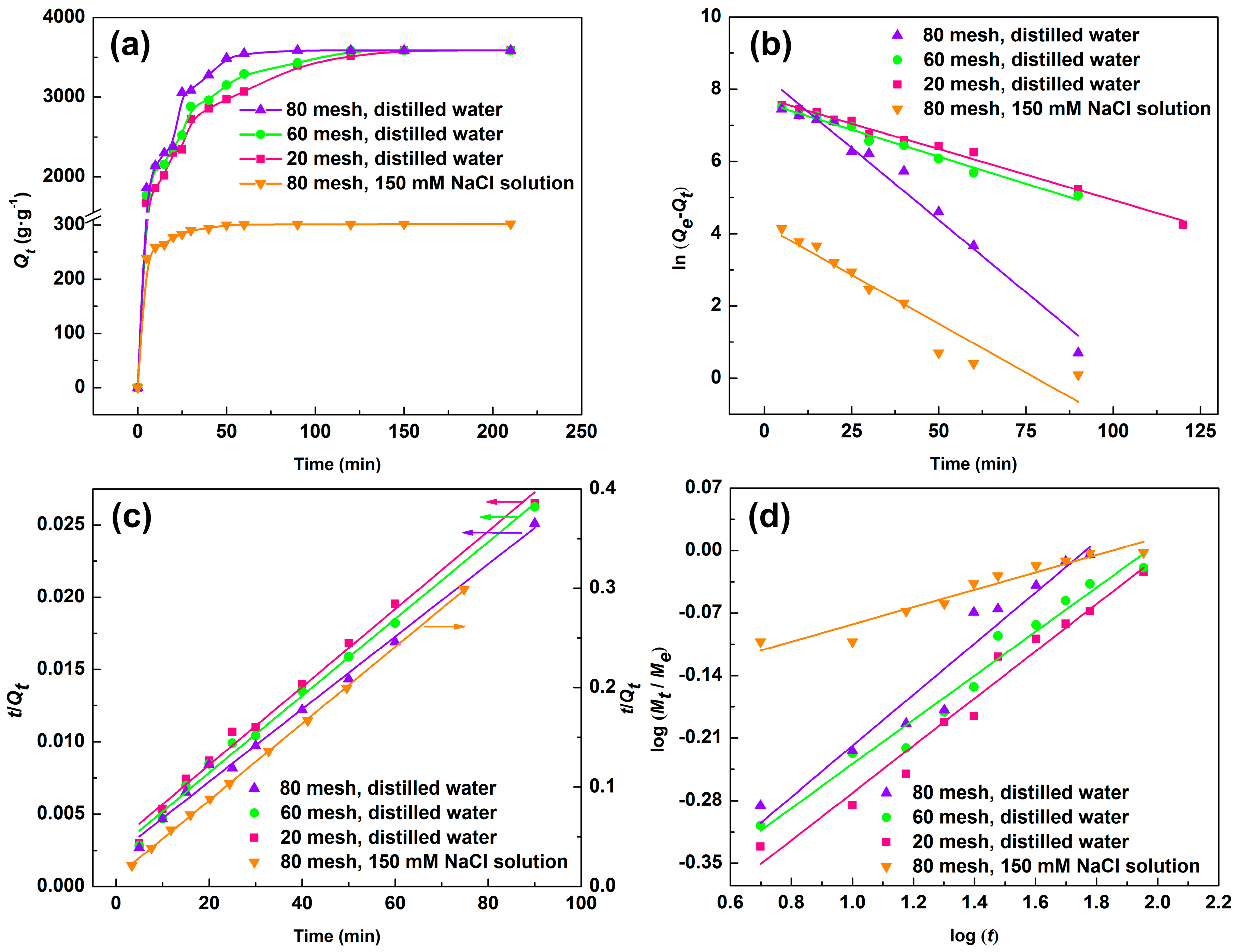
3.4. Water Absorption
3.4.1. Effect of Cations
- The water absorption decreased rapidly in low salt concentration (0–50 mM), and decreased gentlely at the concentration above 50 mM. As an ionic hydrogels, the extra cations in media solution caused increased electrostatic repulsion, and changed the osmotic pressure between the media solution and the polymer, which led to low water absorption [34].
- The water absorption order in different chloride salts solution was KCl > NaCl > NH4Cl > AlCl3 > FeCl3 > MgCl2 > CaCl2. The influence of seven cationic on water absorption followed the sequence: K+ > Na+ > NH4+ > Al3+ > Fe3+ > Mg2+ > Ca2+. The monovalent cationic salts had less effect on water absorption than polyvalent salts. This was due to the fact that divalent and trivalent metal ion can form a complexe with −COO– groups containing in polymer SD-PAA and the polyvalent salt solutions had higher ionic strength; the ionic strength order of 150 mM saline solution was Al3+, Fe3+ > Mg2+, Ca2+ > K+, Na+ (Table 2). According to Equation (4), Qe decreased with the increase of ionic strength. Interestingly, Qe in trivalent cationic solution were higher than in divalent cationic solution, while, as shown in Table 2, the ionic strength of trivalent cationic solution was stronger. It may be because that trivalent cationic affected the charge density of polymer more than divalent, which led to stronger water absorption.
- For monovalent metal ion K+ (ionic radius was138 pm) and Na+ (ionic radius was102 pm), the larger the radius, the higher water absorption was (K+ > Na+), while for divalent and trivalent metal ion Ca2+, Mg2+, Fe3+, and Al3+ (their ionic radius were 99.0, 72.0, 64.5, and 53.5 pm, respectively), the larger the radius, the less water absorption was (Ca2+ < Mg2+ < Fe3+ < Al3+). This may be due to the coordination interaction of these metal ions with the –COO− groups on polymer chains. So, these metal ions acted as crosslinking agents of the polymer, which prompted the polymer, absorbed more water. Moreover, for monovalent cation, the water absorption declined severer in polyatomic solution (NH4+) than in single-atomic solution. Above all, salt sensitivity of SD-PAA in different cations solution was Ca2+ > Mg2+ > Fe3+ > Al3+ > NH4+ > Na+ > K+ (Figure 4c).
3.4.2. Effect of Anions
3.4.3. Effect of Particle Size and Salt Solution
3.5. Kinetic Analysis
3.5.1. Swelling Kinetic
3.5.2. Diffusion Kinetic
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ni, B.; Liu, M.; Lu, S.; Xie, L.; Zhang, X.; Wang, Y. Novel Slow-Release Multielement Compound Fertilizer with Hydroscopicity and Moisture Preservation. Ind. Eng. Chem. Res. 2010, 49, 4546–4552. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, T.; Wang, A. Preparation, Swelling, and Slow-Release Characteristics of Superabsorbent Composite Containing Sodium Humate. Ind. Eng. Chem. Res. 2008, 47, 1766–1773. [Google Scholar] [CrossRef]
- Wang, X.; Lu, S.; Gao, C.; Xu, X.; Wei, Y.; Bai, X.; Feng, C.; Gao, N.; Liu, M.; Wu, L. Biomass-based multifunctional fertilizer system featuring controlled-release nutrient, water-retention and amelioration of soil. RSC Adv. 2014, 4, 18382–18390. [Google Scholar] [CrossRef]
- Tang, H.; Chen, H.; Duan, B.; Lu, A.; Zhang, L. Swelling behaviors of superabsorbent chitin/carboxymethylcellulose hydrogels. J. Mater. Sci. 2014, 49, 2235–2242. [Google Scholar] [CrossRef]
- Loo, S.-L.; Krantz, W.B.; Fane, A.G.; Gao, Y.; Lim, T.-T.; Hu, X. Bactericidal Mechanisms Revealed for Rapid Water Disinfection by Superabsorbent Cryogels Decorated with Silver Nanoparticles. Environ. Sci. Technol. 2015, 49, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, N.C. Synthesis of a novel pH responsive phyllosilicate loaded polymeric hydrogel based on poly(acrylic acid-co-N-vinylpyrrolidone) and polyethylene glycol for drug delivery: Modelling and kinetics study for the sustained release of an antibiotic drug. RSC Adv. 2015, 5, 18312–18327. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Secrieru, E.; Schroefl, C. Effect of superabsorbent polymers (SAPs) on rheological properties of fresh cement-based mortars—Development of yield stress and plastic viscosity over time. Cem. Concr. Res. 2015, 67, 52–65. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jensen, O.M. Chloride migration in concrete with superabsorbent polymers. Cem. Concr. Comp. 2015, 55, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Herrero, R.; Lodeiro, P.; García-Casal, L.J.; Vilariño, T.; Rey-Castro, C.; David, C.; Rodríguez, P. Full description of copper uptake by algal biomass combining an equilibrium NICA model with a kinetic intraparticle diffusion driving force approach. Bioresour. Technol. 2011, 102, 2990–2997. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.-L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-J.; Wang, J.-S.; Liu, Y.-G.; Li, X.; Zeng, G.-M.; Bao, Z.-L.; Zeng, X.-X.; Chen, A.-W.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.; Venditti, R.A.; Pawlak, J.J.; Salam, A.; Hubbe, M.A. Novel Hemicellulose-Chitosan Biosorbent for Water Desalination and Heavy Metal Removal. ACS Sustain. Chem. Eng. 2013, 1, 1102–1109. [Google Scholar] [CrossRef]
- Research, T.M. Superabsorbent Polymers Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2014–2020. Available online: https://www.decisiondatabases.com/ip/47-superabsorbent-polymers-market-report (accessed on 31 October 2018).
- Liu, J.; Su, Y.; Li, Q.; Yue, Q.; Gao, B. Preparation of wheat straw based superabsorbent resins and their applications as adsorbents for ammonium and phosphate removal. Bioresour. Technol. 2013, 143, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, Z.; Zhao, T.; Liu, M.; Hu, M.; Li, J. Synthesis, Characterization, and Swelling Behaviors of Salt-Sensitive Maize Bran–Poly(acrylic acid) Superabsorbent Hydrogel. J. Agric. Food Chem. 2014, 62, 8867–8874. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.A.A.; Ismail, H.; Ariffin, A. Graft copolymerization of polyDADMAC to cassava starch: Evaluation of process variables via central composite design. Ind. Crop. Prod. 2015, 65, 535–545. [Google Scholar] [CrossRef]
- Wang, D.J.; Chen, H.; Xu, H.; Sun, J.M.; Xu, Y.Y. Preparation of Wheat Straw Matrix-g-Polyacrylonitrile-Based Adsorbent by SET-LRP and Its Applications for Heavy Metal Ion Removal. ACS Sustain. Chem. Eng. 2014, 2, 1843–1848. [Google Scholar] [CrossRef]
- Office of the Chief Economist, Agriculture Marketing Service Farm Service Agence. World Agricultural Supply and Demand Estimates; United States Department of Agriculture: Washington, DC, USA, 2015; p. WASDE-541-3.
- Roy, S.G.; Haldar, U.; De, P. Remarkable Swelling Capability of Amino Acid Based Cross-Linked Polymer Networks in Organic and Aqueous Medium. ACS Appl. Mater. Interfaces 2014, 6, 4233–4241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.; Peng, J.; Li, J.; Zhai, M. New Lipophilic Polyelectrolyte Gels Containing Quaternary Ammonium Salt with Superabsorbent Capacity for Organic Solvents. ACS Appl. Mater. Interfaces 2014, 6, 14894–14902. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.B.; Madras, G. Reversible Swelling/Deswelling Characteristics of Ethylene Glycol Dimethacrylate Cross-Linked Poly(acrylic acid-co-sodium acrylate-co-acrylamide) Superabsorbents. Ind. Eng. Chem. Res. 2011, 50, 10918–10927. [Google Scholar] [CrossRef]
- Noppakundilograt, S.; Pheatcharat, N.; Kiatkamjornwong, S. Multilayer-Coated NPK Compound Fertilizer Hydrogel with Controlled Nutrient Release and Water Absorption. J. Appl. Polym. Sci. 2015, 132, 11. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Zhu, C.; Guo, J.; Zhao, C.; Liao, Y.; Guan, Q. UV-initiated polymerization of hydrophobically associating cationic flocculants: Synthesis, characterization, and dewatering properties. Chem. Eng. J. 2013, 234, 318–326. [Google Scholar] [CrossRef]
- Sharma, K.; Kaith, B.S.; Kumar, V.; Kalia, S.; Kumar, V.; Swart, H.C. Synthesis and biodegradation studies of gamma irradiated electrically conductive hydrogels. Polym. Degrad. Stabil. 2014, 107, 166–177. [Google Scholar] [CrossRef]
- Zhang, W.; Sha, Z.; Huang, Y.; Bai, Y.; Xi, N.; Zhang, Y. Glow discharge electrolysis plasma induced synthesis of cellulose-based ionic hydrogels and their multiple response behaviors. RSC Adv. 2015, 5, 6505–6511. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Zhang, J.; Wang, C.; Li, Q. Preparation and characterization of chitosan–poly(vinyl alcohol)/bentonite nanocomposites for adsorption of Hg(II) ions. Chem. Eng. J. 2014, 251, 404–412. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R.; Tharun, A.R. Investigation of the Extraction of Hemoglobin by Adsorption onto Nanocellulose-Based Superabsorbent Composite Having Carboxylate Functional Groups from Aqueous Solutions: Kinetic, Equilibrium, and Thermodynamic Profiles. Ind. Eng. Chem. Res. 2013, 52, 11016–11028. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, L.; Zhang, S.; Wu, R. Preparation and swelling behaviors of a high temperature resistant superabsorbent using tetraallylammonium chloride as crosslinking agent. React. Funct. Polym. 2015, 87, 15–21. [Google Scholar] [CrossRef]
- Gu, S.; Zhou, J.; Luo, Z.; Wang, Q.; Ni, M. A detailed study of the effects of pyrolysis temperature and feedstock particle size on the preparation of nanosilica from rice husk. Ind. Crop. Prod. 2013, 50, 540–549. [Google Scholar] [CrossRef]
- Zaharia, A.; Sarbu, A.; Radu, A.-L.; Jankova, K.; Daugaard, A.; Hvilsted, S.; Perrin, F.-X.; Teodorescu, M.; Munteanu, C.; Fruth-Oprisan, V. Preparation and characterization of polyacrylamide-modified kaolinite containing poly acrylic acid-co-methylene bisacrylamide nanocomposite hydrogels. Appl. Clay Sci. 2015, 103, 46–54. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Z.; Liu, M.; Zhang, Y.; Hu, M.; Li, J. Synthesis and properties of a superabsorbent from an ultraviolet-irradiated waste nameko mushroom substrate and poly(acrylic acid). J. Appl. Polym. Sci. 2014, 131, 40471. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Mahdavinia, G. MBA-crosslinked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Carbohydr. Polym. 2006, 66, 386–395. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; p. 589. [Google Scholar]
- Zhao, Y.; Su, H.; Fang, L.; Tan, T. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Alcaraz-Espinoza, J.J.; Chavez-Guajardo, A.E.; Medina-Llamas, J.C.; Andrade, C.A.; de Melo, C.P. Hierarchical Composite Polyaniline-(Electrospun Polystyrene) Fibers Applied to Heavy Metal Remediation. ACS Appl. Mater. Interfaces 2015, 7, 7231–7240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, Y.; Mu, B.; Wang, A. Attapulgite/bentonite interactions for methylene blue adsorption characteristics from aqueous solution. Chem. Eng. J. 2014, 237, 403–410. [Google Scholar] [CrossRef]
- Cai, X.Q.; Li, J.H.; Zhang, Z.; Yang, F.F.; Dong, R.C.; Chen, L.X. Novel Pb2+ Ion Imprinted Polymers Based on Ionic Interaction via Synergy of Dual Functional Monomers for Selective Solid-Phase Extraction of Pb2+ in Water Samples. ACS Appl. Mater. Interfaces 2014, 6, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Study on partition and release of molecules in superabsorbent thermosensitive nanocomposites. Polymer 2012, 53, 445–453. [Google Scholar] [CrossRef]


| Sample | C (%) | N (%) | H (%) |
|---|---|---|---|
| SD | 38.48 | 0.76 | 5.17 |
| PAA | 33.84 | 0.13 | 4.97 |
| SD-PAA | 37.11 | 0.53 | 5.00 |
| Solution (150 mM) | Ionic Strength a (mol-ion dm−3) | Qe (g·g−1) |
|---|---|---|
| KCl | 0.15 | 339.0 |
| NaCl | 0.15 | 302.0 |
| NH4Cl | 0.15 | 45.9 |
| CaCl2 | 0.45 | 14.7 |
| MgCl2 | 0.45 | 22.6 |
| FeCl3 | 0.90 | 39.2 |
| AlCl3 | 0.90 | 41.3 |
| Na2SO4 | 0.45 | 53.4 |
| Na3PO4 | 0.90 | 36.9 |
| Sample (mesh) | Qe,exp g·g−1 | Pseudo-First Order Kinetic | Pseudo-Second Order Kinetic | Diffusion Kinetic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K1 (min–1) | Qe, cal (g g–1) | R2 | K2 (10−5 g·g−1·min−1) | Qe, cal (g·g−1) | R2 | n | k | ||
| 20 a | 3587 | 0.9899 | 0.0283 | 2336 | 0.9900 | 2.43 | 3707 | 0.9698 | 0.2643 | 0.2912 |
| 60 a | 3587 | 0.9812 | 0.0300 | 2060 | 0.9933 | 2.80 | 3756 | 0.9668 | 0.2470 | 0.3266 |
| 80 a | 3587 | 0.9685 | 0.0800 | 4343 | 0.9919 | 3.84 | 3984 | 0.9335 | 0.2862 | 0.3127 0.6609 |
| 80 b | 302.0 | 0.9036 | 0.0541 | 67.6 | 0.9998 | 163.3 | 308.6 | 0.9260 | 0.0971 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhang, M.; Shangguan, L. Synthesis of a Novel and Salt Sensitive Superabsorbent Hydrogel Using Soybean Dregs by UV-Irradiation. Materials 2018, 11, 2198. https://doi.org/10.3390/ma11112198
Fan Y, Zhang M, Shangguan L. Synthesis of a Novel and Salt Sensitive Superabsorbent Hydrogel Using Soybean Dregs by UV-Irradiation. Materials. 2018; 11(11):2198. https://doi.org/10.3390/ma11112198
Chicago/Turabian StyleFan, Yisa, Mingyue Zhang, and Linjian Shangguan. 2018. "Synthesis of a Novel and Salt Sensitive Superabsorbent Hydrogel Using Soybean Dregs by UV-Irradiation" Materials 11, no. 11: 2198. https://doi.org/10.3390/ma11112198




