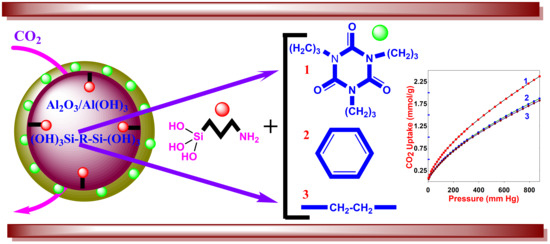
Development of Alumina–Mesoporous Organosilica Hybrid Materials for Carbon Dioxide Adsorption at 25 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
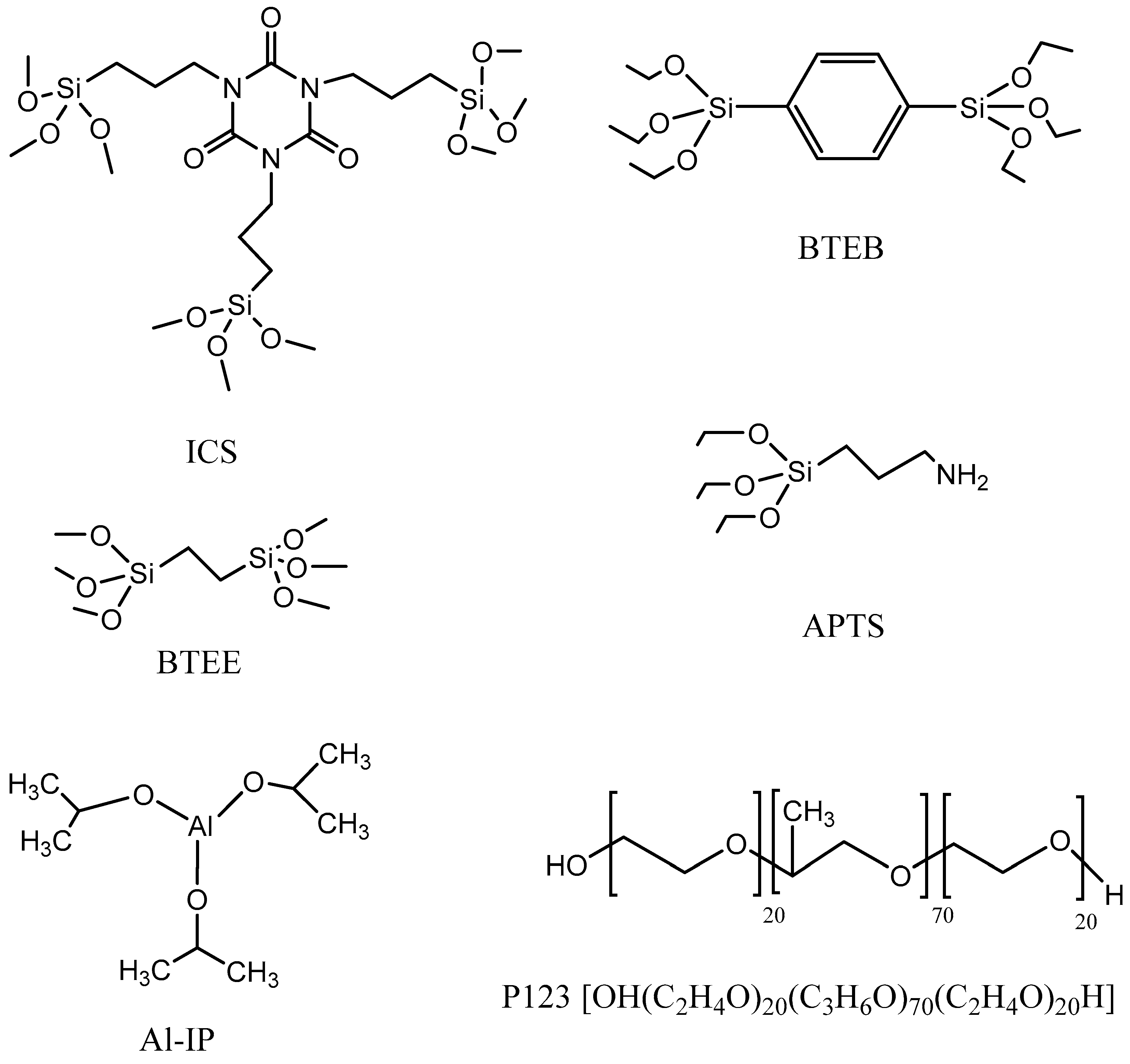
2.2. Synthesis Method
2.3. Characterization of Materials
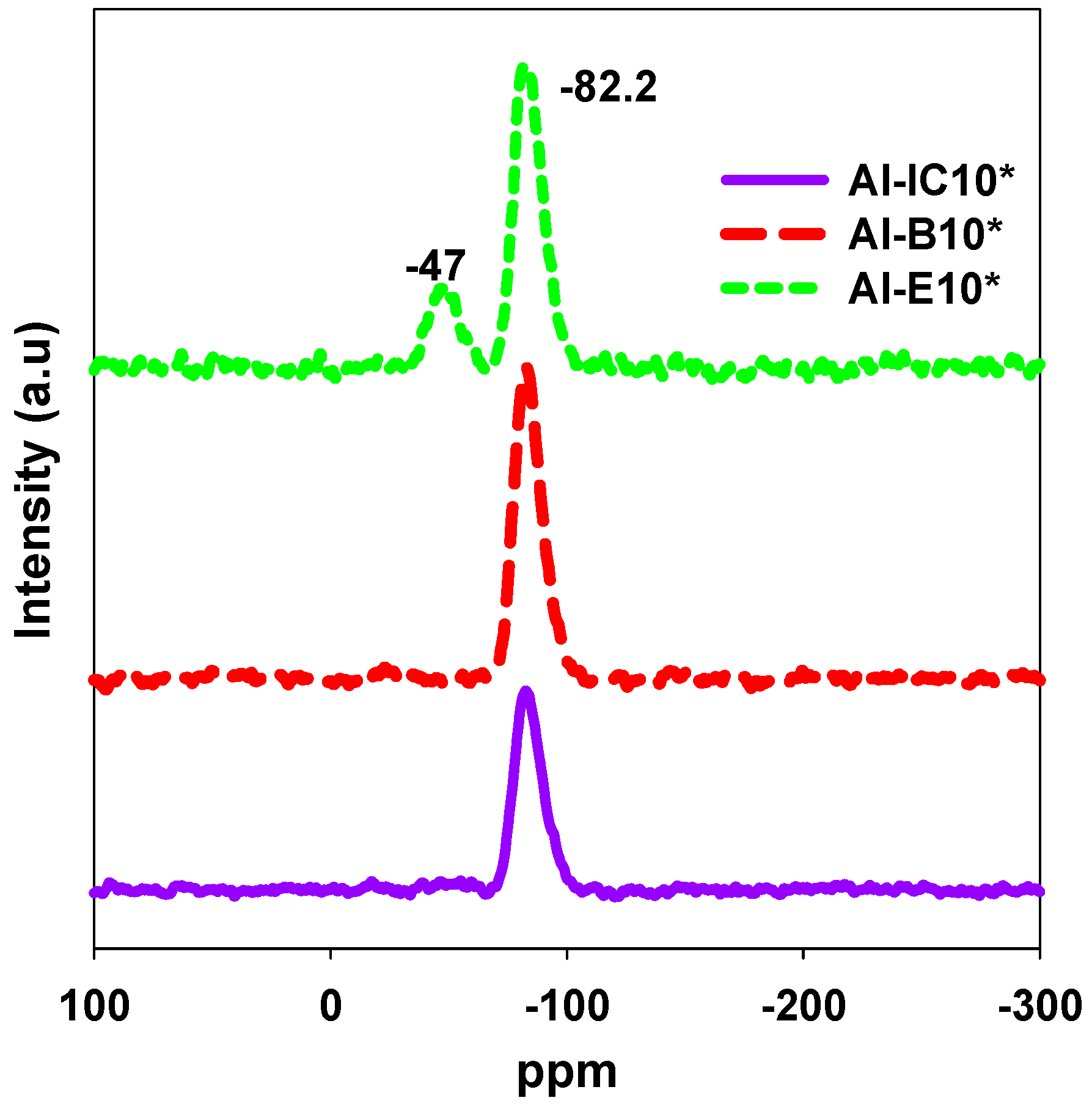
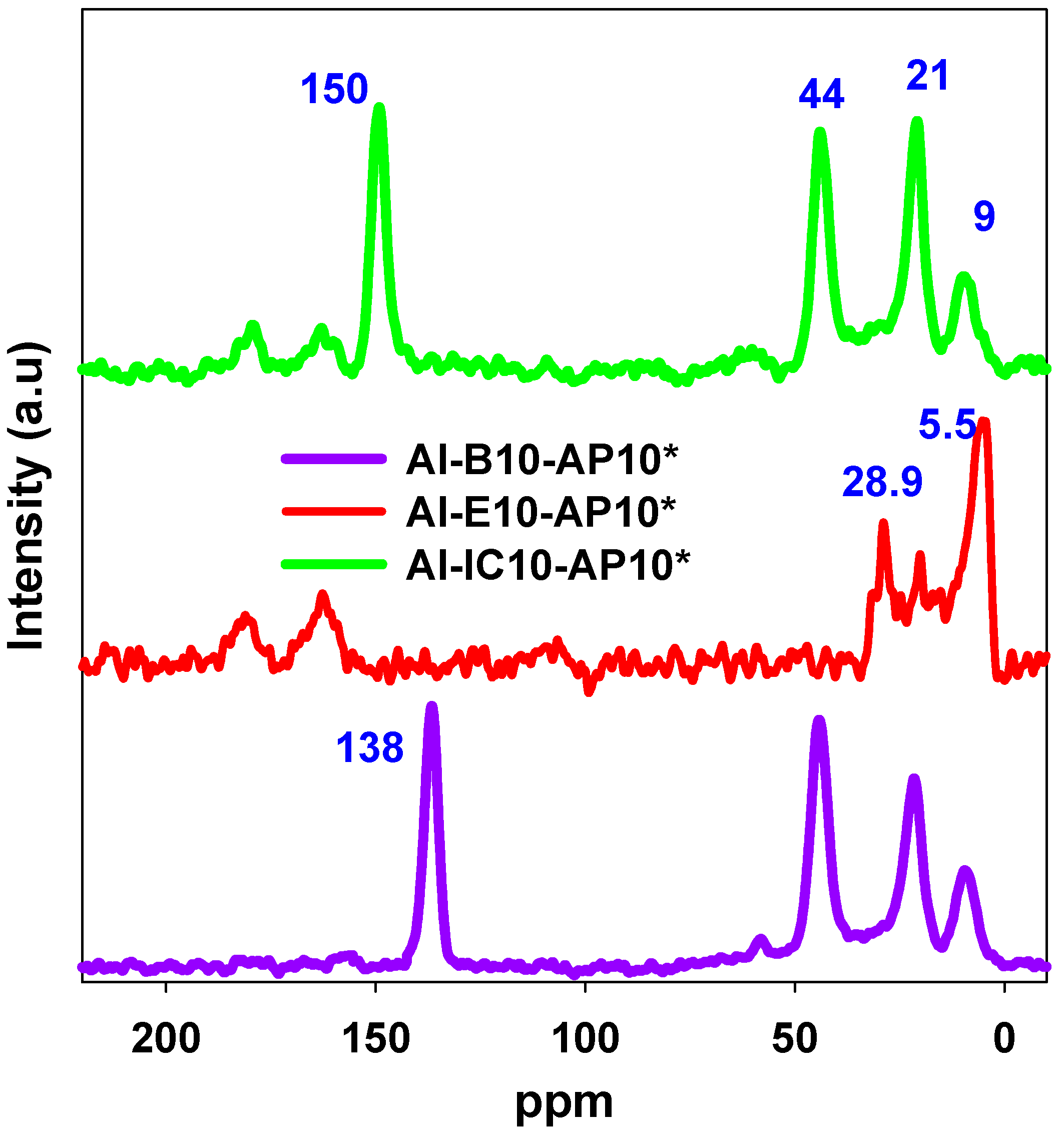
2.4. NMR Analysis
2.5. CO2 Adsorption Measurements
2.6. Calculation of Surface Properties
3. Results and Discussion
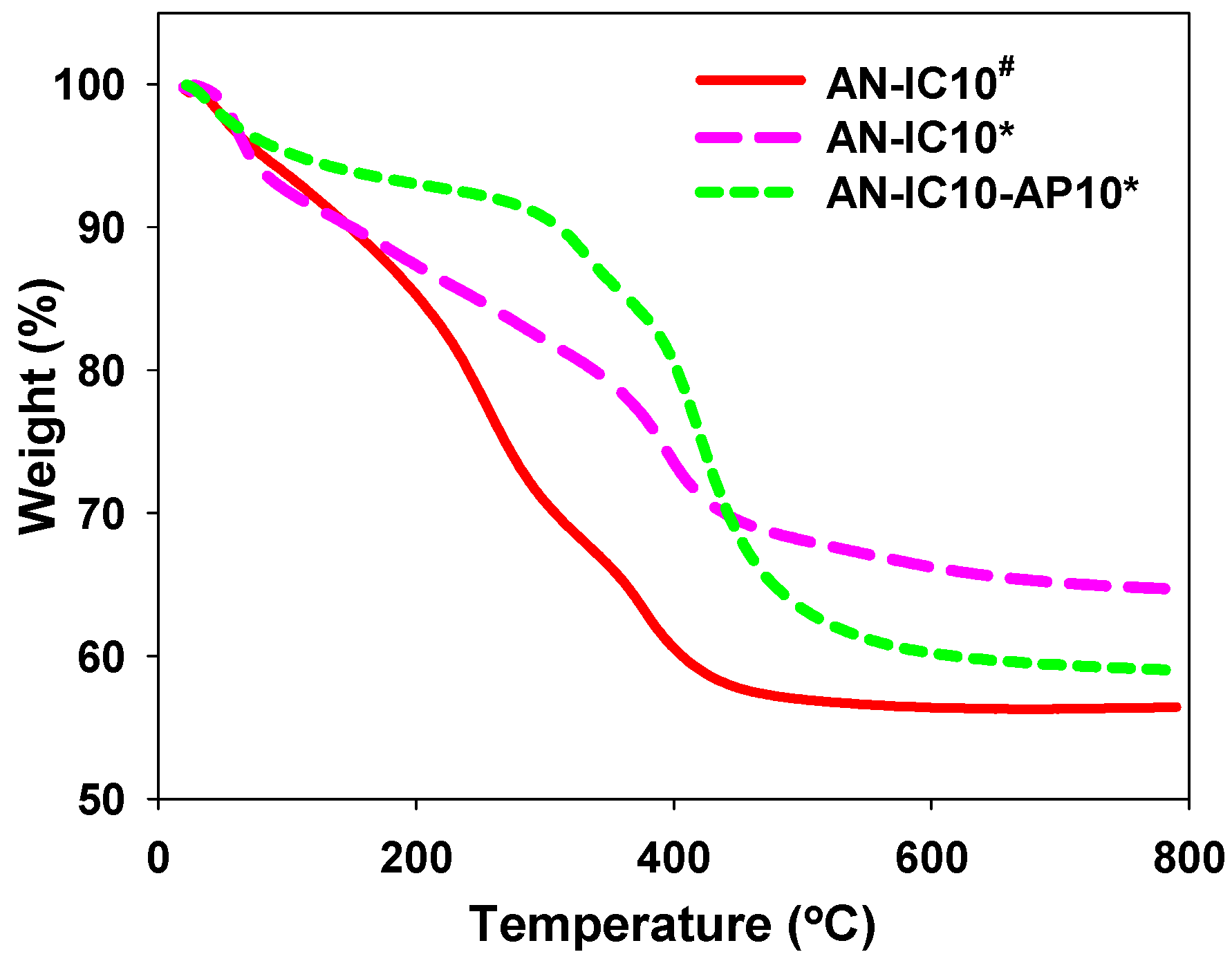
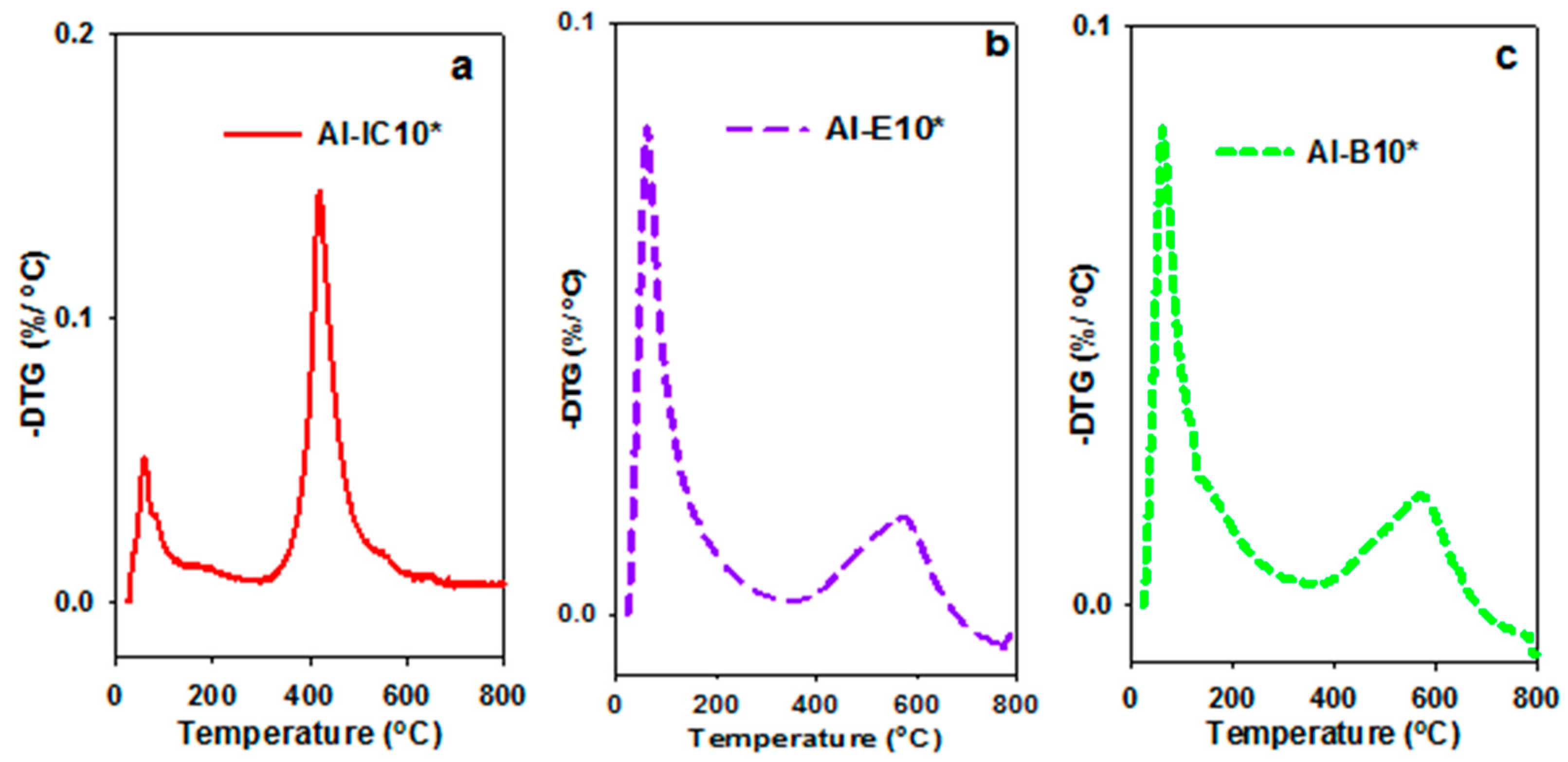
3.1. Properties of Al–MO Hybrid Composites
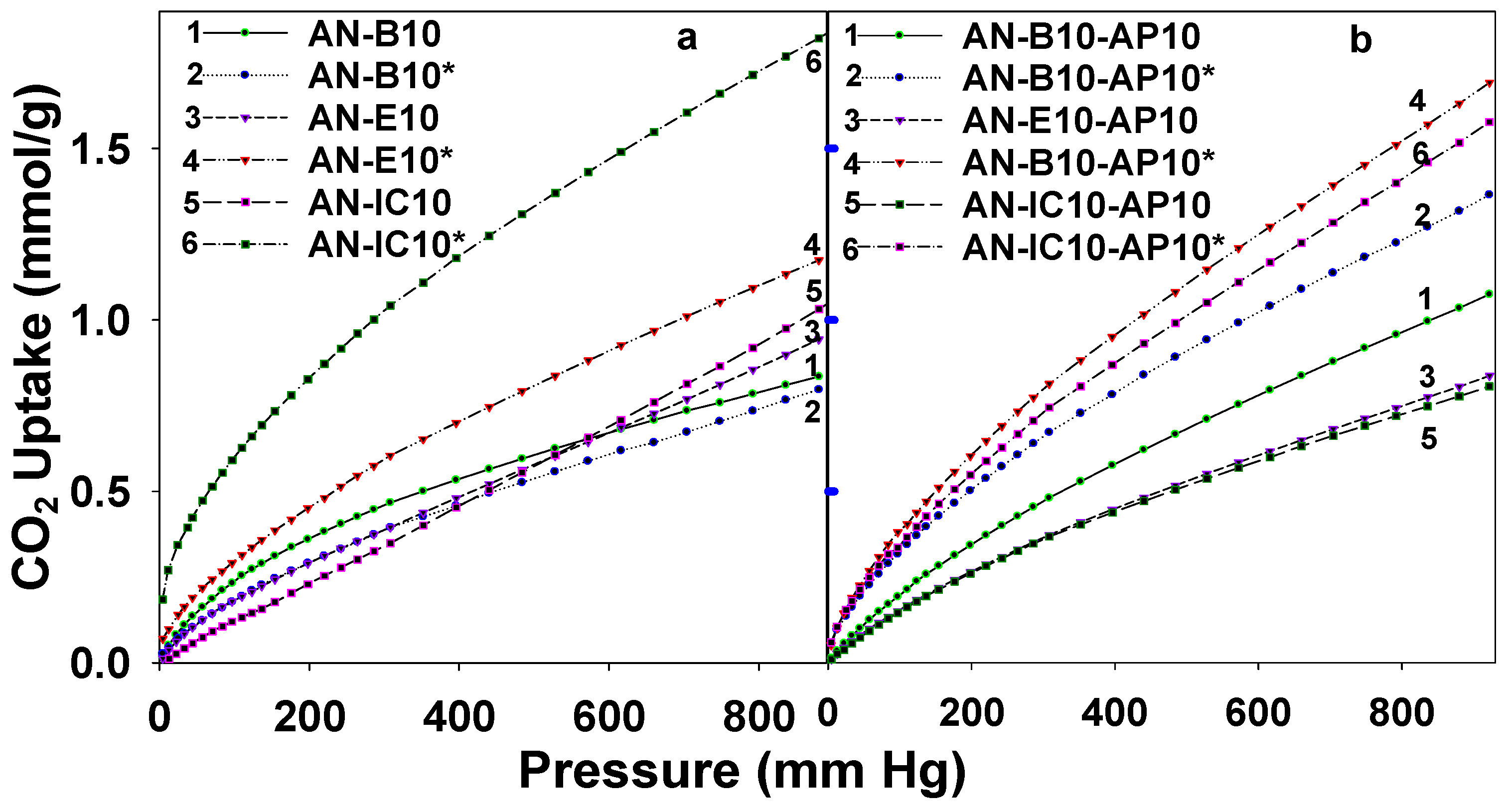
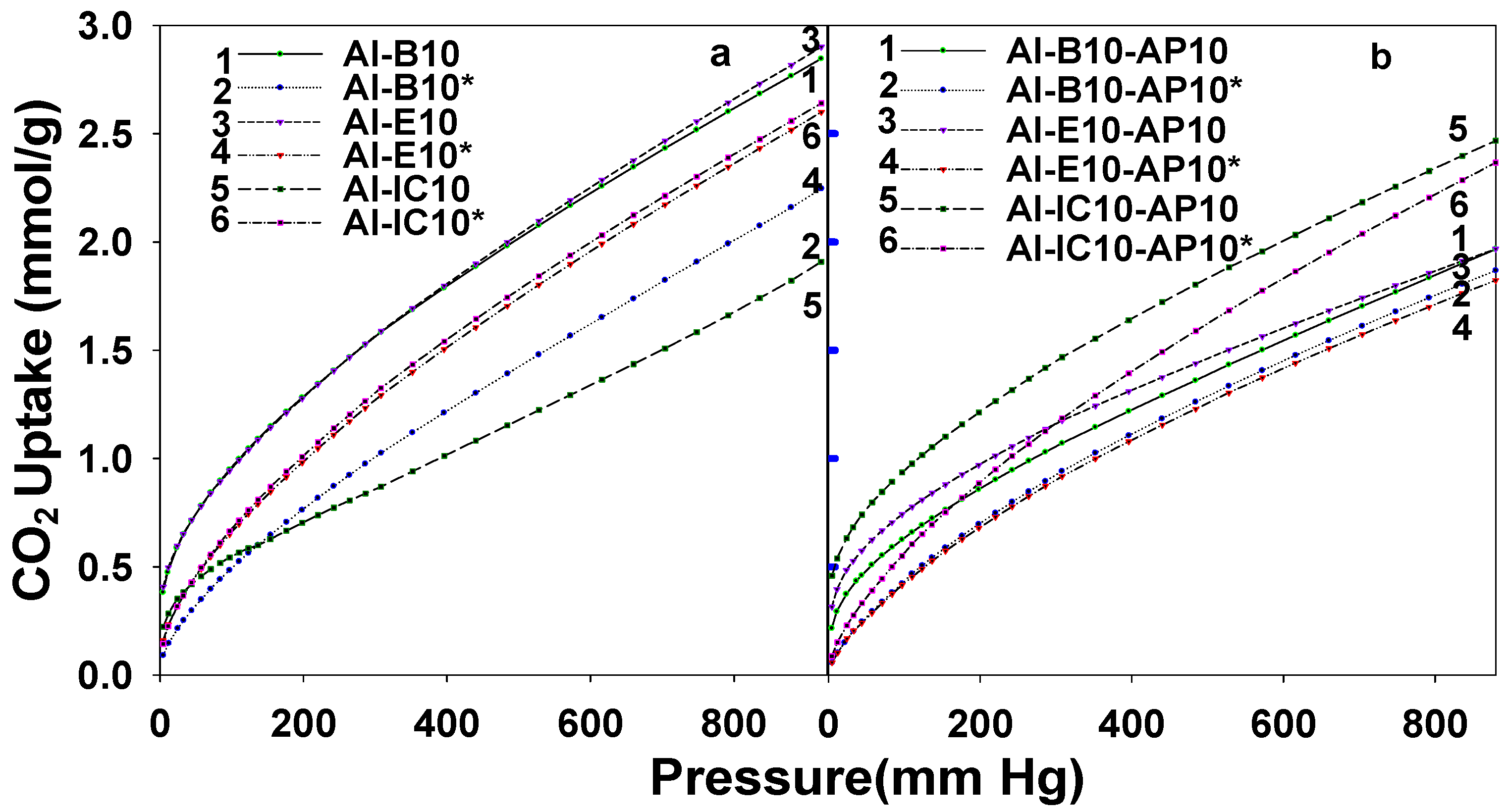
3.2. CO2 Sorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dassanayake, R.S.; Gunathilake, C.; Dassanayake, A.C.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized nanocrystalline cellulose–mesoporous silica composites for carbon dioxide sorption at ambient and elevated temperatures. J. Mater. Chem. A 2017, 5, 7462–7473. [Google Scholar] [CrossRef]
- Burwell, R.L. Section 1—Definitions and Terminology. In Manual of Symbols and Terminology for Physicochemical Quantities and Units–Appendix II; Burwell, R.L., Ed.; Pergamon Press: Oxford, UK, 1976; pp. 74–86. [Google Scholar]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Lou, H.; Goto, M.; Hirose, T. A new PSA process as an extension of the Petlyuk distillation concept. Sep. Purif. Technol. 1999, 15, 31–40. [Google Scholar] [CrossRef]
- Kikkinides, E.S.; Yang, R.T.; Cho, S.H. Concentration and recovery of carbon dioxide from flue gas by pressure swing adsorption. Ind. Eng. Chem. Res. 1993, 32, 2714–2720. [Google Scholar] [CrossRef]
- Burchell, T.D.; Judkins, R.R.; Rogers, M.R.; Williams, A.M. A novel process and material for the separation of carbon dioxide and hydrogen sulfide gas mixtures. Carbon 1997, 35, 1279–1294. [Google Scholar] [CrossRef]
- Rutherford, S.W.; Do, D.D. Adsorption dynamics of carbon dioxide on a carbon molecular sieve 5A. Carbon 2000, 38, 1339–1350. [Google Scholar] [CrossRef]
- Cinke, M.; Li, J.; Bauschlicher, C.W.; Ricca, A.; Meyyappan, M. CO2 adsorption in single-walled carbon nanotubes. Chem. Phys. Lett. 2003, 376, 761–766. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.; Shao, Q.; Lu, L.; Lu, X.; Jiang, S.; Shen, W. Simulations of Binary Mixture Adsorption of Carbon Dioxide and Methane in Carbon Nanotubes: Temperature, Pressure, and Pore Size Effects. J. Phys. Chem. C 2007, 111, 11912–11920. [Google Scholar] [CrossRef]
- Inui, T.; Okugawa, Y.; Yasuda, M. Relationship between properties of various zeolites and their carbon dioxide adsorption behaviors in pressure swing adsorption operation. Ind. Eng. Chem. Res. 1988, 27, 1103–1109. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P. Adsorption of CO2, N2, and O2 on Natural Zeolites. Energy Fuels 2003, 17, 571–576. [Google Scholar] [CrossRef]
- Demessence, A.; D’Alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 Binding in a Water-Stable, Triazolate-Bridged Metal−Organic Framework Functionalized with Ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef] [PubMed]
- Yazaydın, A.Ö.; Snurr, R.Q.; Park, T.-H.; Koh, K.; Liu, J.; LeVan, M.D.; Benin, A.I.; Jakubczak, P.; Lanuza, M.; Galloway, D.B.; et al. Screening of Metal−Organic Frameworks for Carbon Dioxide Capture from Flue Gas Using a Combined Experimental and Modeling Approach. J. Am. Chem. Soc. 2009, 131, 18198–18199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.C.; Chae, H.J.; Lee, S.J.; Park, Y.H.; Ryu, C.K.; Yi, C.K.; Kim, J.C. Novel regenerable potassium-based dry sorbents for CO2 capture at low temperatures. J. Mol. Catal. B Enzym. 2009, 56, 179–184. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, B.Y.; Lee, T.J.; Ryu, C.K.; Ahn, Y.S.; Kim, J.C. CO2 absorption and regeneration of alkali metal-based solid sorbents. Catal. Today 2006, 111, 385–390. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, J.C. Dry Potassium-Based Sorbents for CO2 Capture. Catal. Surv. Asia 2007, 11, 171–185. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, Y.M.; Ryu, C.Y.; Chae, H.J.; Ragupathy, D.; Jung, S.Y.; Lee, J.B.; Ryu, C.K.; Kim, J.C. Development of new aluminium oxide-modified sorbents for CO2 sorption and regeneration at temperatures below 200 °C. Fuel 2011, 90, 1465–1470. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. A comparison of two methods for producing CO2 capture adsorbents. Energy Procedia 2009, 1, 1107–1113. [Google Scholar] [CrossRef]
- Przepiórski, J.; Skrodzewicz, M.; Morawski, A.W. High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Appl. Surf. Sci. 2004, 225, 235–242. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative Study of CO2 Capture by Carbon Nanotubes, Activated Carbons, and Zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Cnen, W.; Bai, H.; Hwang, J.F. Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Drage, T.C.; Arenillas, A.; Smith, K.M.; Pevida, C.; Piippo, S.; Snape, C.E. Preparation of carbon dioxide adsorbents from the chemical activation of urea–formaldehyde and melamine–formaldehyde resins. Fuel 2007, 86, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, P.D.; Chatti, R.V.; Biniwale, R.B.; Labhsetwar, N.K.; Devotta, S.; Rayalu, S.S. Monoethanol Amine Modified Zeolite 13X for CO2 Adsorption at Different Temperatures. Energy Fuels 2007, 21, 3555–3559. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Kuo, S.-C.; Zeng, W. Adsorption of CO2 on Amine-Functionalized Y-Type Zeolites. Energy Fuels 2010, 24, 1441–1448. [Google Scholar] [CrossRef]
- Lee, S.; Filburn, T.P.; Gray, M.; Park, J.-W.; Song, H.-J. Screening Test of Solid Amine Sorbents for CO2 Capture. Ind. Eng. Chem. Res. 2008, 47, 7419–7423. [Google Scholar] [CrossRef]
- Cho, E.-B.; Kim, D.; Mandal, M.; Gunathilake, C.A.; Jaroniec, M. Benzene-Silica with Hexagonal and Cubic Ordered Mesostructures Synthesized in the Presence of Block Copolymers and Weak Acid Catalysts. J. Phys. Chem. C 2012, 116, 16023–16029. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Song, C. “Molecular Basket” Sorbents for Separation of CO2 and H2S from Various Gas Streams. J. Am. Chem. Soc. 2009, 131, 5777–5783. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.B.; Chun, Y.; Cao, Y.; Dong, X.; Zhu, J.H. CO2 Capture by As-Prepared SBA-15 with an Occluded Organic Template. Adv. Funct. Mater. 2006, 16, 1717–1722. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Knowles, G.P.; Delaney, S.W.; Chaffee, A.L. Diethylenetriamine[propyl(silyl)]-Functionalized (DT) Mesoporous Silicas as CO2 Adsorbents. Ind. Eng. Chem. Res. 2006, 45, 2626–2633. [Google Scholar] [CrossRef]
- Norihito, H.; Katsunori, Y.; Tatsuaki, Y. Adsorption of Carbon Dioxide on Amine Modified SBA-15 in the Presence of Water Vapor. Chem. Lett. 2004, 33, 510–511. [Google Scholar]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Arenillas, A.; Rubiera, F.; Pis, J.J. Application of thermogravimetric analysis to the evaluation of aminated solid sorbents for CO2 capture. J. Therm. Anal. Calorim. 2008, 92, 601–606. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zhou, L.; Huang, D.; Zhou, Y. Adsorption of CO2, CH4 and N2 on ordered mesoporous silica molecular sieve. Chem. Phys. Lett. 2005, 415, 198–201. [Google Scholar] [CrossRef]
- He, Y.; Seaton, N.A. Heats of Adsorption and Adsorption Heterogeneity for Methane, Ethane, and Carbon Dioxide in MCM-41. Langmuir 2006, 22, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of Amine-Tethered Solid Sorbents for Direct CO2 Capture from the Ambient Air. Environ. Sci. Technol. 2011, 45, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Drese, J.H.; Choi, S.; Lively, R.P.; Koros, W.J.; Fauth, D.J.; Gray, M.L.; Jones, C.W. Synthesis–Structure–Property Relationships for Hyperbranched Aminosilica CO2 Adsorbents. Adv. Funct. Mater. 2009, 19, 3821–3832. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef] [PubMed]
- Zeleňák, V.; Badaničová, M.; Halamová, D.; Čejka, J.; Zukal, A.; Murafa, N.; Goerigk, G. Amine-modified ordered mesoporous silica: Effect of pore size on carbon dioxide capture. Chem. Eng. J. 2008, 144, 336–342. [Google Scholar] [CrossRef]
- Zelenak, V.; Halamova, D.; Gaberova, L.; Bloch, E.; Llewellyn, P. Amine-modified SBA-12 mesoporous silica for carbon dioxide capture: Effect of amine basicity on sorption properties. Microporous Mesoporous Mater. 2008, 116, 358–364. [Google Scholar] [CrossRef]
- Burkett, S.L.; Sims, S.D.; Mann, S. Synthesis of hybrid inorganic–organic mesoporous silica by co-condensation of siloxane and organosiloxane precursors. Chem. Commun. 1996, 11, 1367–1368. [Google Scholar] [CrossRef]
- Macquarrie, D.J. Direct preparation of organically modified MCM-type materials. Preparation and characterisation of aminopropyl–MCM and 2-cyanoethyl–MCM. Chem. Commun. 1996, 16, 1961–1962. [Google Scholar] [CrossRef]
- Harlick, P.J.E.; Sayari, A. Applications of Pore-Expanded Mesoporous Silicas. 3. Triamine Silane Grafting for Enhanced CO2 Adsorption. Ind. Eng. Chem. Res. 2006, 45, 3248–3255. [Google Scholar] [CrossRef]
- Knowles, G.P.; Graham, J.V.; Delaney, S.W.; Chaffee, A.L. Aminopropyl-functionalized mesoporous silicas as CO2 adsorbents. Fuel Process. Technol. 2005, 86, 1435–1448. [Google Scholar] [CrossRef]
- Mao, C.-F.; snm Vannice, M.A. High surface area α-aluminium oxide. I.: Adsorption properties and heats of adsorption of carbon monoxide, carbon dioxide, and ethylene. Appl. Catal. A General 1994, 111, 151–173. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, A.E. Adsorption of Carbon Dioxide on Basic Aluminium oxide at High Temperatures. J. Chem. Eng. Data 2000, 45, 1093–1095. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Kim, H.-J.; Jones, C.W. Mesoporous Aluminium Oxide-Supported Amines as Potential Steam-Stable Adsorbents for Capturing CO2 from Simulated Flue Gas and Ambient Air. Energy Fuels 2011, 25, 5528–5537. [Google Scholar] [CrossRef]
- Jeon, H.; Ahn, S.H.; Kim, J.H.; Min, Y.J.; Lee, K.B. Templated synthesis of mesoporous aluminium oxides by graft copolymer and their CO2 adsorption capacities. J. Mater. Sci. 2011, 46, 4020–4025. [Google Scholar] [CrossRef]
- Li, L.; Wen, X.; Fu, X.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. MgO/Al2O3 Sorbent for CO2 Capture. Energy Fuels 2010, 24, 5773–5780. [Google Scholar] [CrossRef]
- Gunathilake, C.; Gangoda, M.; Jaroniec, M. Mesoporous isocyanurate-containing organosilica–aluminium oxide composites and their thermal treatment in nitrogen for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. A 2013, 1, 8244–8252. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867. [Google Scholar] [CrossRef]
- Cai, W.; Yu, J.; Anand, C.; Vinu, A.; Jaroniec, M. Facile Synthesis of Ordered Mesoporous Aluminium Oxide and Aluminium Oxide-Supported Metal Oxides with Tailored Adsorption and Framework Properties. Chem. Mater. 2011, 23, 1147–1157. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of Large Pore MCM-41 Molecular Sieves to Improve Pore Size Analysis Using Nitrogen Adsorption Measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Morris, S.M.; Fulvio, P.F.; Jaroniec, M. Ordered Mesoporous Alumina-Supported Metal Oxides. J. Am. Chem. Soc. 2008, 130, 15210–15216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hua, C.; Madden, D.G.; O’Nolan, D.; Chen, K.-J.; Keane, L.-A.J.; Perry, J.J.; Zaworotko, M.J. Hybrid ultramicroporous materials (HUMs) with enhanced stability and trace carbon capture performance. Chem. Commun. 2017, 53, 5946–5949. [Google Scholar] [CrossRef] [PubMed]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Bromberg, L.; Su, X.; Hatton, T.A. Aldehyde Self-Condensation Catalysis by Aluminum Aminoterephthalate Metal–Organic Frameworks Modified with Aluminum Isopropoxide. Chem. Mater. 2013, 25, 1636–1642. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous alumina–zirconia–organosilica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A 2015, 3, 2707–2716. [Google Scholar] [CrossRef]
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized microcrystalline cellulose–mesoporous silica composites for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. A 2016, 4, 4808–4819. [Google Scholar] [CrossRef]
- Hanif, A.; Dasgupta, S.; Nanoti, A. High temperature CO2 adsorption by mesoporous silica supported magnesium aluminum mixed oxide. Chem. Eng. J. 2015, 280, 703–710. [Google Scholar] [CrossRef]
- Granados-Correa, F.; Bonifacio-Martinez, J.; Hernandez-Mendoza, H.; Bulbulian, S. Capture of CO2 on gamma-Al2O3 materials prepared by solution-combustion and ball-milling processes. J. Air Waste Manag. Assoc. 2016, 66, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Thote, J.A.; Chatti, R.V.; Iyer, K.S.; Kumar, V.; Valechha, A.N.; Labhsetwar, N.K.; Biniwale, R.B.; Yenkie, M.K.; Rayalu, S.S. N-doped mesoporous alumina for adsorption of carbon dioxide. J. Environ. Sci. 2012, 24, 1979–1984. [Google Scholar] [CrossRef]
- Wang, J.; Mei, X.; Huang, L.; Zheng, Q.; Qiao, Y.; Zang, K.; Mao, S.; Yang, R.; Zhang, Z.; Gao, Y.; et al. Synthesis of layered double hydroxides/graphene oxide nanocomposite as a novel high-temperature CO2 adsorbent. J. Energy Chem. 2015, 24, 127–137. [Google Scholar] [CrossRef]


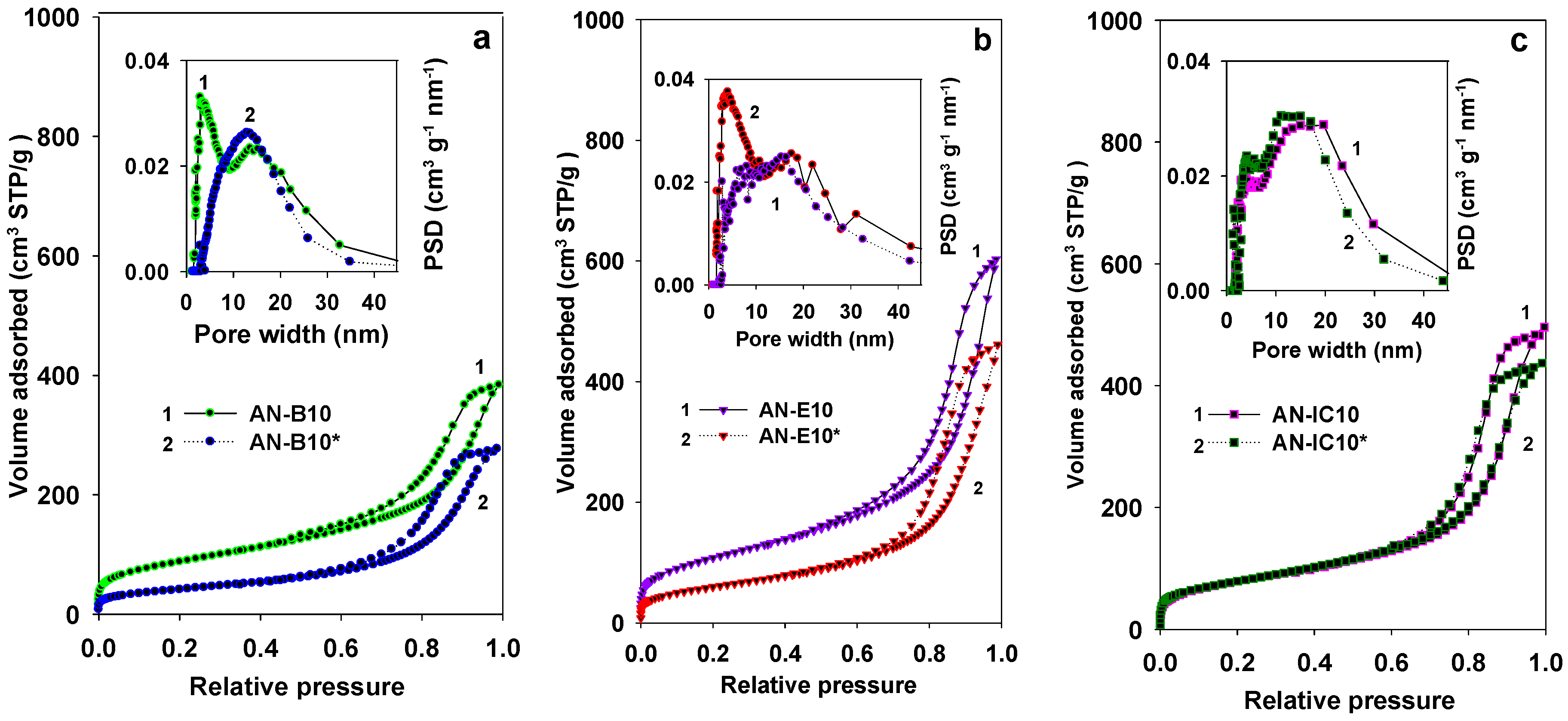
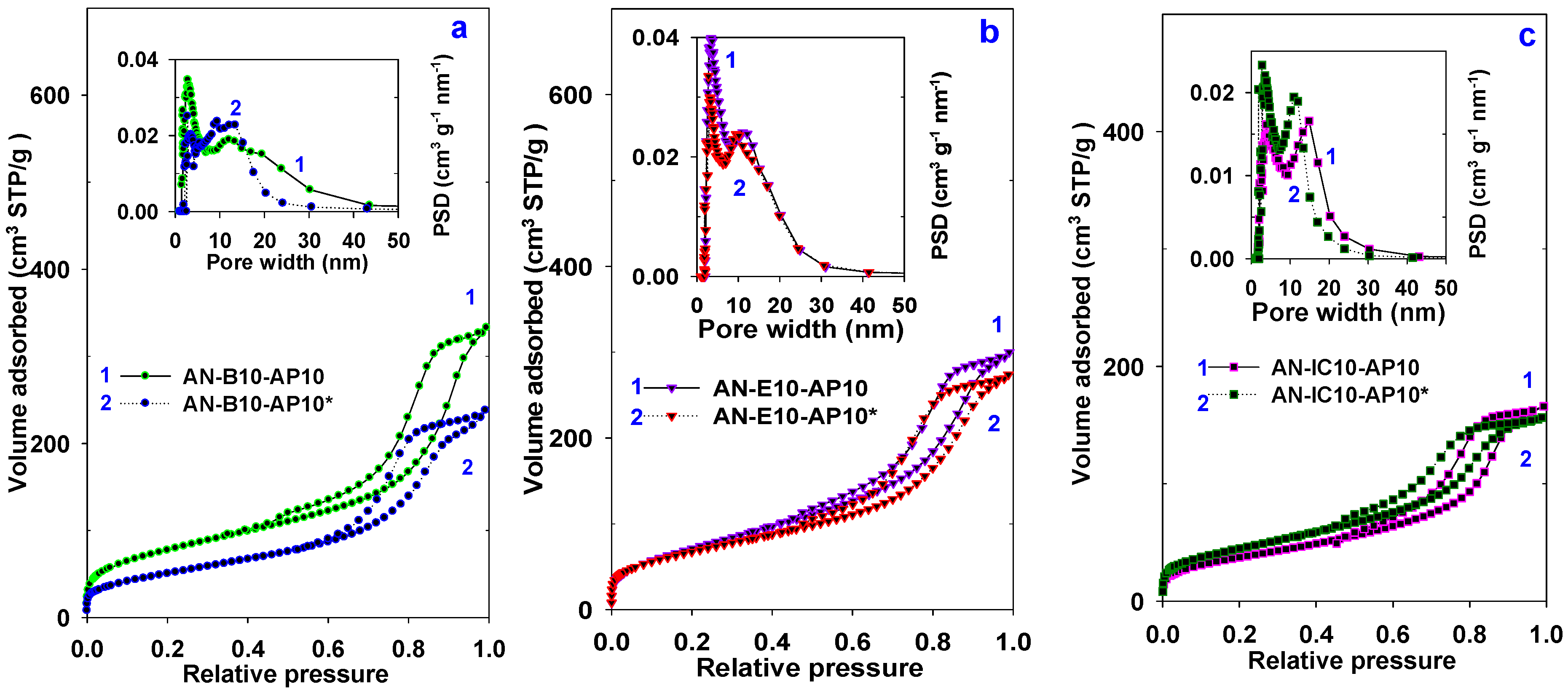
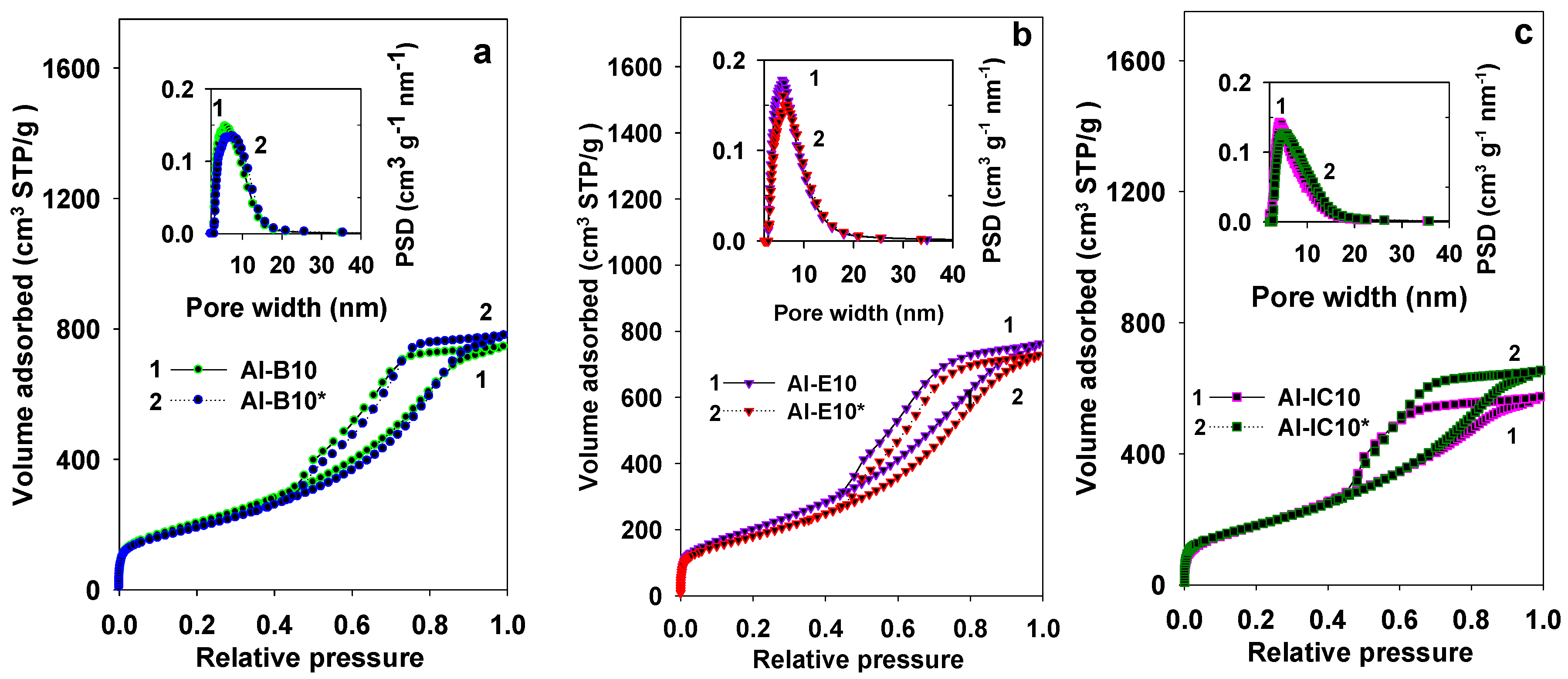


| Samples | SBET (m2/g) | Vsp (cc/g) | Vt (cc/g) | Vmi (cc/g) | Wmax (nm) | nCO2 (mmol/g) | n*CO2 (µmol/m2) |
|---|---|---|---|---|---|---|---|
| AN-B10 | 319 | 0.60 | 0.63 | 0.02 | 2.9/13.6 | 0.78 | 2.45 |
| AN-B10* | 150 | 0.42 | 0.44 | <0.01 | 12.0# | 0.73 | 4.87 |
| AN-E10 | 393 | 0.91 | 0.94 | <0.01 | 4.1/17.0 | 1.09 | 2.77 |
| AN-E10* | 219 | 0.67 | 0.72 | <0.01 | 15.9# | 0.85 | 3.88 |
| AN-IC10 | 289 | 0.74 | 0.77 | 0.01 | 4.0/16.4# | 0.92 | 3.18 |
| AN-IC10* | 286 | 0.68 | 0.68 | <0.01 | 4.0/13.2# | 1.72 | 6.01 |
| AN-B10-AP10 | 284 | 0.50 | 0.52 | 0.02 | 2.9/12.3 | 0.96 | 3.38 |
| AN-B10-AP10* | 186 | 0.36 | 0.38 | <0.01 | 9.4/13.3 | 1.22 | 6.56 |
| AN-E10-AP10 | 265 | 0.46 | 0.48 | <0.01 | 3.4/11.6# | 0.74 | 2.79 |
| AN-E10-AP10* | 247 | 0.42 | 0.43 | <0.01 | 3.2/9.8# | 1.51 | 6.11 |
| AN-IC10-AP10 | 138 | 0.26 | 0.26 | <0.01 | 4.1/14.8 | 0.72 | 5.21 |
| AN-IC10-AP10* | 163 | 0.24 | 0.24 | <0.01 | 3.6/11.1 | 1.40 | 8.59 |
| AI-B10 | 742 | 1.14 | 1.24 | 0.05 | 5.1 | 2.60 | 3.50 |
| AI-B10* | 684 | 1.20 | 1.28 | 0.03 | 6.1 | 1.99 | 2.91 |
| AI-E10 | 740 | 1.18 | 1.27 | 0.03 | 5.7 | 2.64 | 3.57 |
| AI-E10* | 652 | 1.12 | 1.19 | 0.02 | 6.1 | 2.35 | 3.60 |
| AI-IC10 | 664 | 0.89 | 0.95 | 0.04 | 4.1 | 1.66 | 2.50 |
| Al-IC10* | 655 | 1.01 | 1.06 | 0.02 | 4.7 | 2.39 | 3.65 |
| AI-B10-AP10 | 654 | 0.94 | 1.00 | 0.02 | 4.2 | 1.83 | 2.80 |
| AI-B10-AP10* | 452 | 0.76 | 0.78 | <0.01 | 5.6 | 1.74 | 3.85 |
| AI-E10-AP10 | 664 | 0.97 | 1.03 | 0.02 | 4.0 | 1.86 | 2.80 |
| AI-E10-AP10* | 509 | 0.86 | 0.90 | <0.01 | 5.1 | 1.70 | 3.34 |
| AI-IC10-AP10 | 713 | 0.91 | 0.98 | 0.03 | 4.0 | 2.33 | 3.27 |
| AI-IC10-AP10* | 618 | 0.87 | 0.90 | 0.02 | 5.4 | 2.21 | 3.58 |
| Material | Temperature (°C) | Pressure (atm) | CO2 Uptake (mmol/g) | Ref. |
|---|---|---|---|---|
| Al–Mg oxide | 60 (13%H2O) | 0.99 | 1.36 | [49] |
| Al2O3–silica | 120 | 0.99 | 2.20 | [50] |
| Al–Zr–mixed oxide–silica | 0 25 60 120 | 1 1 1 1 | 1.83 1.39 2.60 2.37 | [58] |
| Al–Mg mixed oxide–silica | 300 | 0.99 | 0.46 | [60] |
| γ-Al2O3 | 60 | 9.9 | 1.94 | [61] |
| N-doped γ-Al2O3 | 55 | NG | 0.67 | [62] |
| Al–Mg mixed oxide–nitrate–graphene oxide | 60 | 1 | 1.00 | [63] |
| Al2O3–silica | 25 | 1 | 2.64 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilake, C.; Dassanayake, R.S.; Kalpage, C.S.; Jaroniec, M. Development of Alumina–Mesoporous Organosilica Hybrid Materials for Carbon Dioxide Adsorption at 25 °C. Materials 2018, 11, 2301. https://doi.org/10.3390/ma11112301
Gunathilake C, Dassanayake RS, Kalpage CS, Jaroniec M. Development of Alumina–Mesoporous Organosilica Hybrid Materials for Carbon Dioxide Adsorption at 25 °C. Materials. 2018; 11(11):2301. https://doi.org/10.3390/ma11112301
Chicago/Turabian StyleGunathilake, Chamila, Rohan S. Dassanayake, Chandrakantha S. Kalpage, and Mietek Jaroniec. 2018. "Development of Alumina–Mesoporous Organosilica Hybrid Materials for Carbon Dioxide Adsorption at 25 °C" Materials 11, no. 11: 2301. https://doi.org/10.3390/ma11112301