Fabrication of La2O3 Uniformly Doped Mo Nanopowders by Solution Combustion Synthesis Followed by Reduction under Hydrogen
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of La2O3 Doped MoO2 Precursor
2.3. Hydrogen Reduction of Mo-Based Precursor
2.4. Characterization
3. Results and Discussion
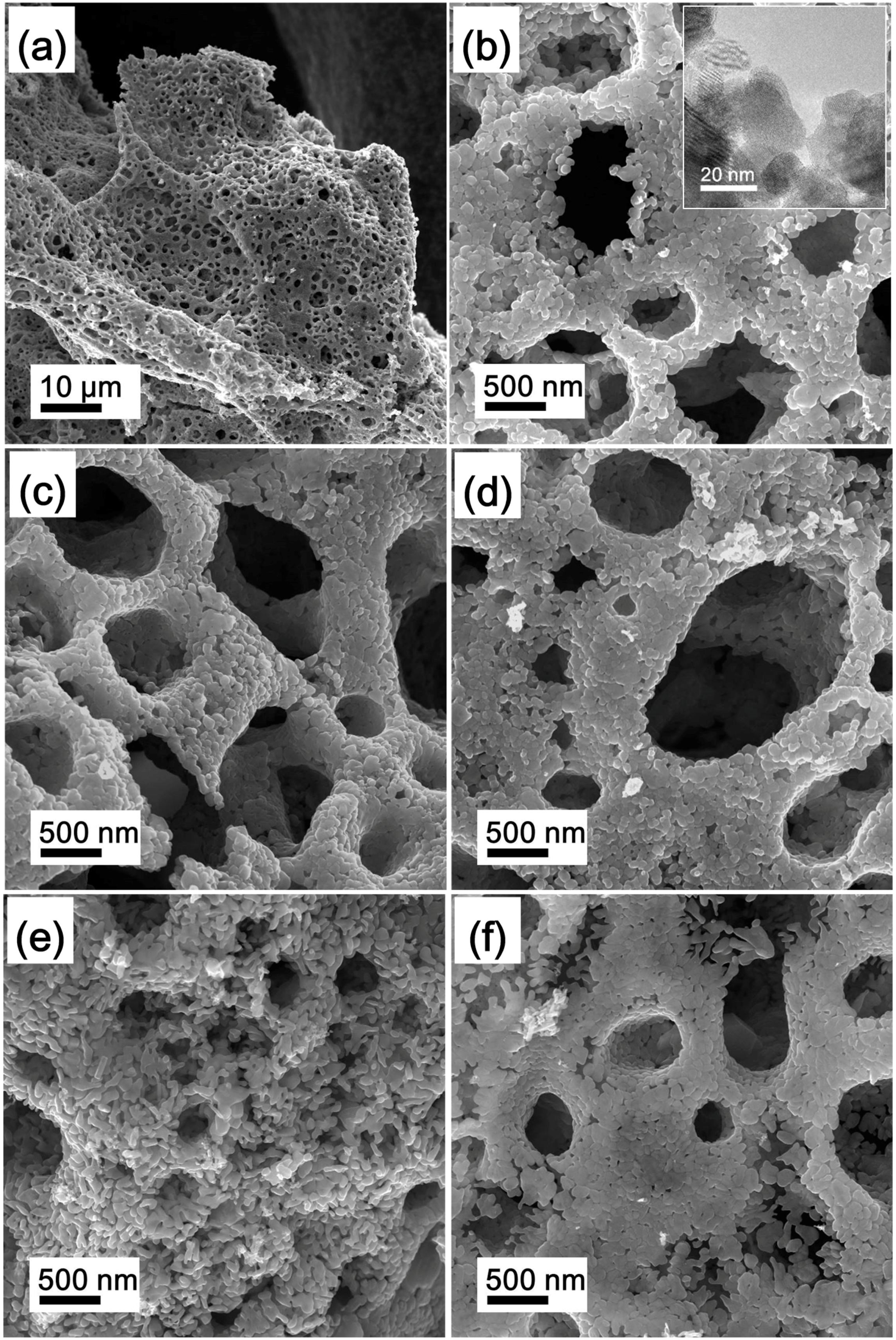
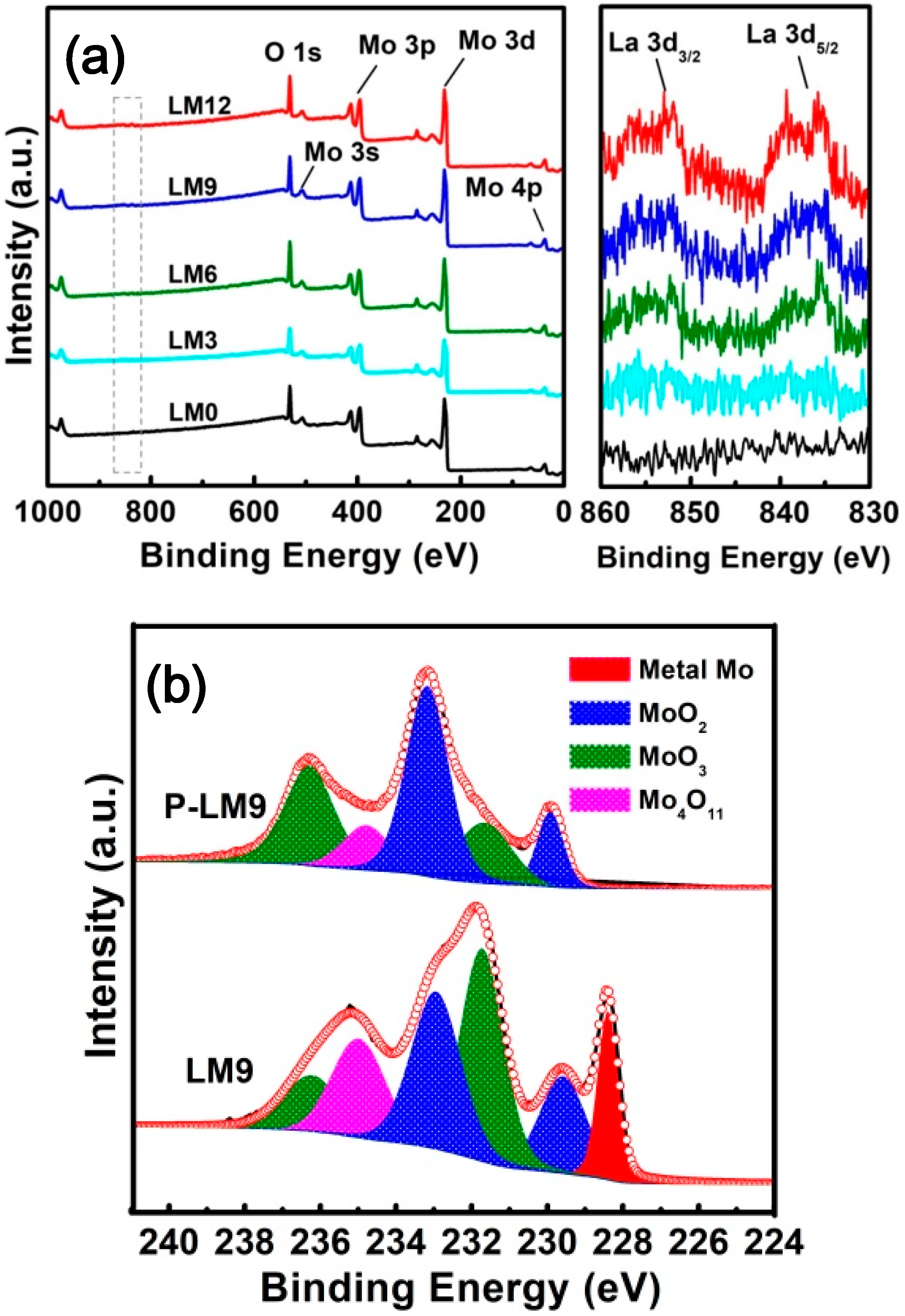
3.1. Phases and Morphology of Precursor
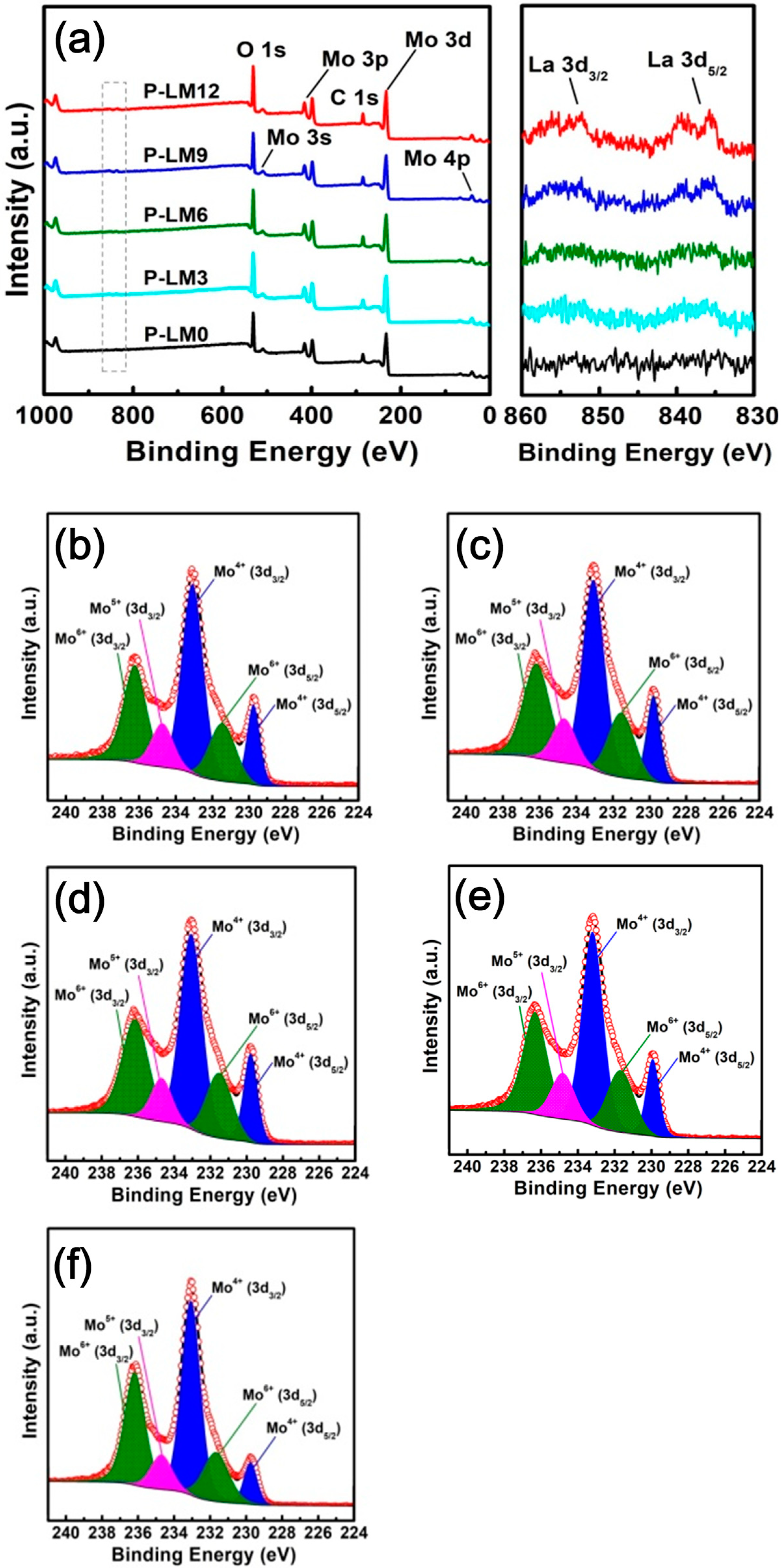
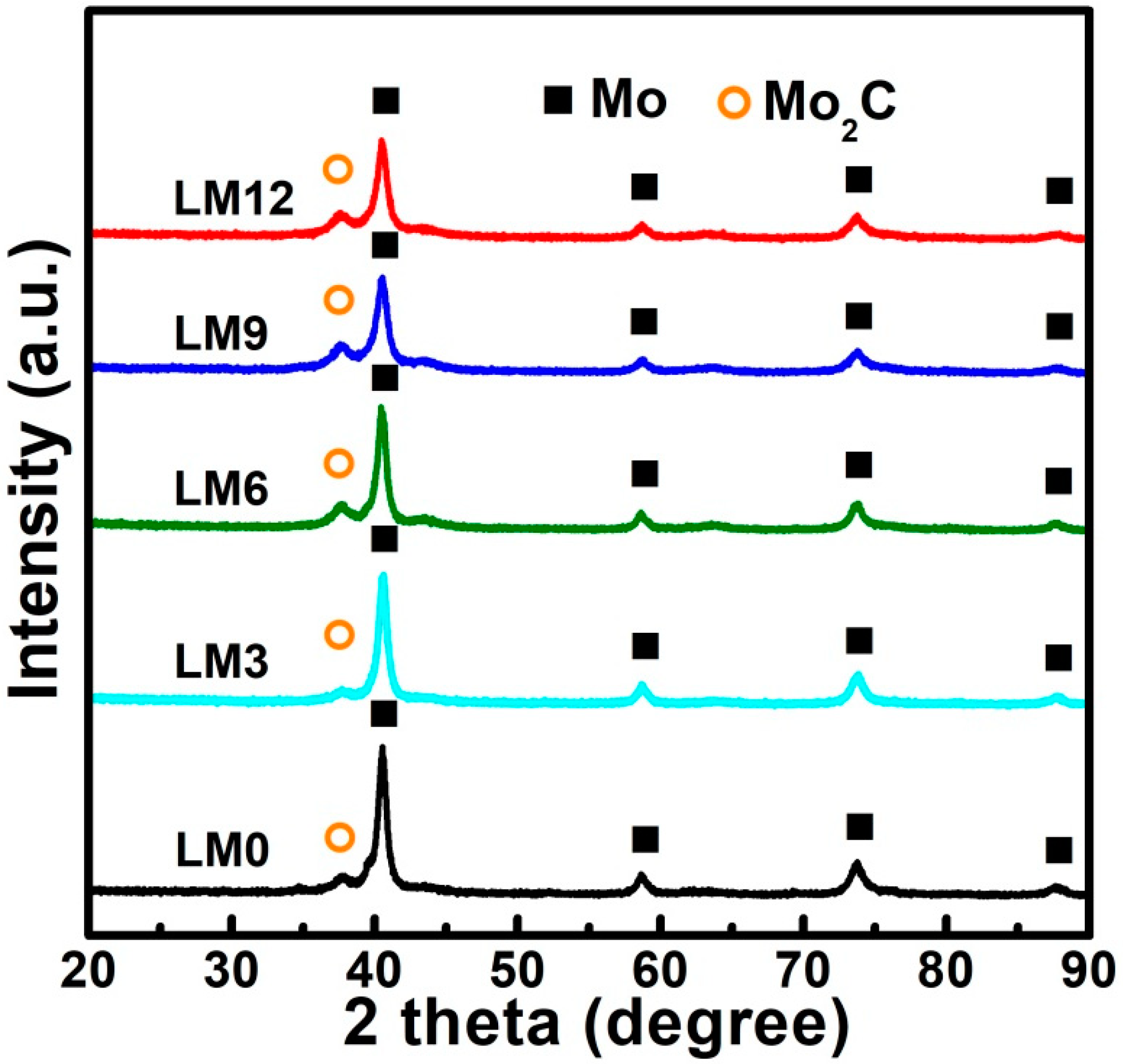
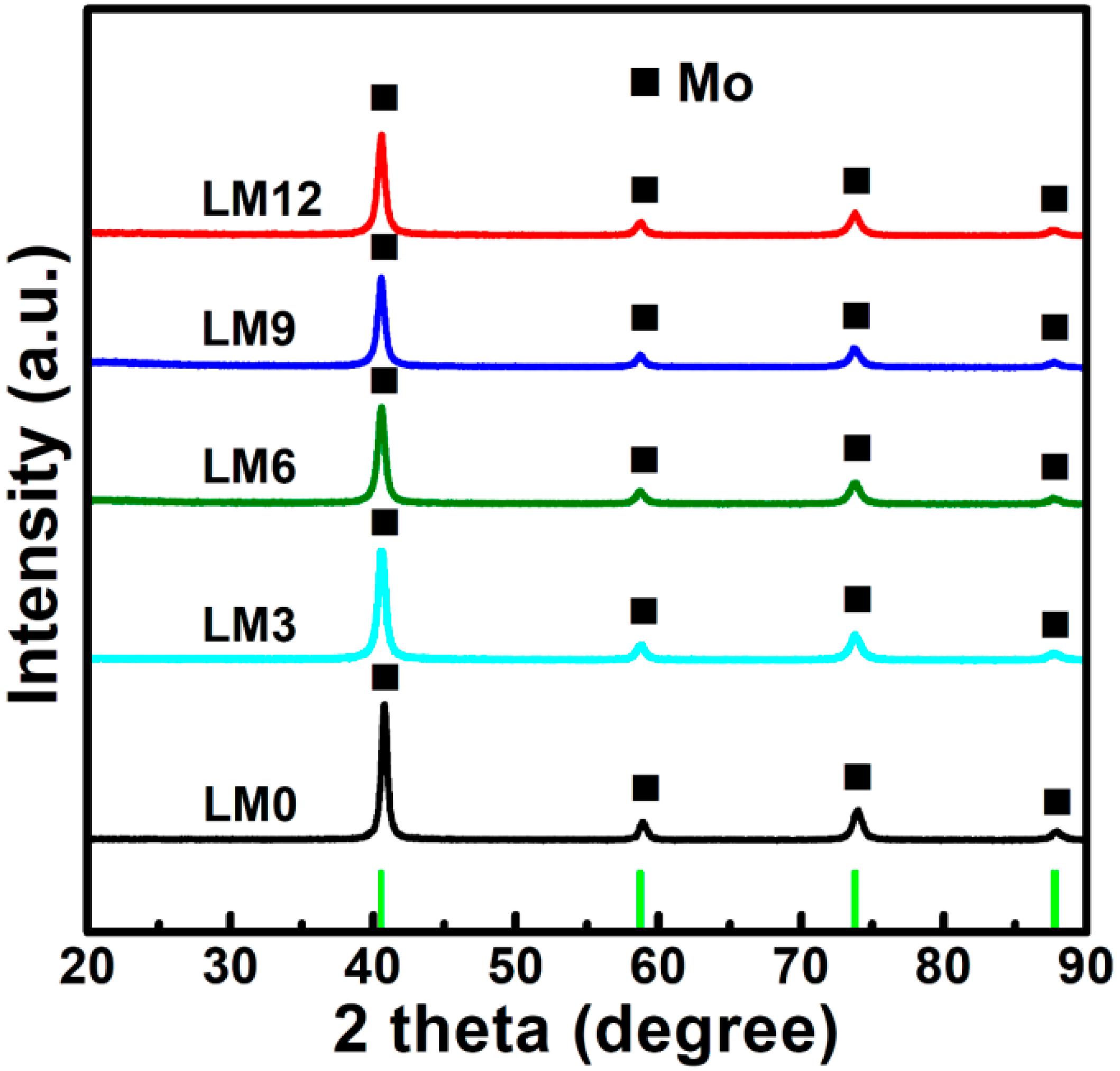
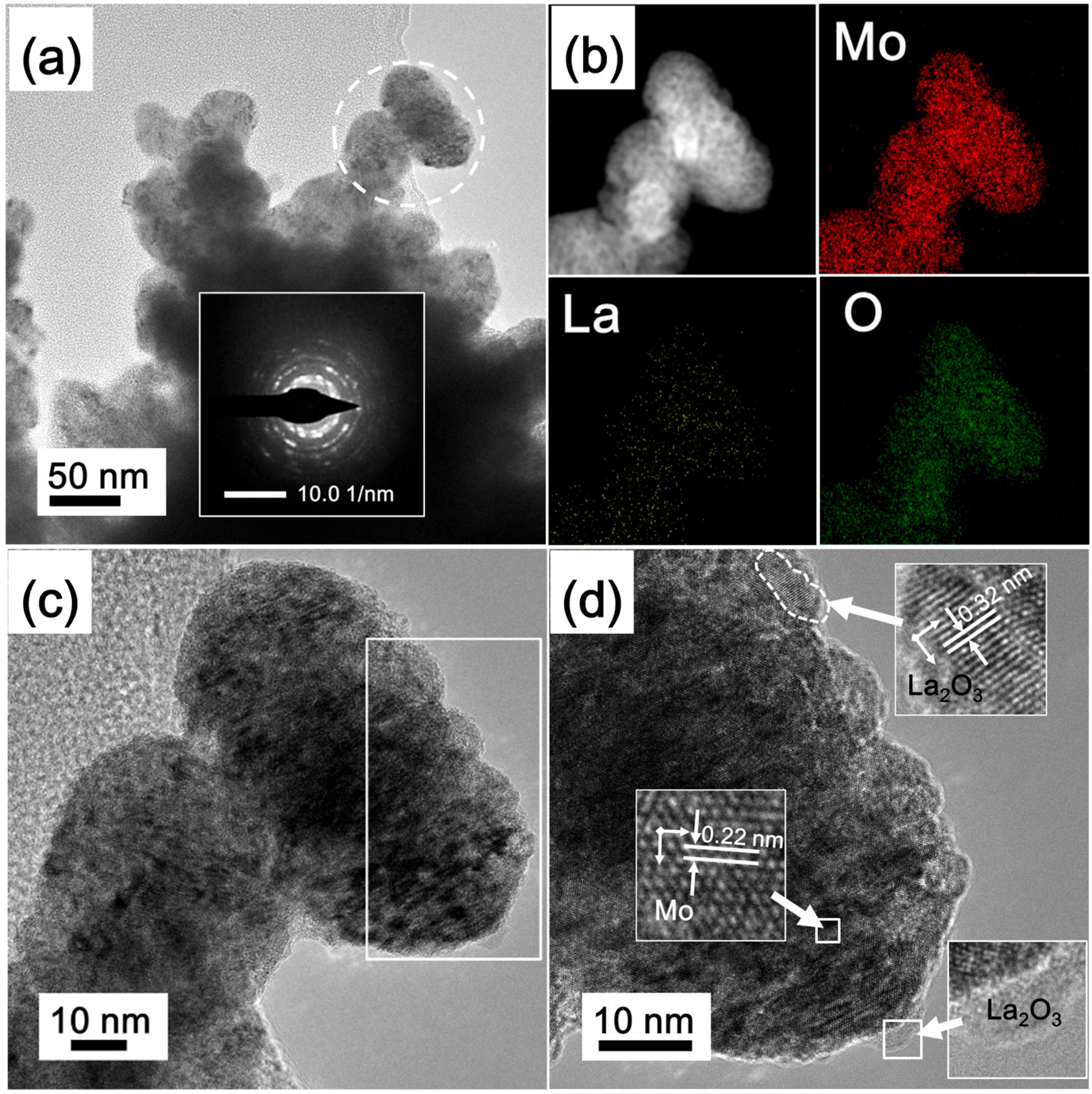
3.2. Preparation of La2O3 Doped Mo Nanopowders
3.3. Formation Mechanism of La2O3 Doped Mo Nanopowders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perepezko, J.H. The hotter the engine, the better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, G.J.; Jiang, F.; Ding, X.D.; Sun, Y.J.; Sun, J.; Ma, E. Nanostructured high-strength molybdenum alloys with unprecedented tensile ductility. Nat. Mater. 2013, 12, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Lenchuk, O.; Rohrer, J.; Albe, K. Atomistic modelling of zirconium and silicon segregation at twist and tilt grain boundaries in molybdenum. J. Mater. Sci. 2016, 51, 1873–1881. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Wei, S.; Pan, K.; Hu, Y. Preparation and characterization of Mo/Al2O3 composites. Int. J. Refract. Met. Hard Mater. 2016, 54, 186–195. [Google Scholar] [CrossRef]
- El-Genk, M.S.; Tournier, J.M. A review of refractory metal alloys and mechanically alloyed-oxide dispersion strengthened steels for space nuclear power systems. J. Nucl. Mater. 2005, 340, 93–112. [Google Scholar] [CrossRef]
- Conduit, B.D.; Jones, N.G.; Stone, H.J.; Conduit, G.J. Probabilistic design of a molybdenum-base alloy using a neural network. Scr. Mater. 2018, 146, 82–86. [Google Scholar] [CrossRef]
- Wang, K.S.; Tan, J.F.; Hu, P.; Yu, Z.T.; Yang, F.; Hu, B.L.; Song, R.; He, H.C.; Volinsky, A.A. La2O3 effects on TZM alloy recovery, recrystallization and mechanical properties. Mater. Sci. Eng. A 2015, 636, 415–420. [Google Scholar] [CrossRef]
- Cheng, P.M.; Zhang, G.J.; Zhang, J.Y.; Liu, G.; Sun, J. Coupling effect of intergranular and intragranular particles on ductile fracture of Mo-La2O3 alloys. Mater. Sci. Eng. A 2015, 640, 320–329. [Google Scholar] [CrossRef]
- Wang, L.; Liu, G.; Sun, J. Effects of La2O3 and annealing temperature on grain size and mechanical properties of Mo alloys. Mater. Res. Express 2017, 4, 116515. [Google Scholar] [CrossRef]
- Yang, X.; Tan, H.; Lin, N.; Li, Z.; He, Y. The influences of La doping method on the microstructure and mechanical properties of Mo alloys. Int. J. Refract. Met. Hard Mater. 2015, 51, 301–308. [Google Scholar] [CrossRef]
- Yang, X.; Tan, H.; Lin, N.; Li, Z.; He, Y. Effects of the lanthanum content on the microstructure and properties of the molybdenum alloy. Int. J. Refract. Met. Hard Mater. 2016, 61, 179–184. [Google Scholar] [CrossRef]
- Zhang, G.J.; Sun, Y.J.; Zuo, C.; Wei, J.F.; Sun, J. Microstructure and mechanical properties of multi-components rare earth oxide-doped molybdenum alloys. Mater. Sci. Eng. A 2008, 483, 350–352. [Google Scholar] [CrossRef]
- Cockeram, B.V. The fracture toughness and toughening mechanism of commercially available unalloyed molybdenum and oxide dispersion strengthened molybdenum with an equiaxed, large grain structure. Metall. Mater. Trans. A 2009, 40, 2843–2860. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Jia, Y.L.; Wang, M.P.; Li, Z.; Wang, Z.X. The microstructure and texture of Mo-La2O3 alloys with high transverse ductility. J. Alloy Compd. 2014, 589, 531–538. [Google Scholar] [CrossRef]
- Endo, M.; Kimura, K.; Udagawa, T.; Tanabe, S.; Seto, H. The effects of doping molybdenum wire with rare-earth elements. High Temp. High Press. 1990, 21, 129–137. [Google Scholar]
- Zhang, J.; Liu, L.; Zhou, M.; Hu, Y.; Zuo, T. Fracture toughness of sintered Mo-La2O3 alloy and the toughening mechanism. Int. J. Refract. Met. Hard Mater. 1999, 17, 405–409. [Google Scholar]
- Chen, P.; Qin, M.; Zhang, D.; Chen, Z.; Jia, B.; Wan, Q.; Wu, H.; Qu, X. Combustion synthesis and excellent photocatalytic degradation properties of W18O49. CrystEngComm 2015, 17, 5889–5894. [Google Scholar] [CrossRef]
- Huang, M.; Qin, M.; Chen, P.; Jia, B.; Chen, Z.; Li, R.; Liu, Z.; Qu, X. Facile preparation of network-like porous hematite (α-Fe2O3) nanosheets via a novel combustion-based route. Ceram. Int. 2016, 42, 10380–10388. [Google Scholar] [CrossRef]
- Bakrania, S.D.; Miller, T.A.; Perez, C.; Wooldridge, M.S. Combustion of multiphase reactants for the synthesis of nanocomposite materials. Combust. Flame 2007, 148, 76–87. [Google Scholar] [CrossRef]
- Wu, H.; Qin, M.; Chu, A.; Cao, Z.; Chen, P.; Liu, Y.; Qu, X. Effect of urea on the synthesis of Al-doped ZnO nanoparticle and its adsorptive properties for organic pollutants. Mater. Res. Bull. 2016, 75, 78–82. [Google Scholar] [CrossRef]
- Cao, Z.; Qin, M.; Gu, Y.; Jia, B.; Chen, P.; Qu, X. Synthesis and characterization of Sn-doped hematite as visible light photocatalyst. Mater. Res. Bull. 2016, 77, 41–47. [Google Scholar] [CrossRef]
- Chen, P.; Qin, M.; Liu, Y.; Jia, B.; Cao, Z.; Wan, Q.; Qu, X. Superior optical properties of Fe3+–W18O49 nanoparticles prepared by solution combustion synthesis. New J. Chem. 2015, 39, 1196–1201. [Google Scholar] [CrossRef]
- Gu, S.; Qin, M.; Zhang, H.; Ma, J.; Wu, H.; Qu, X. Facile solution combustion synthesis of MoO2 nanoparticles as efficient photocatalysts. CrystEngComm 2017, 19, 6516–6526. [Google Scholar] [CrossRef]
- Gangwar, B.P.; Palakollu, V.; Singh, A.; Kanvah, S.; Sharma, S. Combustion synthesized La2O3 and La(OH)3: recyclable catalytic activity towards Knoevenagel and Hantzsch reactions. RSC Adv. 2014, 4, 55407–55416. [Google Scholar] [CrossRef]
- Singh, A.; Palakollu, V.; Pandey, A.; Kanvah, S.; Sharma, S. Green synthesis of 1,4-benzodiazepines over La2O3 and La(OH)3 catalysts: possibility of Langmuir–Hinshelwood adsorption. RSC Adv. 2016, 6, 103455–103462. [Google Scholar] [CrossRef]
- Nowicki, W.; Piskuła, Z.S.; Kuźma, P.; Kirszensztejn, P. Synthesis and characterization of a binary system La2O3–SiO2 prepared by combustion method. J. Sol Gel Sci. Technol. 2017, 82, 574–580. [Google Scholar] [CrossRef]
- Gu, S.; Qin, M.; Zhang, H.; Ma, J.; Qu, X. Preparation of Mo nanopowders through hydrogen reduction of a combustion synthesized foam-like MoO2 precursor. Int. J. Refract. Met. Hard Mater. 2018, 76, 90–98. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Wang, J.S.; Chou, K.C. Study on hydrogen reduction of ultrafine MoO2 to produce ultrafine Mo. J. Phys. Chem. C 2016, 120, 4097–4103. [Google Scholar] [CrossRef]
- Ressler, T.; Jentoft, R.E.; Wienold, J.; Günter, M.M.; Timpe, O. In stu XAS and XRD studies on the formation of Mo suboxides during reduction of MoO3. J. Phys. Chem. B 2000, 104, 6360–6370. [Google Scholar] [CrossRef]
- Liang, C.; Ying, P.; Li, C. Nanostructured β-Mo2C prepared by carbothermal hydrogen reduction on ultrahigh surface area carbon material. Chem. Mater. 2002, 14, 3148–3151. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, M.; Xing, Y.; Wang, S.; Zhang, W.; Lv, F.; Li, Y.; Zhang, Y.; Wang, W.; Guo, S. A Universal strategy for intimately coupled carbon nanosheets/MoM nanocrystals (M = P, S, C, and O) hierarchical hollow nanospheres for hydrogen evolution catalysis and sodium-ion storage. Adv. Mater. 2018, 30, 1706085. [Google Scholar] [CrossRef] [PubMed]




| Precursor | La2O3 Doping Content (wt.%) | AHM (mol) | NH4NO3 (mol) | C2H5O2N (mol) | La(NO3)3 (mol) |
|---|---|---|---|---|---|
| P-LM0 | 0 | 0.01 | 0.2 | 0.10 | 0 |
| P-LM3 | 0.3 | 0.01 | 0.2 | 0.10 | 0.00012 |
| P-LM6 | 0.6 | 0.01 | 0.2 | 0.10 | 0.00025 |
| P-LM9 | 0.9 | 0.01 | 0.2 | 0.10 | 0.00037 |
| P-LM12 | 1.2 | 0.01 | 0.2 | 0.10 | 0.00050 |
| Precursor | MoO2 (wt.%) | MoO3 (wt.%) | Mo4O11 (wt.%) |
|---|---|---|---|
| P-LM0 | 52.6 | 38.8 | 8.6 |
| P-LM3 | 51.7 | 38.7 | 9.6 |
| P-LM6 | 51.0 | 39.1 | 9.9 |
| P-LM9 | 48.9 | 41.6 | 9.3 |
| P-LM12 | 48.6 | 42.8 | 8.6 |
| Precursors | P-LM0 | P-LM3 | P-LM6 | P-LM9 | P-LM12 |
|---|---|---|---|---|---|
| Elements C (wt.%) | 0.27 | 0.17 | 0.20 | 0.18 | 0.13 |
| Elements La (wt.%) | 0 | 0.13 | 0.38 | 0.59 | 0.81 |
| Content | Process | Samples | ||||
|---|---|---|---|---|---|---|
| LM0 | LM3 | LM6 | LM9 | LM12 | ||
| C (wt.%) | H2/600 °C | 0.286 | 0.219 | 0.241 | 0.236 | 0.183 |
| Ar/500 °C + H2/600 °C | 0.046 | 0.043 | 0.053 | 0.051 | 0.042 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Qin, M.; Zhang, H.; Ma, J. Fabrication of La2O3 Uniformly Doped Mo Nanopowders by Solution Combustion Synthesis Followed by Reduction under Hydrogen. Materials 2018, 11, 2385. https://doi.org/10.3390/ma11122385
Gu S, Qin M, Zhang H, Ma J. Fabrication of La2O3 Uniformly Doped Mo Nanopowders by Solution Combustion Synthesis Followed by Reduction under Hydrogen. Materials. 2018; 11(12):2385. https://doi.org/10.3390/ma11122385
Chicago/Turabian StyleGu, Siyong, Mingli Qin, Houan Zhang, and Jidong Ma. 2018. "Fabrication of La2O3 Uniformly Doped Mo Nanopowders by Solution Combustion Synthesis Followed by Reduction under Hydrogen" Materials 11, no. 12: 2385. https://doi.org/10.3390/ma11122385





