The Shape-Memory Effect of Hindered Phenol (AO-80)/Acrylic Rubber (ACM) Composites with Tunable Transition Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparations
2.3. Methods
3. Results
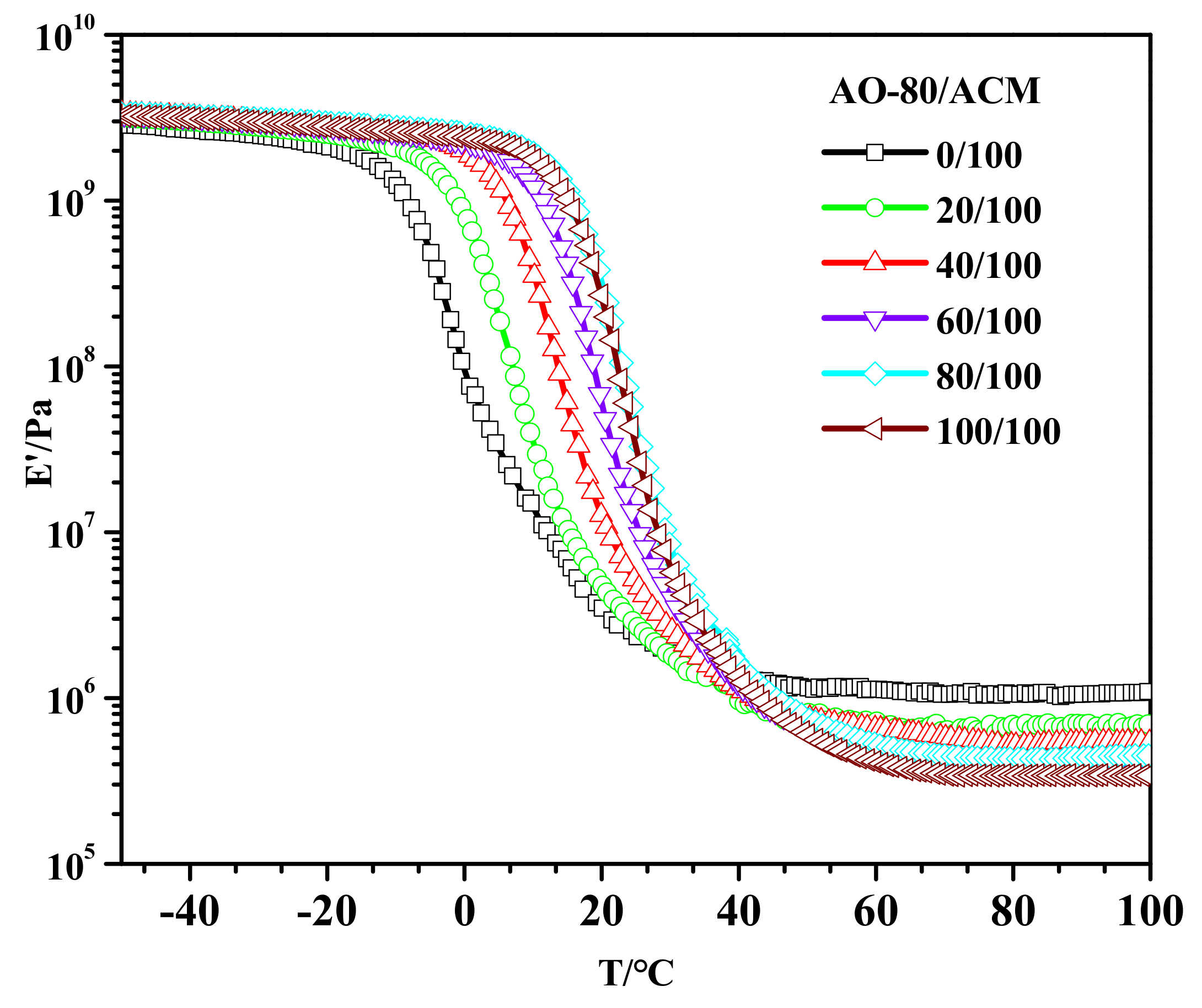
3.1. Tg of AO-80/ACM Rubber Composites
3.2. FT-IR of AO-80/ACM Rubber Composites
3.3. Static Mechanical Properties of AO-80/ACM Rubber Composites
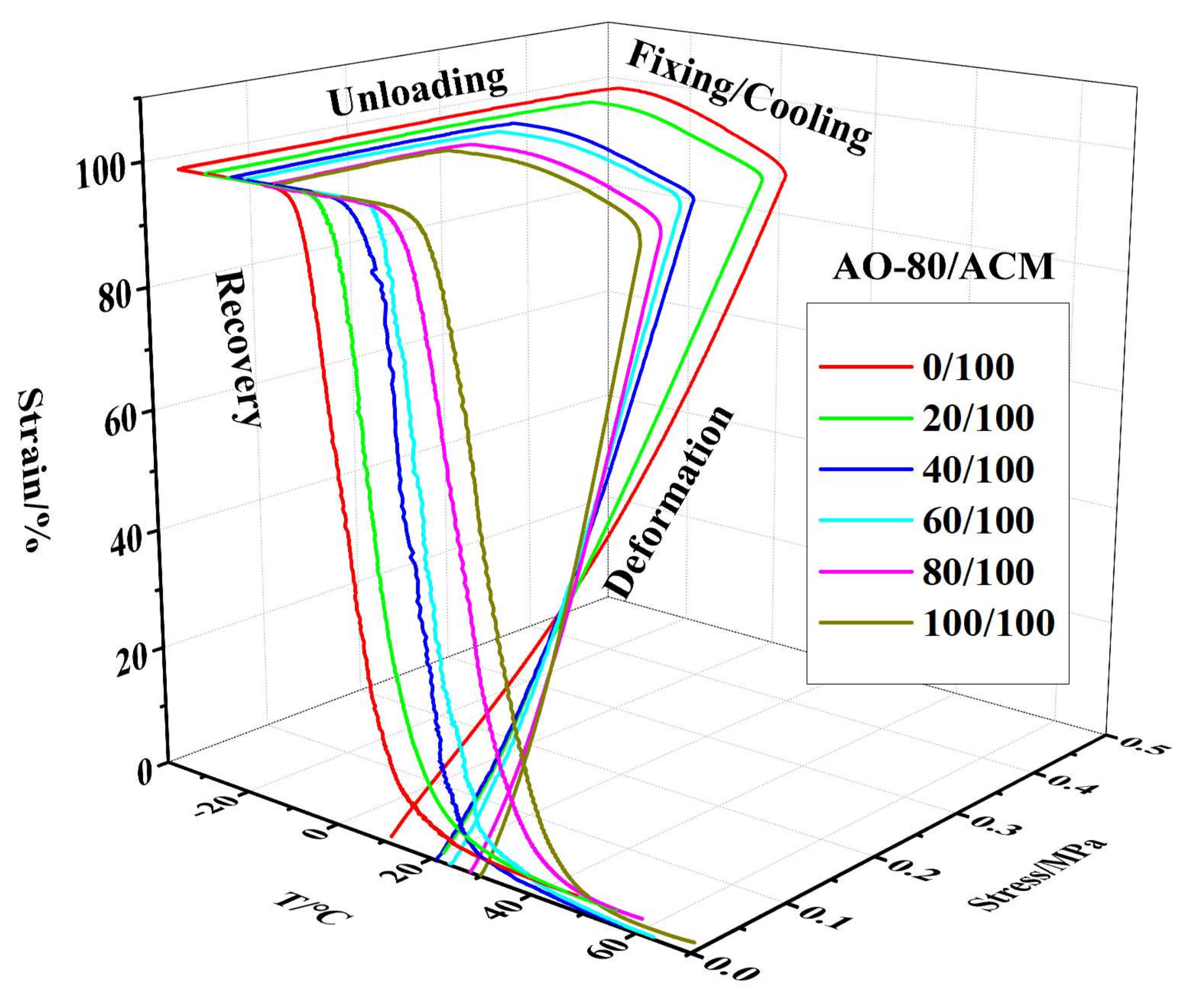
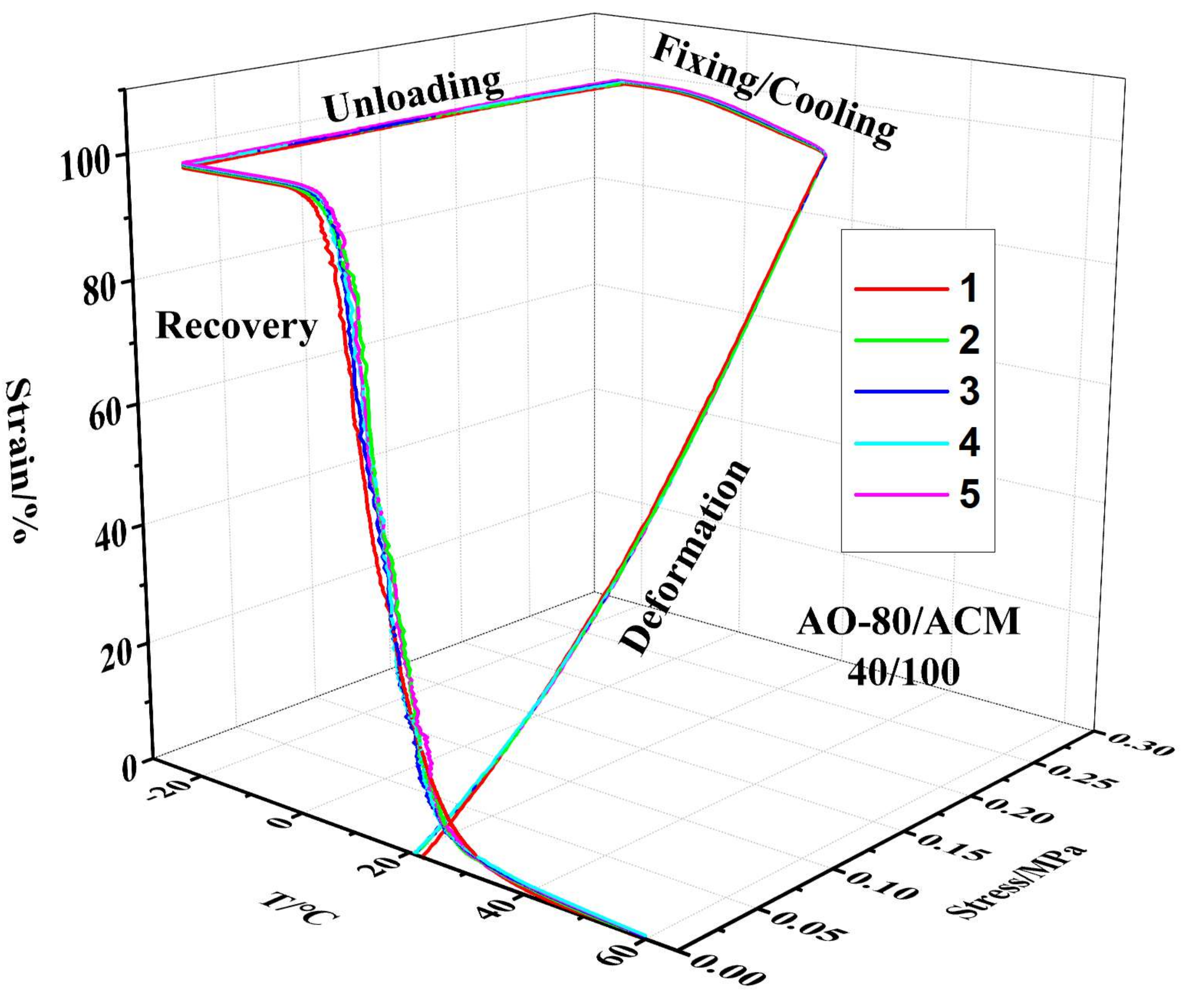
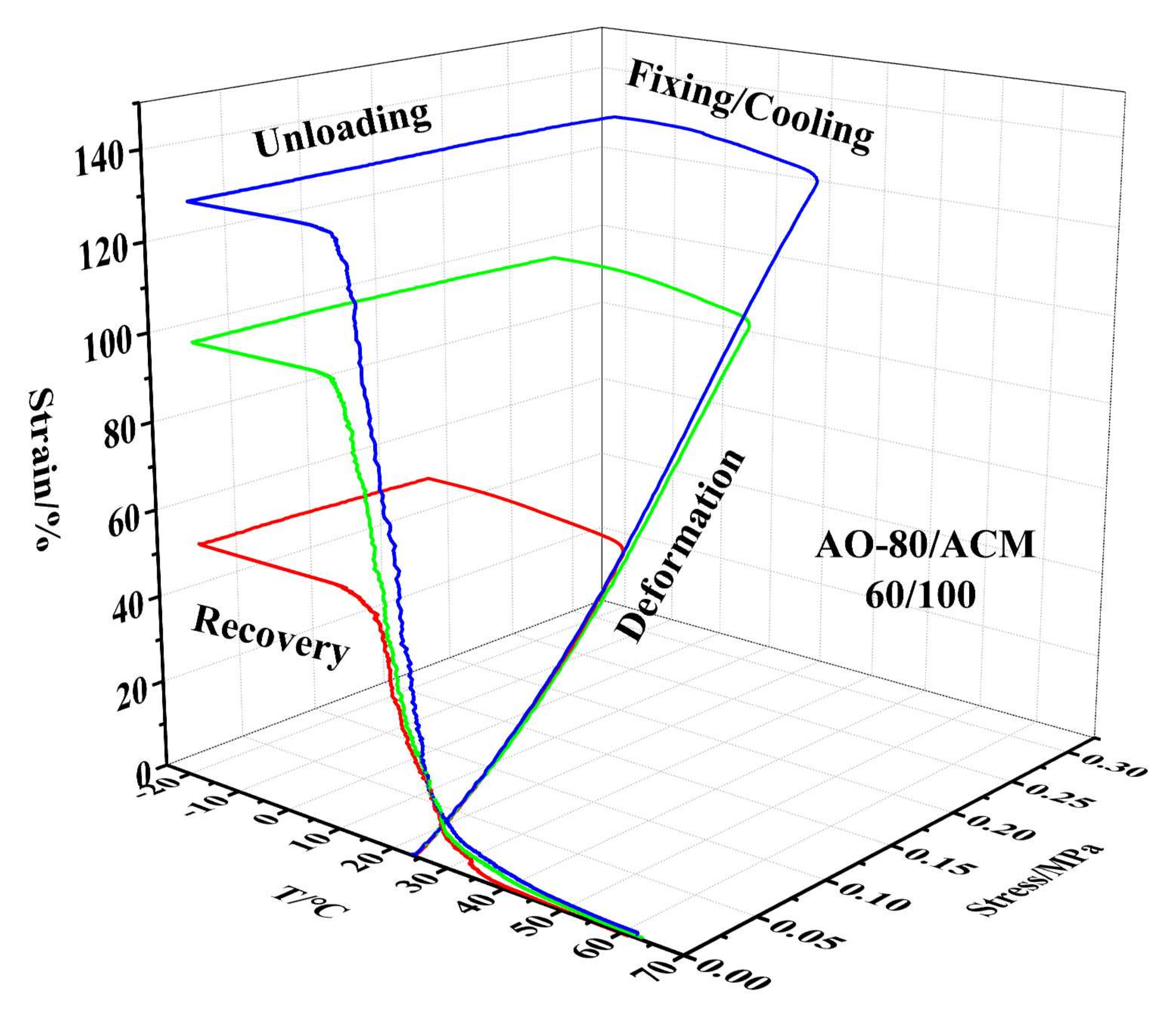
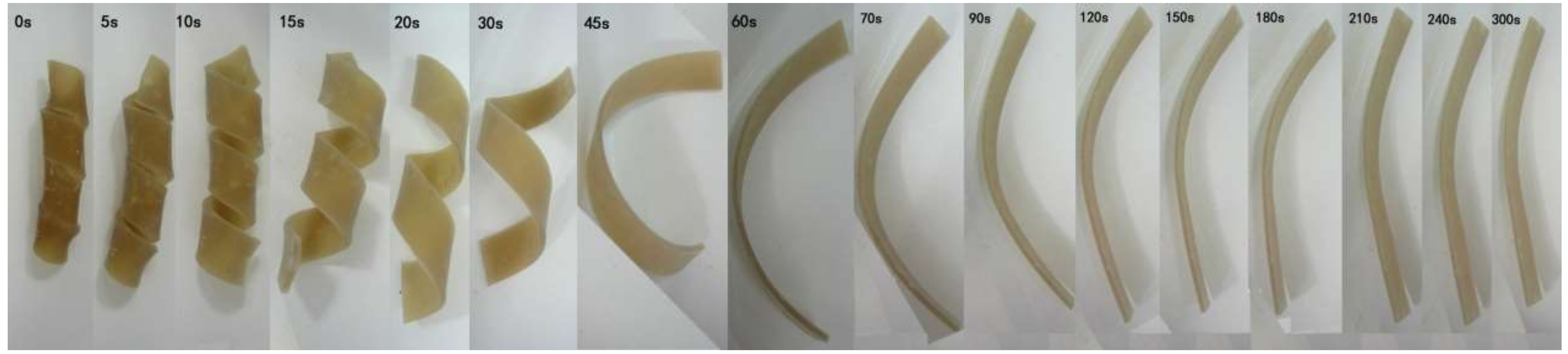
3.4. Shape-Memory Effect of AO-80/ACM Rubber Composite
3.5. Dynamic Mechanical Properties of AO-80/ACM Rubber Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiss, R.A.; Izzo, E.; Mandelbaum, S. New Design of Shape Memory Polymers: Mixtures of an Elastomeric Ionomer and Low Molar Mass Fatty Acids and Their Salts. Macromolecules 2008, 41, 2978–2980. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-responsive shape memory materials: A review. Mater. Des. 2012, 33, 577–640. [Google Scholar] [CrossRef]
- Leng, J.S.; Lan, X.; Liu, Y.L.; Du, S.Y. Shape-memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Lendlein, A.; Jiang, H.Y.; Jünger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, Q.; Loredana, S.; Anna, S.E. Shape memory epoxy foams for space applications. Mater. Lett. 2012, 69, 20–23. [Google Scholar] [CrossRef]
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Devices 2010, 7, 357–379. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.P.; Gall, K.; Dunn, M.L.; Mcculskey, P. Thermomechanics of shape memory polymer nanocomposites. Mech. Mater. 2004, 36, 929–940. [Google Scholar] [CrossRef]
- Squeo, E.A.; Quadrini, F. Shape memory epoxy foams by solid-state foaming. Smart Mater. Struct. 2010, 19, 533–536. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Han, C.M.; Tan, H.F.; Du, X.W. Thermal, mechanical and shape memory properties of shape memory epoxy resin. Mater. Sci. Eng. A 2010, 527, 2510–2514. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.M.; Li, C.; Li, L. Effects of moisture on the thermomechanical properties of a polyurethane shape memory polymer. Polymer 2006, 47, 1348–1356. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; An, L.; Li, C. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005, 86, 114105–114108. [Google Scholar] [CrossRef]
- Lee, K.M.; Koerner, H.; Vaia, R.A.; Bunning, T.J.; White, T.J. Light-activated shape memory of glassy, Azobenzene liquid crystalline polymer networks. Soft Matter 2011, 7, 4318–4324. [Google Scholar] [CrossRef]
- Koerner, H.; Price, G.; Pearce, N.A.; Alxander, M.; Vaia, R.A. Remotely actuated polymer nanocomposites-stress-recovery of carbon-nanotube-filled thermoplastic elastomers. Nat. Mater. 2004, 3, 115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Gong, X.L.; Kang, Y.; Jiang, Z.C.; Zhang, S.; Li, B.J. Light-, pH- and thermal-responsive hydrogels with the triple-shape memory effect. Chem. Commun. 2016, 52, 10609–10612. [Google Scholar] [CrossRef]
- Yang, J.; Wen, H.; Zhuo, H.; Chen, S.; Ban, J. A new type of photo-thermo staged-responsive shape-memory polyurethanes network. Polymers 2017, 9, 287–297. [Google Scholar] [CrossRef]
- Ji, F.L.; Zhu, Y.; Hu, J.L.; Liu, Y.; Yeung, L.Y.; Ye, G.D. Smart polymer fibers with shape memory effect. Smart Mater. Struct. 2006, 15, 1547. [Google Scholar] [CrossRef]
- Du, F.P.; Ye, E.Z.; Yang, W.; Shen, T.H.; Tang, C.Y.; Xie, X.L.; Zhou, X.P.; Law, W.C. Electroactive shape memory polymer based on optimized multi-walled carbon nanotubes/polyvinyl alcohol nanocomposites. Compos. Part B 2015, 68, 170–175. [Google Scholar] [CrossRef]
- Lu, H.B.; Liu, Y.J.; Gou, J.H.; Leng, J.S.; Du, S.Y. Synergistic effect of carbon nanofiber and carbon nanopaper on shape memory polymer composite. Appl. Phys. Lett. 2010, 96, 879. [Google Scholar] [CrossRef]
- Lu, H.B.; Liu, Y.J.; Gou, J.H.; Leng, J.S.; Du, S.Y. Synergistic effect of carbon nanofiber and sub-micro filamentary nickel nanostrand on the shape memory polymer nanocomposite. Smart Mater. Struct. 2011, 20, 035017–035023. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; Dong, Z.Q.; Fan, M.M.; Liu, Y.; Li, J.H.; Wang, Y.F.; Yuan, Q.J. pH-induced shape-memory polymers. Macromol. Rapid Commun. 2012, 33, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xiao, P.; Gu, J.; Wen, X.; Xu, J.; Zhao, C.; Zhang, J.; Chen, T. Self-healable macro-/microscopic shape memory hydrogels based on supramolecular interactions. Chem. Commun. 2014, 50, 12277–12280. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zheng, J.; Wen, X.; Cai, Z.; Zhang, J.; Chen, T. Ph- and sugar-induced shape memory hydrogel based on reversible phenylboronic acid–diol ester bonds. Macromol. Rapid Commun. 2015, 36, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.; Li, H.Z.; Lu, Z.; Rehman, S.; Siddig, M.; Yang, H.Y. A shape memory hydrogel induced by the interactions between metal ions and phosphate. Soft Matter 2014, 10, 972. [Google Scholar] [CrossRef]
- Ahn, S.; Deshmukh, P.; Kasi, R.M. Shape Memory Behavior of Side-Chain Liquid Crystalline Polymer Networks Triggered by Dual Transition Temperatures. Macromolecules 2010, 43, 7330–7340. [Google Scholar] [CrossRef]
- Liu, C.D.; Chun, S.B.; Mather, P.T.; Zhang, L.; Haley, E.H.; Coughlin, E.B. Chemically Cross-Linked Polycyclooctene: Synthesis, Characterization, and Shape Memory Behavior. Macromolecules 2002, 35, 9868–9874. [Google Scholar] [CrossRef]
- Liu, G.; Ding, X.; Cao, Y.; Zheng, Z.H.; Peng, Y.X. Shape Memory of Hydrogen-Bonded Polymer Network/Poly(ethylene glycol) Complexes. Macromolecules 2014, 37, 2228–2232. [Google Scholar] [CrossRef]
- Cao, Y.P.; Guan, Y.; Du, J.; Luo, J.; Peng, Y.X.; Chan, A.S.C. Hydrogen-bonded polymer network-poly(ethylene glycol) complexes with shape memory effect. J. Mater. Chem. 2002, 12, 2957–2960. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Li, J.; Viveros, J.A.; Wrue, M.H.; Anthamatten, M. Shape-memory effects in polymer networks containing reversibly associating side-groups. Adv. Mater. 2007, 19, 2851–2855. [Google Scholar] [CrossRef]
- Ware, T.; Hearon, K.; Lonnecker, A.; Wooley, K.L.; Maitland, D.J.; Voit, W. Triple-Shape Memory Polymers Based on Self-Complementary Hydrogen Bonding. Macromolecules 2012, 45, 1062. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.B.; Liu, Y.J.; Leng, J.S. Nanocomposites of epoxy-based shape memory polymer and thermally reduced graphite oxide: Mechanical, thermal and shape memory characterizations. Compos. Part B 2016, 91, 75–82. [Google Scholar] [CrossRef]
- Lendlein, A.; Behl, M. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Cao, Y.J.; Zou, H.; Li, J.; Zhang, L.Q. Structure and Dynamic Properties of Nitrile- Butadiene Rubber /Hindered Phenol Composites. J. Appl. Polym. Sci. 2011, 123, 3696–3702. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Xiang, P.; Cao, Y.J.; Tian, M.; Fond, H.; Jin, R.G.; Zhang, L.Q. Nitrile butadiene rubber/hindered phenol nanocomposites with improved strength and high damping performance. Polymer 2007, 48, 6056–6063. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Kelch, S.; Lendlein, A. Polymers Move in Response to Light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Ramanan, S.P.; Jose, S.; Siengchin, S.; Magueresse, A.; Janke, A.; Pionteck, J. Shape memory properties of epoxy/PPO-PEO-PPO triblock copolymer blends with tunable thermal transitions and mechanical characteristics. Ind. Eng. Chem. Res. 2017, 56, 14069–14077. [Google Scholar] [CrossRef]
- Kumar, K.S.S.; Biju, R.; Nair, C.P.R. Progress in shape memory epoxy resins. React. Funct. Polym. 2013, 73, 421–430. [Google Scholar] [CrossRef]
- Yu, R.; Yang, X.; Zhang, Y.; Zhao, X.; Wu, X.; Zhao, T.; Zhao, Y.; Huang, W. Three-dimensional printing of shape memory composites with epoxy-acrylate hybrid photopolymer. ACS Appl. Mater. Interfaces 2017, 9, 1820–1829. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Liu, Y.; Leng, J.; Bhattacharyya, D. Electrical actuation properties of reduced graphene oxide paper/epoxy-based shape memory composites. Compos. Sci. Technol. 2015, 106, 20–24. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Kéki, S. Review of Progress in Shape Memory Epoxies and Their Composites. Polymers 2018, 10, 34. [Google Scholar] [CrossRef]
- Xiao, D.L.; Zhao, X.Y.; Feng, Y.P.; Xiang, P.; Zhang, L.Q.; Wang, W.M. The structure and dynamic properties of thermoplastic polyurethane elastomer/hindered phenol hybrids. J. Appl. Polym. Sci. 2010, 116, 2143–2150. [Google Scholar] [CrossRef]
- Ghobadi, E.; Heuchel, M.; Kratz, K.; Lendlein, A. Atomistic Simulation of the Shape-Memory Effect in Dry and Water Swollen Poly[(rac-lactide)-co-glycolide] and Copolyester Urethanes Thereof. Macromol. Chem. Phys. 2014, 215, 65–75. [Google Scholar] [CrossRef]
- Ghobadi, E.; Heuchel, M.; Kratz, K.; Lendlein, A. Simulation of volumetric swelling of degradable poly[(rac-lactide)-co-glycolide] based polyesterurethanes containing different urethane-linkers. J. Appl. Biomater. Funct. Mater. 2013, 10, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Mou, H.Y.; Shen, F.; Xu, H.Y.; Hu, G.H.; Wu, C.F. Hydrogenated nitrile butadiene rubber and hindered phenol composite. II. Characterization of hydrogen bonding. Polym. Eng. Sci. 2011, 51, 201–208. [Google Scholar] [CrossRef]
- Wu, C.F. Microstructural development of a vitrified hindered phenol compound during thermal annealing. Polymer 2003, 44, 1697–1703. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lu, Y.L.; Xiao, D.L.; Wu, S.Z.; Zhang, L.Q. Thermoplastic Ternary Hybrids of Polyurethane, Hindered Phenol and Hindered Amine with Selective Two-Phase Dispersion. Macromol. Mater. Eng. 2009, 294, 345–351. [Google Scholar] [CrossRef]
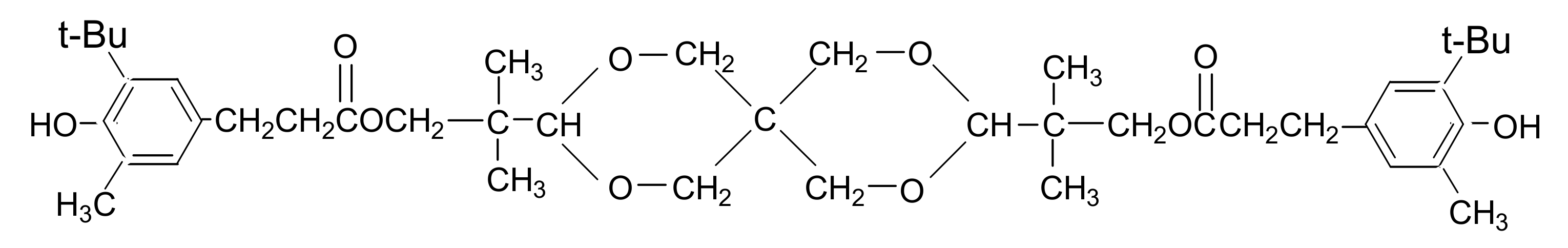





| Properties | Loadings of AO-80/phr | |||||
|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | 80 | 100 | |
| Hardness (Shore A) | 41 ± 0 | 48 ± 0 | 68 ± 0 | 78 ± 0 | 93 ± 0 | 95 ± 0 |
| Tensile strength (MPa) | 1.5 ± 0.2 | 1.9 ± 0.1 | 4.0 ± 0.2 | 7.7 ± 0.1 | 8.2 ± 0.1 | 9.2 ± 0.2 |
| Elongation at break (%) | 210 ± 9 | 248 ± 11 | 295 ± 12 | 336 ± 8 | 369 ± 8 | 377 ± 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.-k.; Chen, S.; Zhao, X.-y.; Guo, M.-m.; Zhang, L.-q. The Shape-Memory Effect of Hindered Phenol (AO-80)/Acrylic Rubber (ACM) Composites with Tunable Transition Temperature. Materials 2018, 11, 2461. https://doi.org/10.3390/ma11122461
Hu S-k, Chen S, Zhao X-y, Guo M-m, Zhang L-q. The Shape-Memory Effect of Hindered Phenol (AO-80)/Acrylic Rubber (ACM) Composites with Tunable Transition Temperature. Materials. 2018; 11(12):2461. https://doi.org/10.3390/ma11122461
Chicago/Turabian StyleHu, Shi-kai, Si Chen, Xiu-ying Zhao, Ming-ming Guo, and Li-qun Zhang. 2018. "The Shape-Memory Effect of Hindered Phenol (AO-80)/Acrylic Rubber (ACM) Composites with Tunable Transition Temperature" Materials 11, no. 12: 2461. https://doi.org/10.3390/ma11122461





