Synthesis and Preparation of Chitosan/Clay Microspheres: Effect of Process Parameters and Clay Type
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
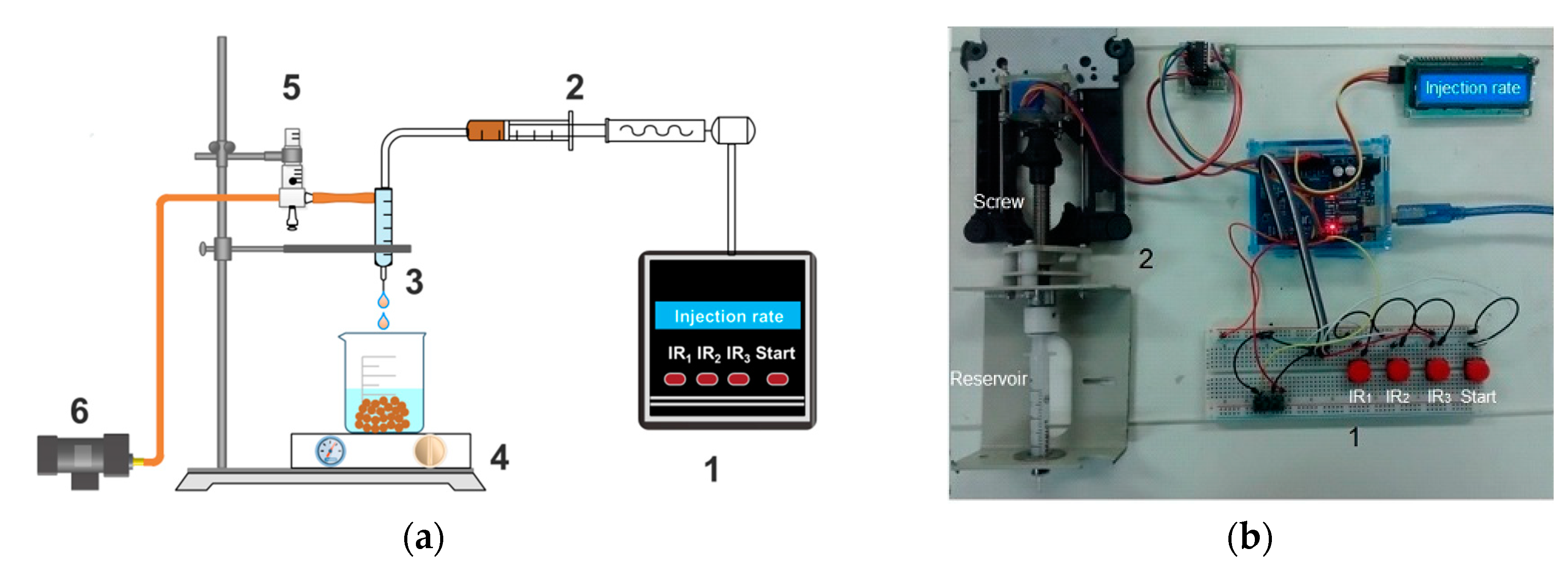
2.2. Preparation of Chitosan/Clay Microspheres
2.3. Characterization
2.3.1. Determination of the Dimensions of Chitosan/Clay Microspheres
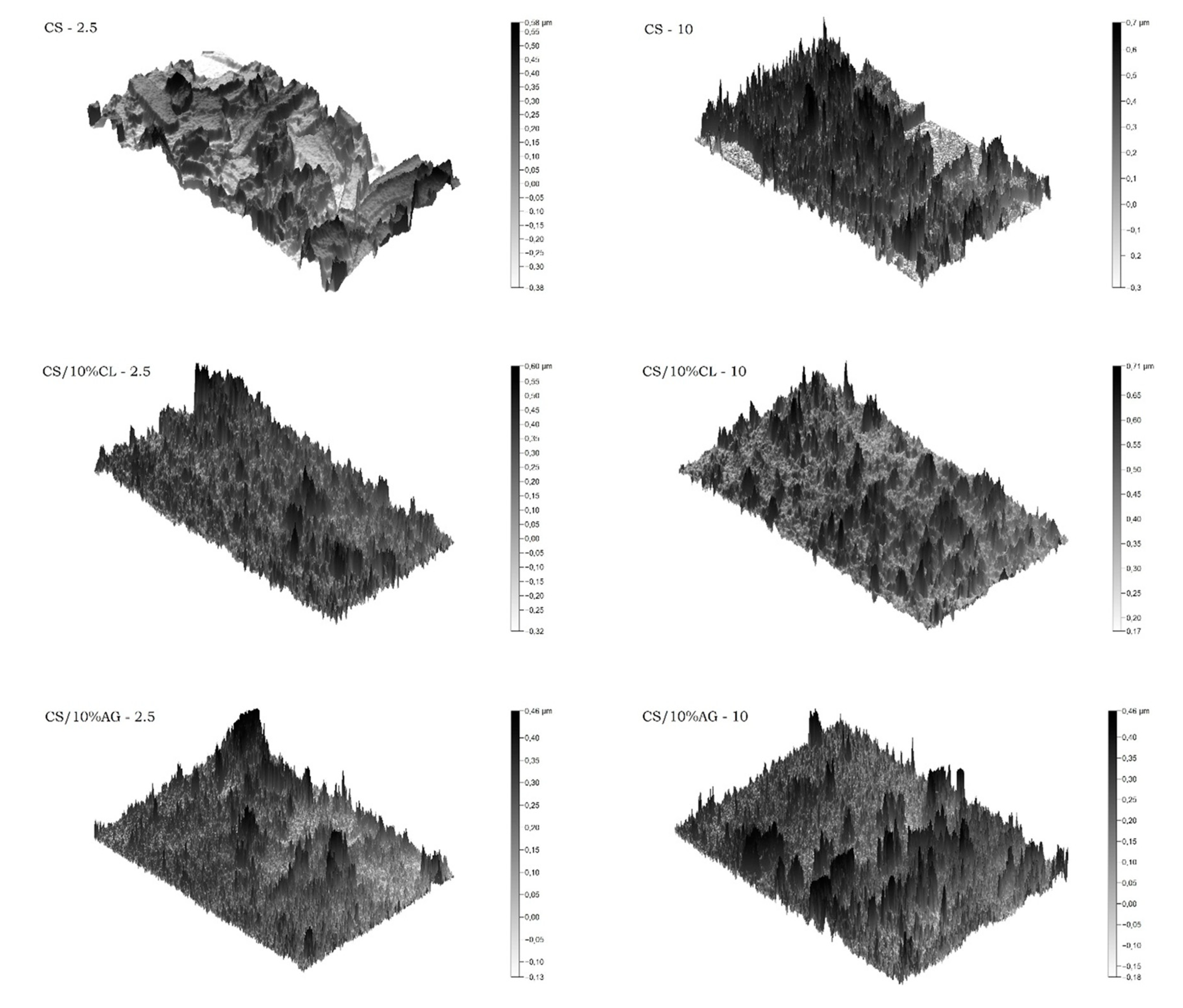
2.3.2. Microspheres Morphology
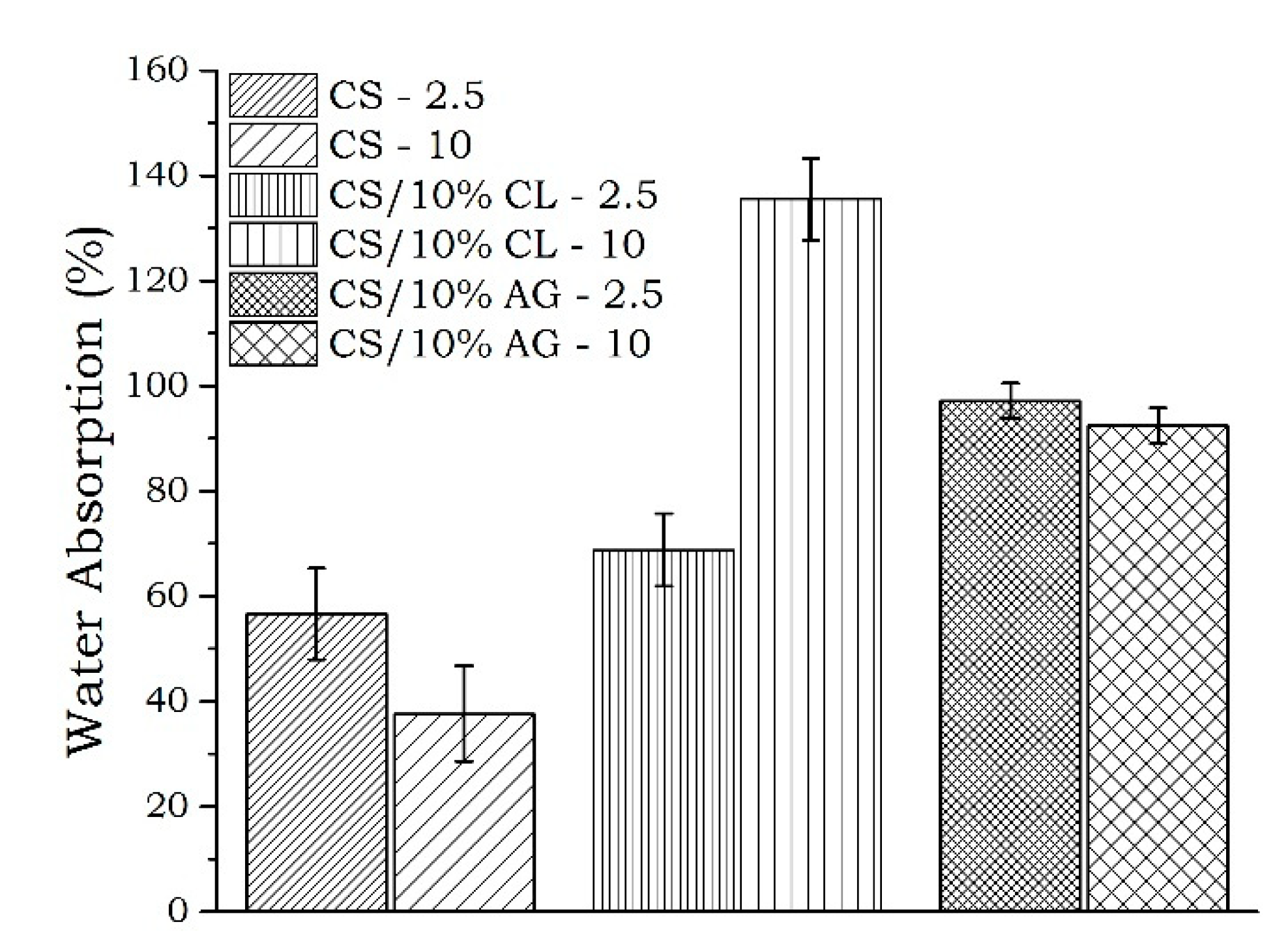
2.3.3. Water Absorption
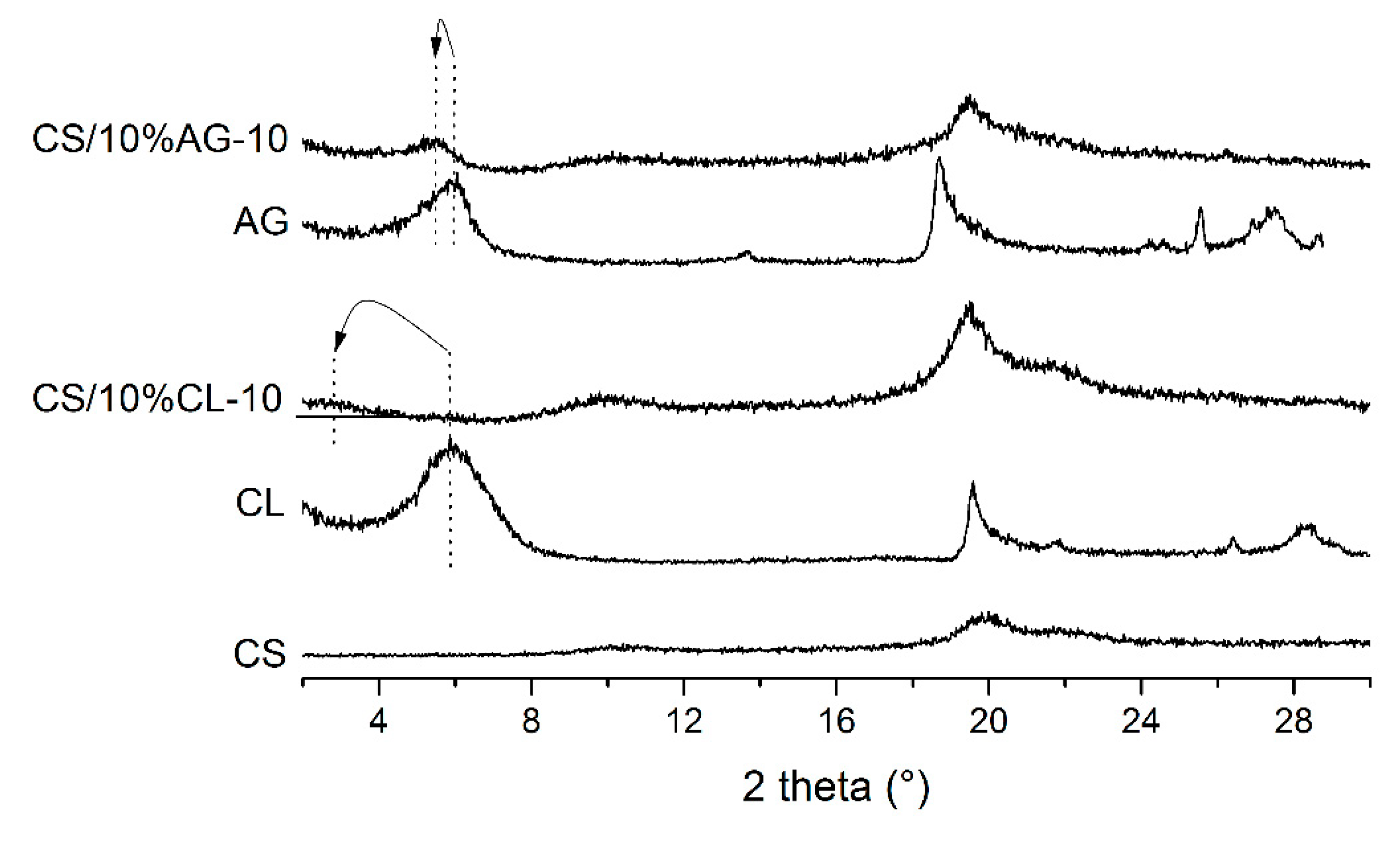
3. Results and Discussion
Optical Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Xue, M.; Hu, S.; Lu, Y.; Zhang, Y.; Jiang, X.; An, S.; Guo, Y.; Zhou, X.; Hou, H.; Jiang, C. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int. J. Pharm. 2015, 495, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Cossu, M.; Gavini, E.; Giunchedi, P.; Dalpiaz, A. Particulate formulations based on chitosan for nose-to-brain delivery of drugs. A review. J. Drug Deliv. Sci. Technol. 2016, 32, 77–87. [Google Scholar] [CrossRef]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ilium, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Remunan-Lopez, C.; Bodmeier, R. Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J. Control. Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Enhancement of alendronate encapsulation in chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2015, 30, 391–396. [Google Scholar] [CrossRef]
- Erel, G.; Kotmakçı, M.; Akbaba, H.; Karadağlı, S.S.; Kantarcı, A.G. Nanoencapsulated chitosan nanoparticles in emulsion-based oral delivery system: In vitro and in vivo evaluation of insulin loaded formulation. J. Drug Deliv. Sci. Technol. 2016, 36, 161–167. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V. Alginate/chitosan microparticles for tamoxifen delivery to the lymphatic system. Int. J. Pharm. 2009, 367, 127–132. [Google Scholar] [CrossRef]
- Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Gallucci, M.C.; Luppi, B. Mucoadhesive buccal tablets based on chitosan/gelatin microparticles for delivery of propranolol hydrochloride. J. Pharm. Sci. 2015, 104, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Parolin, C.; Vitali, B.; Bigucci, F.; Gallucci, M.; Nicoletta, F.; Luppi, B. Microparticles based on chitosan/carboxymethylcellulose polyelectrolyte complexes for colon delivery of vancomycin. Carbohyd. Polym. 2016, 143, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H. Chitosan microparticles. J. Drug Deliv. Sci. Technol. 2010, 20, 15–22. [Google Scholar] [CrossRef]
- Shu, X.; Zhu, K. Controlled drug release properties of ionically cross-linked chitosan beads: The influence of anion structure. Int. J. Pharm. 2002, 233, 217–225. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Siepmann, F.; Siepmann, J.; Pongjanyakul, T. Chitosan-clay nanocomposite microparticles for controlled drug delivery: Effects of the MAS content and TPP crosslinking. J. Drug Deliv. Sci. Technol. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Sorby, D.L.; Liu, G. Effects of adsorbents on drug absorption II: Effect of an antidiarrhea mixture on promazine absorption. J. Pharm. Sci. 1966, 55, 504–510. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Peppas, N. Devices based on intelligent biopolymers for oral protein delivery. Int. J. Pharm. 2004, 277, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Khunawattanakul, W.; Puttipipatkhachorn, S. Physicochemical characterizations and release studies of nicotine–magnesium aluminum silicate complexes. Appl. Clay Sci. 2009, 44, 242–250. [Google Scholar] [CrossRef]
- Rojtanatanya, S.; Pongjanyakul, T. Propranolol–magnesium aluminum silicate complex dispersions and particles: Characterization and factors influencing drug release. Int. J. Pharm. 2010, 383, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Khunawattanakul, W.; Puttipipatkhachorn, S.; Rades, T.; Pongjanyakul, T. Chitosan–magnesium aluminum silicate composite dispersions: Characterization of rheology, flocculate size and zeta potential. Int. J. Pharm. 2008, 351, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Khunawattanakul, W.; Puttipipatkhachorn, S.; Rades, T.; Pongjanyakul, T. Chitosan–magnesium aluminum silicate nanocomposite films: Physicochemical characterization and drug permeability. Int. J. Pharm. 2010, 393, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Khunawattanakul, W.; Puttipipatkhachorn, S.; Rades, T.; Pongjanyakul, T. Novel chitosan–magnesium aluminum silicate nanocomposite film coatings for modified-release tablets. Int. J. Pharm. 2011, 407, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Rácz, I.; Marcovich, N.E. Structure and properties of nanocomposite films based on sodium caseinate and nanocellulose fibers. J. Food Eng. 2011, 103, 76–83. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef]
- Sinha, V.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.M.; Niu, G.C.C.; Chang, S.J.; Kuo, C.H.; Bair, M.S. A one-step method for fabricating chitosan microspheres. J. Appl. Polym. Sci. 2004, 94, 2150–2157. [Google Scholar] [CrossRef]
- Jameela, S.; Jayakrishnan, A. Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery vehicle: Studies on the in vitro release of mitoxantrone and in vivo degradation of microspheres in rat muscle. Biomaterials 1995, 16, 769–775. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999, 187, 53–65. [Google Scholar] [CrossRef]
- Denkbaş, E.B.; Kilicay, E.; Birlikseven, C.; Öztürk, E. Magnetic chitosan microspheres: Preparation and characterization. React. Funct. Polym. 2002, 50, 225–232. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Gu, Y.-H.; Zhou, Q.-Z.; Ma, G.-H.; Wan, Y.-H.; Su, Z.-G. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf. B 2006, 50, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Ma, G.-H.; Su, Z.-G. Preparation of uniform sized chitosan microspheres by membrane emulsification technique and application as a carrier of protein drug. J. Control. Release 2005, 106, 62–75. [Google Scholar] [CrossRef]
- Barbosa, H.D.C.; Santos, B.F.F.D.; Tavares, A.A.; Barbosa, R.C.; Fook, M.V.L.; Canedo, E.L.; Silva, S.M.D.L. Inexpensive Apparatus for Fabricating Microspheres for 5-Fluorouracil Controlled Release Systems. Int. J. Chem. Eng. 2018, 2018. [Google Scholar] [CrossRef]
- Il’Ina, A.; Varlamov, V. Hydrolysis of chitosan in lactic acid. Appl. Biochem. Microbiol. 2004, 40, 300–303. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Leite, I.F.; Soares, A.P.; Carvalho, L.H.; Raposo, C.M.O.; Malta, O.M.L.; Silva, S.M.L. Characterization of pristine and purified organobentonites. J. Therm. Anal. Calorim. 2009, 100, 563–569. [Google Scholar] [CrossRef]
- Utracki, L.A. Clay-Containing Polymeric Nanocomposites; Rapra Technology: Shropshire, UK, 2004; Volume 1. [Google Scholar]
- Depan, D.; Kumar, A.P.; Singh, R.P. Preparation and characterization of novel hybrid of chitosan-g-lactic acid and montmorillonite. J. Biomed. Mater. Res. A 2006, 78, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Orrego, C.E.; Valencia, J.S. Preparation and characterization of chitosan membranes by using a combined freeze gelation and mild crosslinking method. Bioproc. Biosyst. Eng. 2009, 32, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Van de Belt, H.; Neut, D.; Uges, D.; Schenk, W.; Van Horn, J.; Van der Mei, H.; Busscher, H. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000, 21, 1981–1987. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohyd. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Baskar, D.; Kumar, T.S. Effect of deacetylation time on the preparation, properties and swelling behavior of chitosan films. Carbohyd. Polymers 2009, 78, 767–772. [Google Scholar] [CrossRef]
- Luo, D.; Sang, L.; Wang, X.; Xu, S.; Li, X. Low temperature, pH-triggered synthesis of collagen–chitosan–hydroxyapatite nanocomposites as potential bone grafting substitutes. Mater. Lett. 2011, 65, 2395–2397. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.; Peppley, B.; Bui, V.T. Ionic conductivity of chitosan membranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]
- Baklagina, Y.; Klechkovskaya, V.; Kononova, S.; Petrova, V.; Poshina, D.; Orekhov, A.; Skorik, Y. Polymorphic Modifications of Chitosan. Crystallogr. Rep. 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Paiva, L.D.; Morales, A.; DÍAZ, F.V. Argilas organofílicas: Características, metodologias de preparação, compostos de intercalação e técnicas de caracterização. Cerâmica 2008, 54, 213–226. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Y.; Szeto, Y.-S.; Liao, L. A novel method to prepare chitosan/montmorillonite nanocomposites in the presence of hydroxy-aluminum oligomeric cations. Compos. Sci. Technol. 2008, 68, 2917–2921. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer–clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Liu, W.G.; Li, F.; Zhao, X.D.; Yao, K.D.; Liu, Q.G. Atom force microscopic characterisation of the interaction forces between bovine serum albumin and cross-linked alkylated chitosan membranes in media of different pH. Polym. Int. 2002, 51, 1459–1463. [Google Scholar] [CrossRef]
- Perugini, P.; Genta, I.; Conti, B.; Modena, T.; Pavanetto, F. Periodontal delivery of ipriflavone: New chitosan/PLGA film delivery system for a lipophilic drug. Int. J. Pharm. 2003, 252, 1–9. [Google Scholar] [CrossRef]
- Liu, K.-H.; Liu, T.-Y.; Chen, S.-Y.; Liu, D.-M. Drug release behavior of chitosan–montmorillonite nanocomposite hydrogels following electrostimulation. Acta Biomater. 2008, 4, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Luo, J.; Lin, B.; Kennedy, J.F. Chitosan/organic rectorite nanocomposite films: Structure, characteristic and drug delivery behaviour. Carbohyd. Polym. 2007, 69, 41–49. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of polysaccharide nanocrystals on structure, properties and drug release kinetics of alginate-based microspheres. Colloids Surf. B 2011, 85, 270–279. [Google Scholar] [CrossRef] [PubMed]
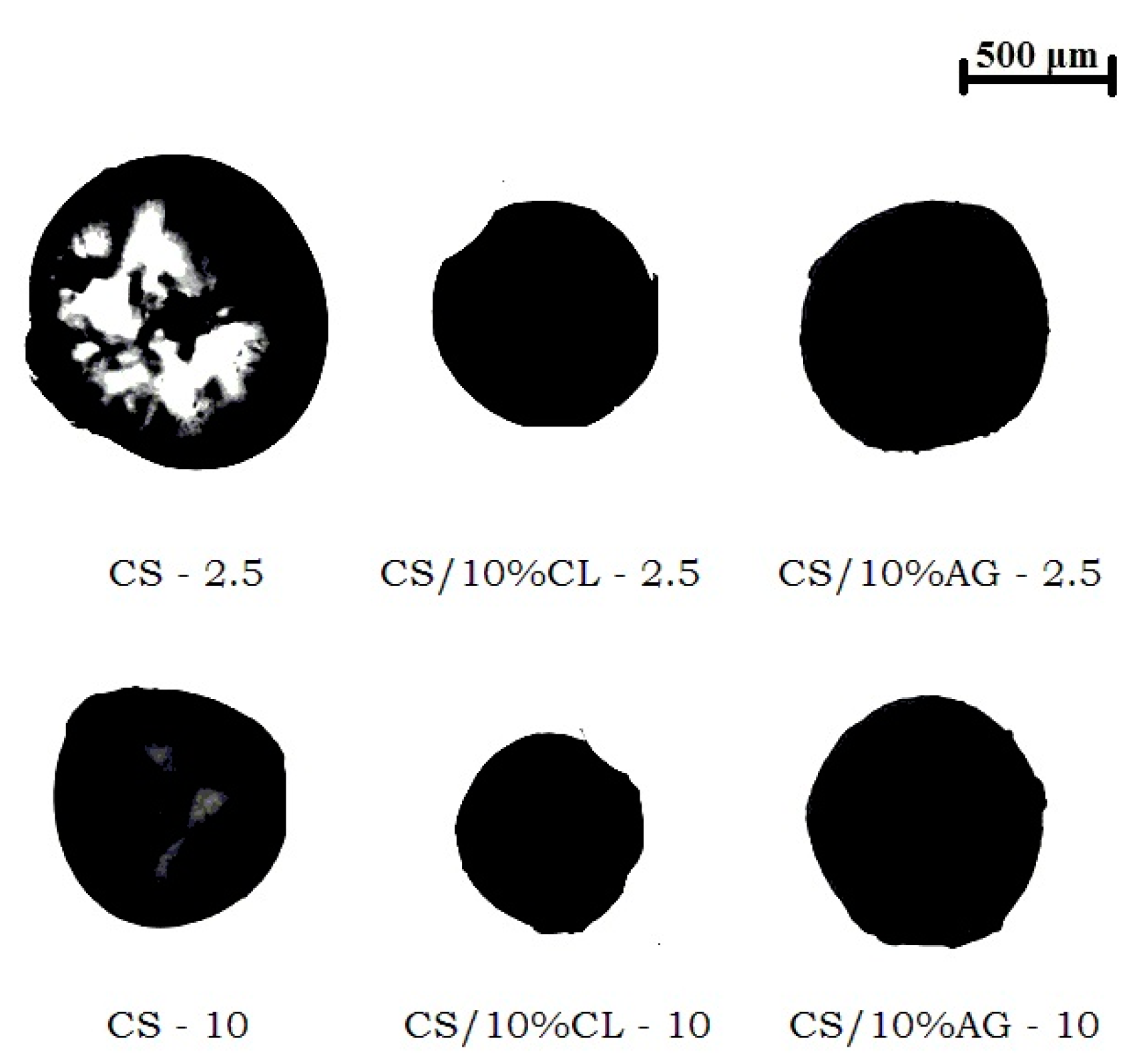

| Sample | Diameter (mm) | Volume (mm3) |
|---|---|---|
| CS-2.5 | 1.23 ± 0.10 | 0.99 ± 0.26 |
| CS-10 | 0.80 ± 0.05 | 0.26 ± 0.05 |
| CS/10%CL-2.5 | 0.56 ± 0.06 | 0.09 ± 0.03 |
| CS/10%CL-10 | 0.39 ± 0.06 | 0.03 ± 0.02 |
| CS/10%AG-2.5 | 0.88 ± 0.11 | 0.38 ± 0.13 |
| CS/10%AG-10 | 0.74 ± 0.10 | 0.22 ± 0.08 |
| Sample | Average Pore Size (nm) |
|---|---|
| CS-2.5 | 207 |
| CS-10 | 358 |
| CS/10%CL-2.5 | 83 |
| CS/10%CL-10 | 117 |
| CS/10%AG-2.5 | 67 |
| CS/10%AG-10 | 61 |
| Sample | Water Absorption (%) |
|---|---|
| CS/10%CL-10 | 135 A |
| CS/10%AG-2.5 | 98 B |
| CS/10%AG-10 | 92 B |
| CS/10%CL-2.5 | 69 C |
| CS-2.5 | 57 CD |
| CS-10 | 38 D |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
F. dos Santos, B.F.; Maciel, M.A.; A. Tavares, A.; Q. B. de Araújo Fernandes, C.; B. de Sousa, W.J.; Lia Fook, M.V.; Farias Leite, I.; De Lima Silva, S.M. Synthesis and Preparation of Chitosan/Clay Microspheres: Effect of Process Parameters and Clay Type. Materials 2018, 11, 2523. https://doi.org/10.3390/ma11122523
F. dos Santos BF, Maciel MA, A. Tavares A, Q. B. de Araújo Fernandes C, B. de Sousa WJ, Lia Fook MV, Farias Leite I, De Lima Silva SM. Synthesis and Preparation of Chitosan/Clay Microspheres: Effect of Process Parameters and Clay Type. Materials. 2018; 11(12):2523. https://doi.org/10.3390/ma11122523
Chicago/Turabian StyleF. dos Santos, Bárbara Fernanda, Matheus Aleixo Maciel, Albaniza A. Tavares, Clarissa Q. B. de Araújo Fernandes, Wladymyr Jefferson B. de Sousa, Marcus Vinícius Lia Fook, Itamara Farias Leite, and Suédina Maria De Lima Silva. 2018. "Synthesis and Preparation of Chitosan/Clay Microspheres: Effect of Process Parameters and Clay Type" Materials 11, no. 12: 2523. https://doi.org/10.3390/ma11122523





