Natural Dyes and Their Derivatives Integrated into Organic Solar Cells
Abstract
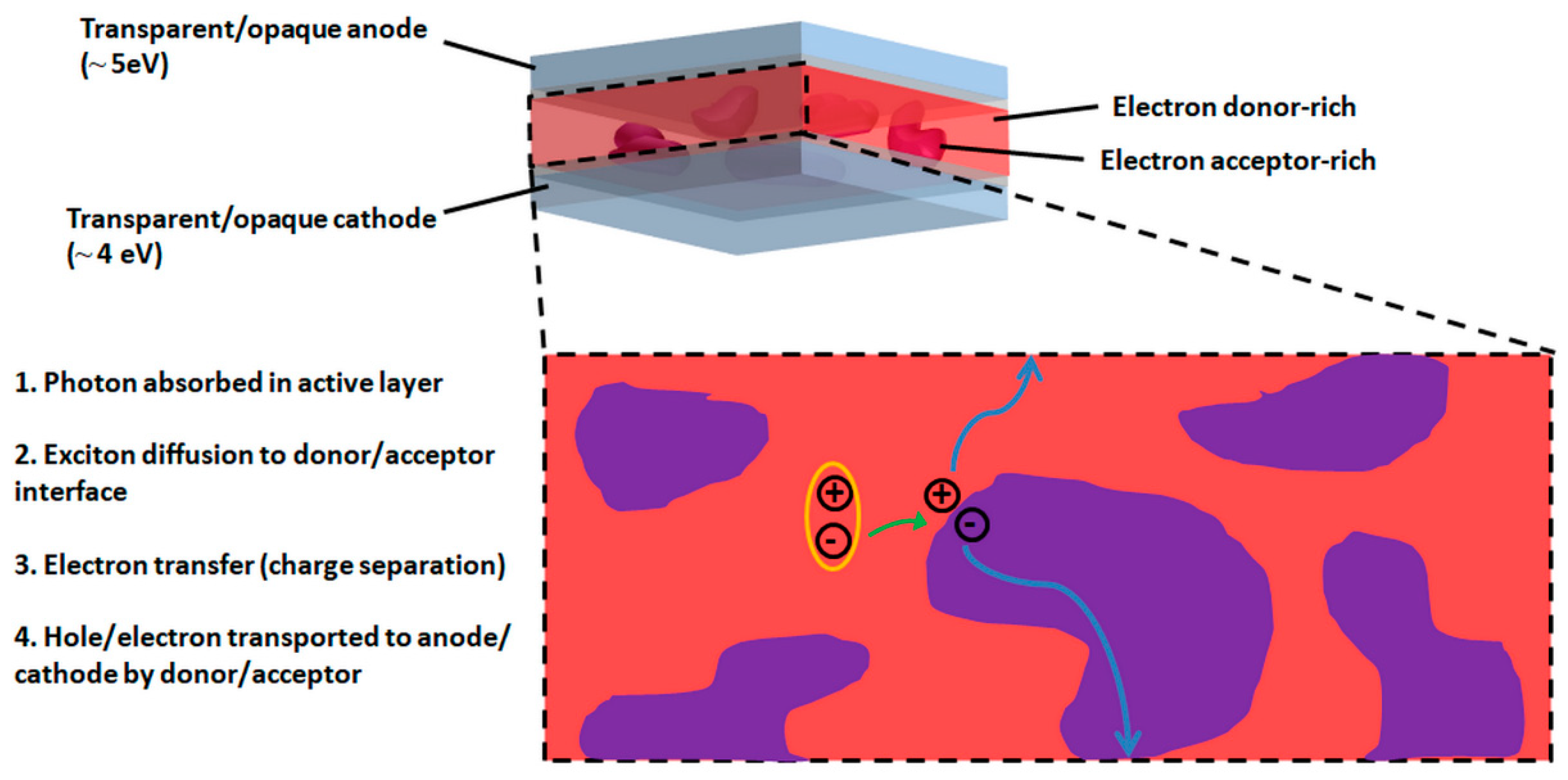
:1. Introduction
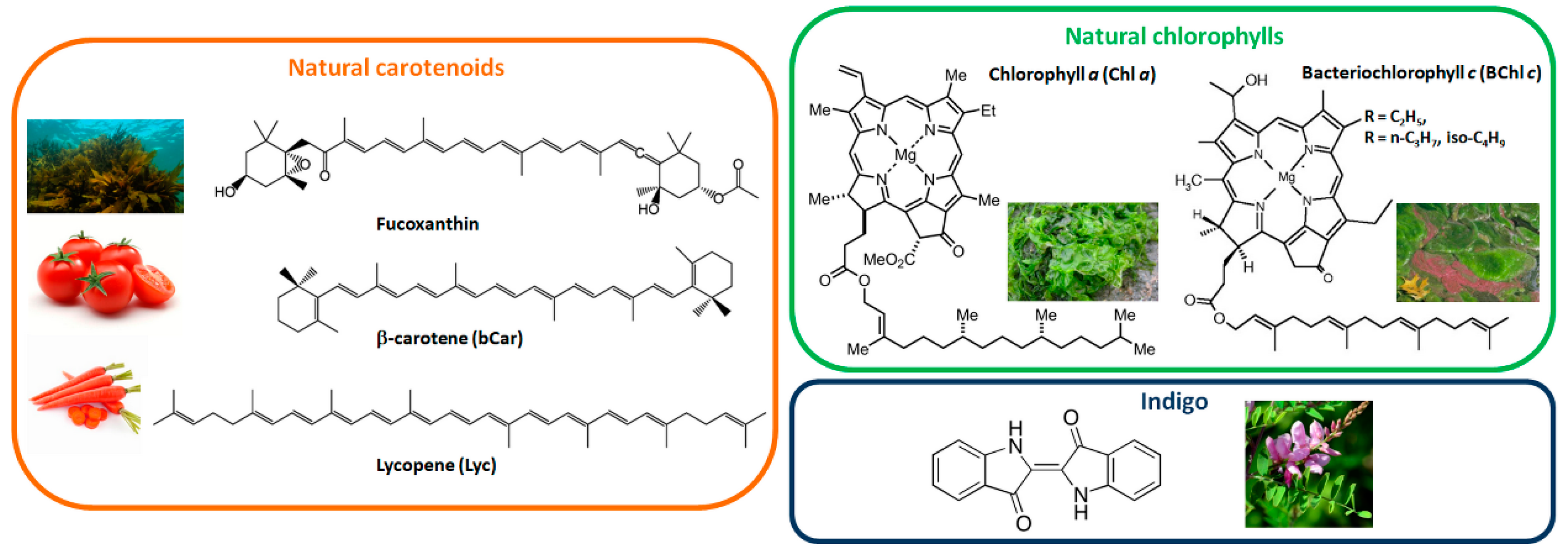
2. As Extracted Natural Dyes in OSC Active Layers
3. Efficient OSCs Employing Natural Dye Derivatives or Synthetic Equivalents as Active Materials
4. OSCs with Natural Complexes as Light-Harvesting Interlayers
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic solar cell materials toward commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef] [PubMed]
- Upama, M.B.; Wright, M.; Elumalai, N.K.; Mahmud, M.A.; Wang, D.; Xu, C.; Uddin, A. High-efficiency semitransparent organic solar cells with non-fullerene acceptor for window application. ACS Photonics 2017, 4, 2327–2334. [Google Scholar] [CrossRef]
- Vohra, V. Can polymer solar cells open the path to sustainable and efficient photovoltaic windows fabrication? Chem. Rec. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.F.; Zaretski, A.V.; Savagatrup, S.; Printz, A.D.; Wilkes, C.D.; Diaz, M.I.; Sawyer, E.J.; Lipomi, D.J. Wearable organic solar cells with high cyclic bending stability: Materials selection criteria. Sol. Energy Mater. Sol. Cells 2016, 144, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Vohra, V.; Kawashima, K.; Kakara, T.; Koganezawa, T.; Osaka, I.; Takimiya, K.; Murata, H. Efficient inverted polymer solar cells employing favourable molecular orientation. Nat. Photonics 2015, 9, 403–408. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Zhu, J.; Hou, J. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv. Mater. 2018, 30, 1800868. [Google Scholar] [CrossRef] [PubMed]
- Hug, H.; Barder, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural pigments in dye-sensitized solar cells. Appl. Energy 2014, 115, 216–225. [Google Scholar] [CrossRef]
- Ravi, S.K.; Udayagiri, V.S.; Suresh, L.; Tan, S.C. Emerging role of the band-structure approach in biohybrid photovoltaics: A path beyond bioelectrochemistry. Adv. Funct. Mater. 2018, 28, 1705305. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Aydin, M.; Kiliçoǧlu, T. Photovoltaic properties of Au/β-carotene/n-Si organic solar cells. J. Phys. Chem. B 2006, 110, 9782–9784. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; Albrecht, A.C. Chlorophyll-a photovoltaic cells. Nature 1975, 254, 507–509. [Google Scholar] [CrossRef]
- Dodelet, J.P.; Brich, J.L.; Leblanc, R. Photovoltaic efficiencies of microcrystalline and anhydrous chlorophyll a. Photochem. Photobiol. 1979, 29, 1135–1145. [Google Scholar] [CrossRef]
- Uehara, K.; Takagishi, K.; Tanaka, M. The Al/Indigo/Au photovoltaic cell. Sol. Cells 1987, 22, 295–301. [Google Scholar] [CrossRef]
- Frolov, L.; Wilner, O.; Carmeli, C.; Carmeli, I. Fabrication of oriented multilayers of photosystem I proteinson solid surfaces by auto-metallization. Adv. Mater. 2008, 20, 263–266. [Google Scholar] [CrossRef]
- Saenger, W.; Jordan, P.; Krauß, N. The assembly of protein subunits and cofactors in photosystem I. Curr. Opin. Struct. Biol. 2002, 12, 244–254. [Google Scholar] [CrossRef]
- Sutikno, M.; Dharmaputera, N.M.; Rahayu, S. Fabrication and characterization of banana flower extract anthocyanin-based organic solar cell. J. Adv. Agric. Technol. 2014, 1, 89–93. [Google Scholar] [CrossRef]
- Laily, A.R.N.; Hasiah, S.; Nik Aziz, N.A.; Dagang, A.N. Poly (3-dodecylthiophene)/natural dye bulk heterojunction organic solar cell: An electrical conductivity, and hall effect study. Procedia Chem. 2016, 19, 2–9. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Montero de Espinosa, V.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and β-carotene extraction from tomato processing waste using supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Chemat-Djenni, Z.; Ferhat, M.A.; Tomao, V.; Chemat, F. Carotenoid extraction from tomato using a green solvent resulting from orange processing waste. J. Essent. Oil Bear. Pl. 2010, 13, 139–147. [Google Scholar] [CrossRef]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono, E.Y.; Goto, M. Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Wang, L.; Wang, Z.; Wang, Y.; Tamai, N.; Hong, Z.; Kido, J. Natural photosynthetic carotenoids for solution-processed organic bulk-heterojunction solar cells. J. Phys. Chem. C 2013, 117, 804–811. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT:PCBM, best seller in polymer photovoltaic research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Sasaki, S.; Ikeuchi, T.; Kido, J.; Wang, X.-F. Natural-photosynthesis-inspired photovoltaic cells using carotenoid aggregates as electron donors and chlorophyll derivatives as electron acceptors. RSC Adv. 2015, 5, 45755–45759. [Google Scholar] [CrossRef]
- Tange, R.; Inai, K.; Sagawa, T.; Yoshikawa, S. Application of self-assembling photosynthetic dye for organic photovoltaics. J. Mater. Res. 2011, 26, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.-J.; Jung, H.-S.; Kim, S.-H.; Han, E.-M.; Vaithianathan, V.; Jenekhe, S.A. Chlorophyll-layer-inserted poly(3-hexyl-thiophene) solar cell having a high light-to-current conversion efficiency up to 1.48%. Appl. Phys. Lett. 2005, 87, 123102. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, R.; Ma, S.; Qu, Z.; Zhang, X.; Zhang, Q. Theoretical studies on the mechanism of primary electron transfer in thephotosynthetic reaction center of Rhodobacter sphaeroides. Photosynth. Res. 2002, 74, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Mustain, M.; Sulistyana, D.; Utari, U.; Purnama, B. Charge carrier mobility analysis of chlorophyll thin film spirulinasp produced by spin coating. Indonesian J. Appl. Phys. 2016, 4, 14–18. [Google Scholar] [CrossRef]
- Brettel, K.; Leibl, W. Electron transfer in photosystem I. Biochim. Biophys. Acta Bioenergy 2001, 1507, 100–114. [Google Scholar] [CrossRef]
- Kazemzadeh, S.; Riazi, G.; Ajeian, R. Novel approach of biophotovoltaic solid state solar cells based on a multilayer of PS1 complexes as an active layer. ACS Sustain. Chem. Eng. 2017, 5, 9836–9840. [Google Scholar] [CrossRef]
- Zeynali, A.; Ghiasi, T.S.; Riazi, G.; Ajeian, R. Organic solar cell based on photosystem I pigment-protein complex, fabrication and optimization. Org. Electron. 2017, 51, 341–348. [Google Scholar] [CrossRef]
- Das, R.; Kiley, P.J.; Segal, M.; Norville, J.; Yu, A.A.; Wang, L.; Trammell, S.A.; Reddick, L.E.; Kumar, R.; Stellacci, F.; et al. Integration of photosynthetic protein molecular complexes in solid-state electronic devices. Nano Lett. 2004, 4, 1079–1083. [Google Scholar] [CrossRef]
- Morvillo, P.; Ricciardi, R.; Nenna, G.; Bobeico, E.; Diana, R.; Minarini, C. Elucidating the origin of the improved current output in inverted polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 152, 51–58. [Google Scholar] [CrossRef]
- Gordiichuk, P.I.; Wetzelaer, G.A.H.; Rimmerman, D.; Gruszka, A.; De Vries, J.W.; Saller, M.; Gautier, D.A.; Catarci, S.; Pesce, D.; Richter, S.; et al. Solid-state biophotovoltaic cells containing photosystem I. Adv. Mater. 2014, 26, 4863–4869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Sasaki, S.; Zhuang, T.; Tamiaki, H.; Zhang, J.-P.; Ikeuchi, T.; Hong, Z.; Kido, J.; Wang, X.-F. Dicyano-functionalized chlorophyll derivatives with ambipolar characteristic for organic photovoltaics. Org. Electron. 2013, 14, 1972–1979. [Google Scholar] [CrossRef]
- Duan, S.; Chen, G.; Li, M.; Chen, G.; Wang, X.-F.; Tamiaki, H.; Sasaki, S. Near-infrared absorption bacteriochlorophyll derivatives as biomaterial electron donor for organic solar cells. J. Photochem. Photobiol. A Chem. 2017, 347, 49–54. [Google Scholar] [CrossRef]
- Duan, S.; Dall’Agnese, C.; Chen, G.; Wang, X.-F.; Tamiaki, H.; Yamamoto, Y.; Ikeuchi, T.; Sasaki, S. Bilayer chlorophyll-based biosolar cells inspired from the Z-scheme process of oxygenic photosynthesis. ACS Energy Lett. 2018, 3, 1708–1712. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Voss, G.; Sariciftci, N.S. 25th anniversary article: progress in chemistry and applications of functional indigos for organic electronics. Adv. Mater. 2013, 25, 6783–6800. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M.; Głowacki, E.D.; Troshin, P.A.; Schwabegger, G.; Leonat, L.; Susarova, D.K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M.A.; et al. Indigo—A natural pigment for high performance ambipolar organic field effect transistors and circuits. Adv. Mater. 2012, 24, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Głowacki, E.D.; Voss, G.; Demirak, K.; Havlicek, M.; Sünger, N.; Okur, A.C.; Monkowius, U.; Gąsiorowski, J.; Leonat, L.; Sariciftci, N.S. A facile protection–deprotection route for obtainingindigo pigments as thin films and their applications inorganic bulk heterojunctions. Chem. Commun. 2013, 49, 6063–6065. [Google Scholar] [CrossRef] [PubMed]
- Stalder, R.; Mei, J.; Subbiah, J.; Grand, C.; Estrada, L.A.; So, F.; Reynolds, J.R. N-type conjugated polyisoindigos. Macromolecules 2011, 44, 6303–6310. [Google Scholar] [CrossRef]
- Mei, J.; Graham, K.R.; Stalder, R.; Reynolds, J.R. Synthesis of isoindigo-based oligothiophenes for molecular bulk heterojunction solar cells. Org. Lett. 2010, 12, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Sonar, P.; Tan, H.-S.; Sun, S.; Lam, Y.M.; Dodabalapur, A. Isoindigo dye incorporated copolymers with naphthalene and anthracene: Promising materials for stable organic field effect transistors. Polym. Chem. 2013, 4, 1983–1994. [Google Scholar] [CrossRef]
- Wang, E.; Ma, Z.; Zhang, Z.; Henriksson, P.; Inganäs, O.; Zhang, F.; Andersson, M.R. An isoindigo-based low band gap polymer for efficient polymer solar cells with high photo-voltage. Chem. Commun. 2011, 47, 4908–4910. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, N.A.; Abdissa, Z.; Mammo, W.; Andersson, M.R.; Schlettwein, D.; Schwoerer, H. Ultrafast excited state dynamics of a bithiophene-isoindigo copolymer obtained by direct arylation polycondensation and its application in indium tin oxide-free solar cells. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1475–1483. [Google Scholar] [CrossRef]
- Stadler, R.; Grand, C.; Subbiah, J.; So, F.; Reynolds, J.R. An isoindigo and dithieno[3,2-b:2’,3’-d]silole copolymer for polymer solar cells. Polym. Chem. 2012, 3, 89–92. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, E.; Jarvid, M.A.; Henriksson, P.; Inganäs, O.; Zhang, F.; Andersson, M.R. Synthesis and characterization of benzodithiophene–isoindigo polymers for solar cells. J. Mater. Chem. 2012, 22, 2306–2314. [Google Scholar] [CrossRef]
- Wang, E.; Ma, Z.; Zhang, Z.; Vandewal, K.; Henriksson, P.; Inganäs, O.; Zhang, F.; Andersson, M.R. An easily accessible isoindigo-based polymer for high-performance polymer solar cells. J. Am. Chem. Soc. 2011, 133, 14244–14247. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Dang, D.; Tang, Z.; Gedefaw, D.; Bergqvist, J.; Zhu, W.; Mammo, W.; Andersson, M.R.; Inganäs, O.; Zhang, F.; et al. A facile method to enhance photovoltaic performance of benzodithiophene-isoindigo polymers by inserting bithiophene spacer. Adv. Energy Mater. 2014, 4, 1301455. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, C.; Chen, G.; Zhou, Z.; Li, Q. Side chain engineering: The effect on the properties of isoindigo-based conjugated polymers contain different length and structure alkyl chains on nitrogen atom. Org. Electron. 2017, 49, 278–285. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, M.; Li, B.; Jiang, C.; Li, Q. High efficiency organic photovoltaic devices based on isoindigo conjugated polymers with a thieno[3,2-b]thiophene π-bridge. J. Mater. Chem. A 2016, 4, 16064–16072. [Google Scholar] [CrossRef]
- Xu, T.; Yu, L. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Sun, R.; Liu, K.; Zhang, Y.; Wang, H.-Q.; Fang, J.; Yang, C. Improved device performance of polymer solar cells by using a thin light-harvesting-complex modified ZnO film as the cathode interlayer. ACS Appl. Mater. Interfaces 2015, 7, 18904–18908. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Kelly, M.A.; You, W.; Yu, L. Status and prospects for ternary organic photovoltaics. Nat. Photonics 2015, 9, 491–500. [Google Scholar] [CrossRef]
- An, Q.; Zhang, F.; Zhang, J.; Tang, W.; Deng, Z.; Hu, B. Versatile ternary organic solar cells: A critical review. Energy Environ. Sci. 2016, 9, 281–322. [Google Scholar] [CrossRef]
- Yao, K.; Liu, C.; Chen, Y.; Chen, L.; Li, F.; Liu, K.; Sun, R.; Wang, P.; Yang, C. Integration of light-harvesting complexes into the polymer bulk heterojunction P3HT/PCBM device for efficient photovoltaic cells. J. Mater. Chem. 2012, 22, 7342–7349. [Google Scholar] [CrossRef]
- Yao, K.; Jiao, H.; Yu, Y.-X.; He, Q.; Li, F.; Wang, X. Nano-bio hybrids of plasmonic metals/photosynthetic proteins for broad-band light absorption enhancement in organic solar cells. J. Mater. Chem. A 2016, 4, 13400–13406. [Google Scholar] [CrossRef]
- Nakano, K.; Suzuki, K.; Chen, Y.; Tajima, K. Roles of energy/charge cascades and intermixed layers at donor/acceptor interfaces in organic solar cells. Sci. Rep. 2016, 6, 29529. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, K.-D.; Heo, J.; Lee, J.Y.; Cha, G.; Seo, B.Y.; Kim, Y.D.; Kim, Y.S.; Choi, S.-Y.; Lim, D.C. Role of additional PCBM layer between ZnO and photoactive layers in inverted bulk heterojunction solar cells. Sci. Rep. 2014, 4, 4306. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lim, J.W.; Kim, J.G.; Wang, H.; Kang, B.-H.; Park, Y.W.; Kim, H.; Jang, Y.J.; Kim, J.; Kim, D.H.; et al. Plasmonic periodic nanodot arrays via laser interference lithography for organic photovoltaic cells with >10% efficiency. ACS Nano 2016, 10, 10143–10151. [Google Scholar] [CrossRef] [PubMed]


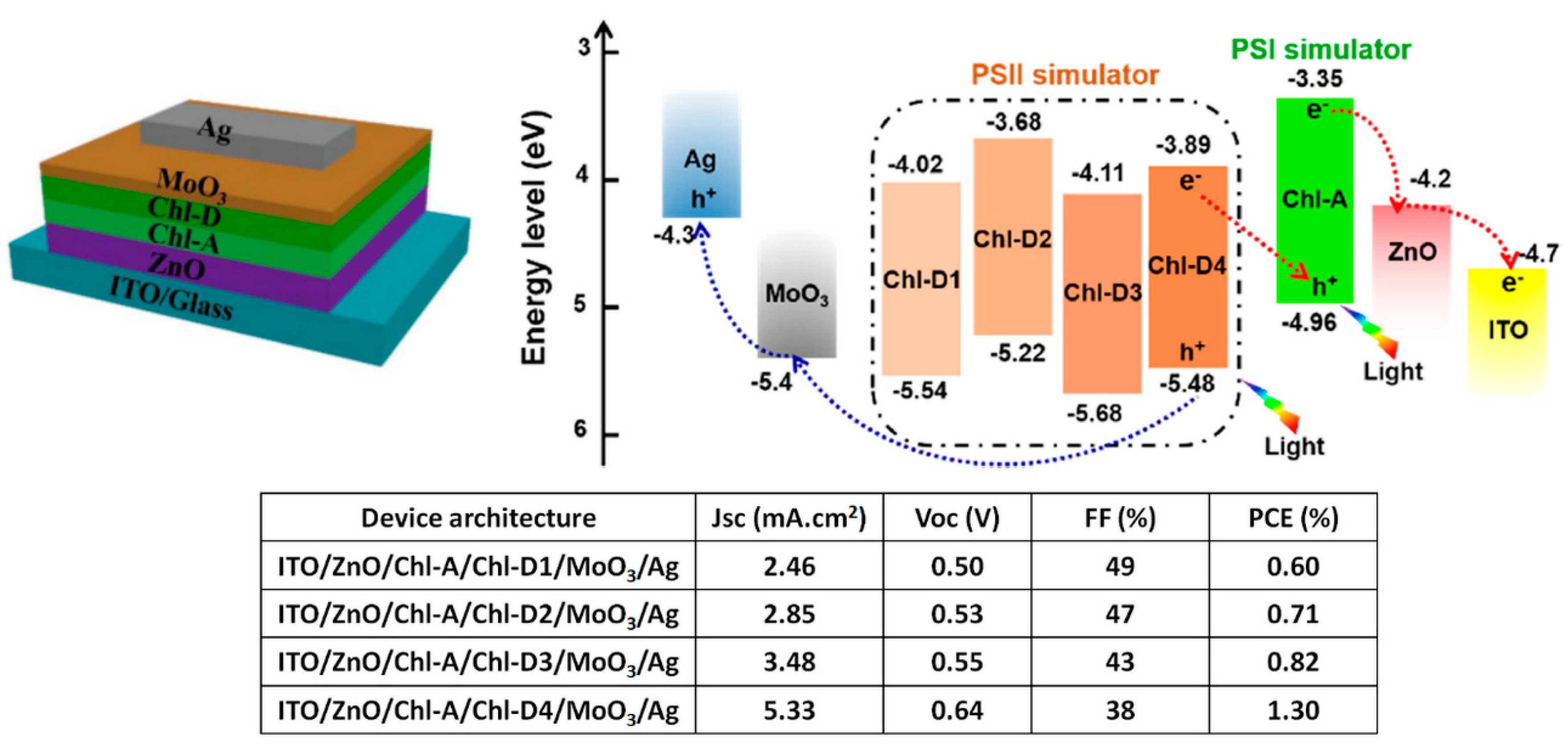

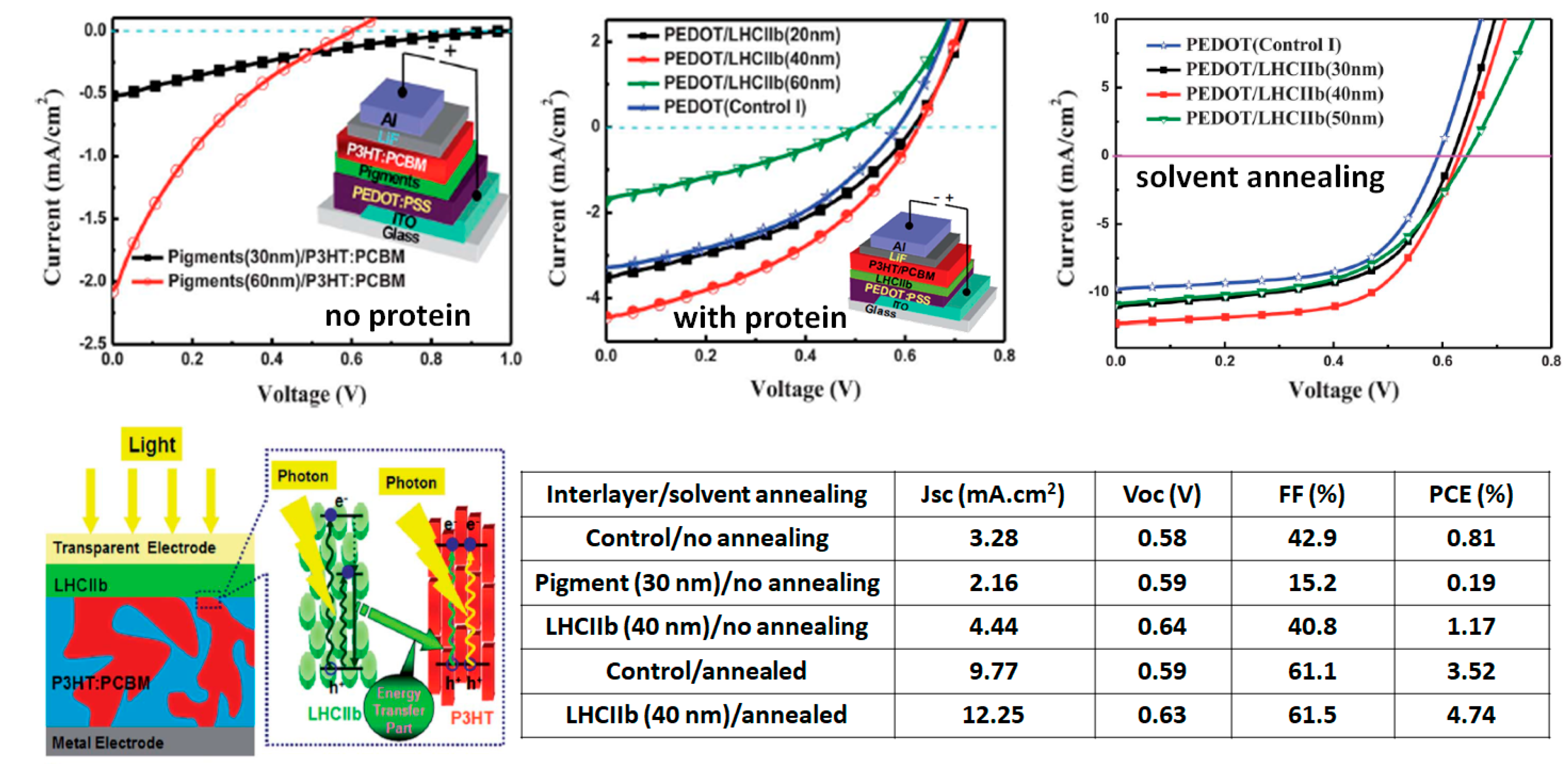
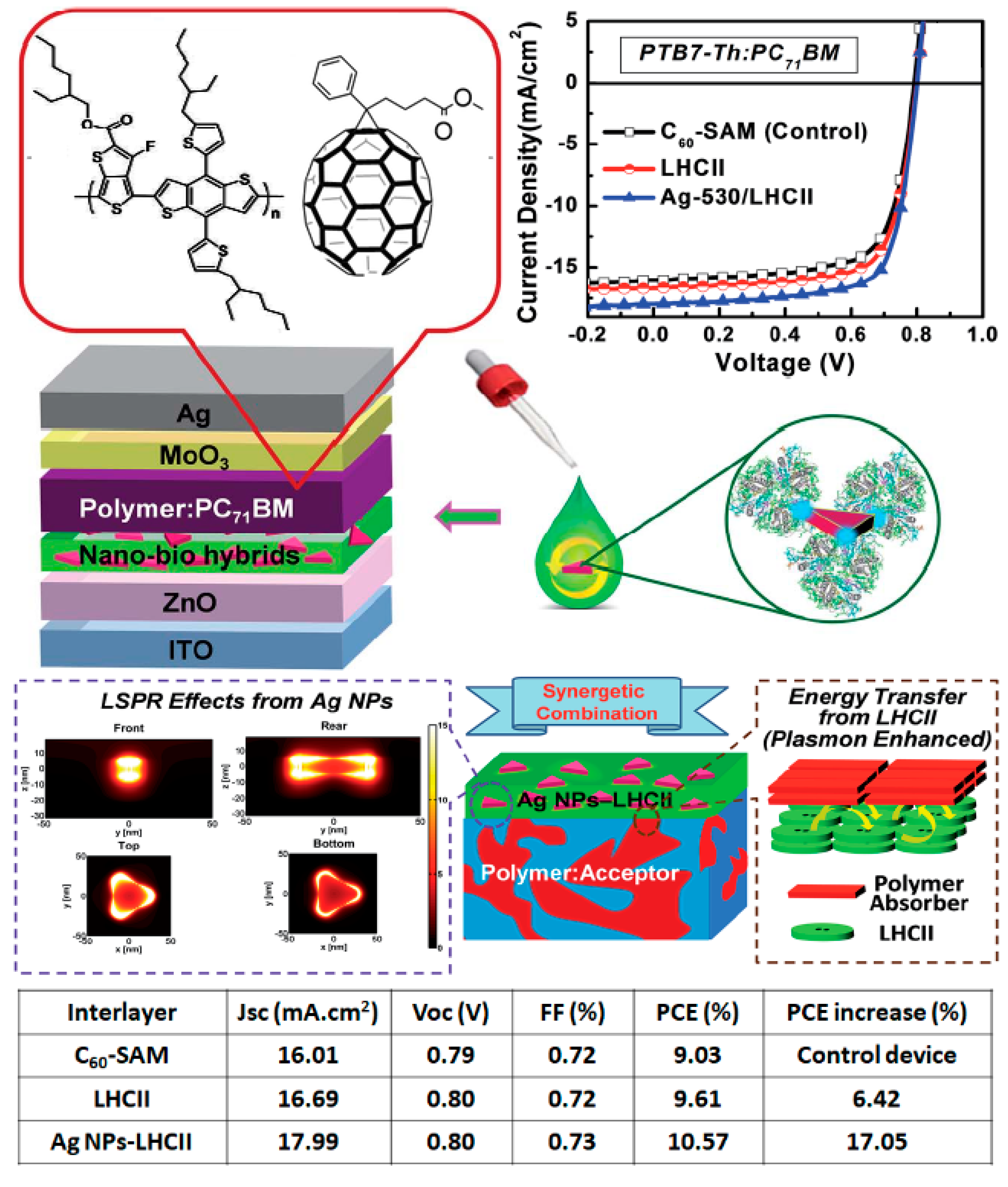
| “Natural” Material | Chemical Modifications | Function | Bottom Electrode; Top Electrode | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|---|
| Carotenoids [20] | Natural (Lyc) | Electron donor | ITO/MoO3; Ca/Al | 1.1 | 0.64 | 55 | 0.38 |
| Carotenoids and Chl [22] | Natural (Lyc) and synthetic equivalent (Chl-D4) | Electron donor; electron acceptor | ITO/MoO3; Ca/Al | 0.23 | 0.85 | 23 | 0.05 |
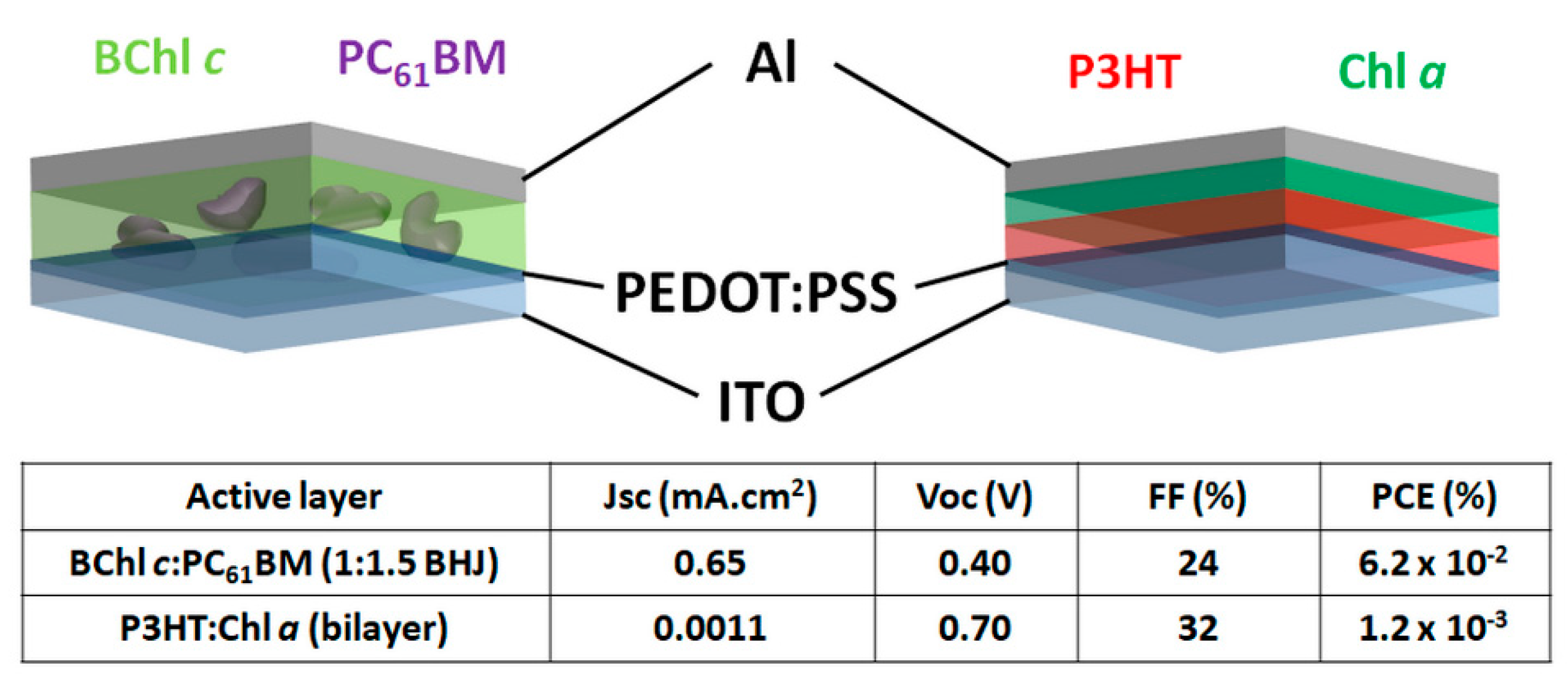
| Chls [24] | Natural (Chla) | Electron acceptor | ITO/PEDOT:PSS; Al | 0.001 | 0.70 | 32 | 0.001 |
| Chls [23] | Natural (BChlc) | Electron donor | ITO/PEDOT:PSS; Al | 0.65 | 0.40 | 24 | 0.06 |
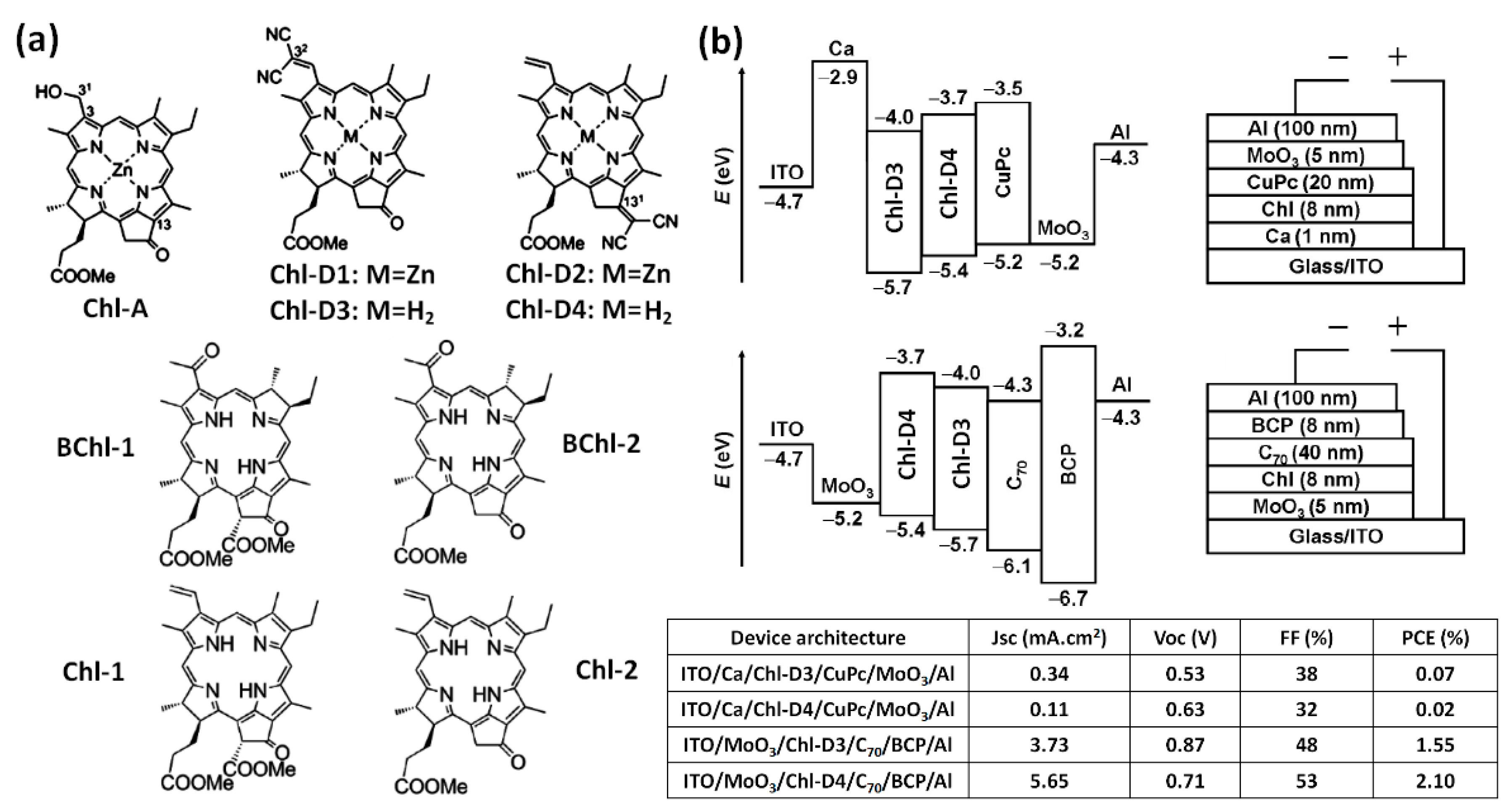
| Chl-D3 [33] | Synthetic equivalent of chlorophyll derivative | Electron acceptor | ITO/Ca; MoO3/Al | 0.34 | 0.53 | 38 | 0.07 |
| Chl-D4 [33] | Synthetic equivalent of chlorophyll derivative | Electron donor | ITO/MoO3; BCP/Al | 5.65 | 0.71 | 53 | 2.10 |
| Chl-A and Chl-D4 [35] | Synthetic equivalent of chlorophyll derivatives | Electron donor; electron acceptor | ITO/ZnO; MoO3/Ag | 5.33 | 0.64 | 38 | 1.30 |
| PSI [29] | Natural | Active layer | ITO/Tyrosine; C60/Au | 3.47 | 0.36 | 33 | 0.52 |
| PSI [32] | Natural | Active layer | ITO/TiOx; PTAA/MoO3/Al | 2.9 | 0.76 | 45 | 0.99 |
| Indigo [38] | Derivative | Electron acceptor | ITO/PEIE; MoOx/Ag | 0.2~0.5 | ~0.35 | - | <0.1 |
| Isoindigo [39] | Homopolymer | Electron acceptor | ITO/PEDOT:PSS; LiF/Al | 1.91 | 0.62 | 41 | 0.47 |
| Isoindigo [49] | Building block for p-type copolymer | Electron donor | ITO/PEDOT:PSS; Ca/Al | 14.4 | 0.79 | 72 | 8.05 |
| LHCIIb [54] | Natural | Antenna interlayer | ITO/PEDOT:PSS; LiF/Al | 12.3 | 0.63 | 62 | 4.74 |
| LHCII [55] | Natural | Antenna interlayer | ITO/ZnO; MoO3/Ag | 16.7 | 0.80 | 72 | 9.6 |
| LHCII [55] | Modified with Ag NPs | Plasmonic interlayer | ITO/ZnO; MoO3/Ag | 18.0 | 0.80 | 73 | 10.6 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vohra, V. Natural Dyes and Their Derivatives Integrated into Organic Solar Cells. Materials 2018, 11, 2579. https://doi.org/10.3390/ma11122579
Vohra V. Natural Dyes and Their Derivatives Integrated into Organic Solar Cells. Materials. 2018; 11(12):2579. https://doi.org/10.3390/ma11122579
Chicago/Turabian StyleVohra, Varun. 2018. "Natural Dyes and Their Derivatives Integrated into Organic Solar Cells" Materials 11, no. 12: 2579. https://doi.org/10.3390/ma11122579





