Microstructural Evolution and Phase Formation in 2nd-Generation Refractory-Based High Entropy Alloys
Abstract
:1. Introduction
2. Results
2.1. Cr20Nb20Ti20V20Zr20 Alloy
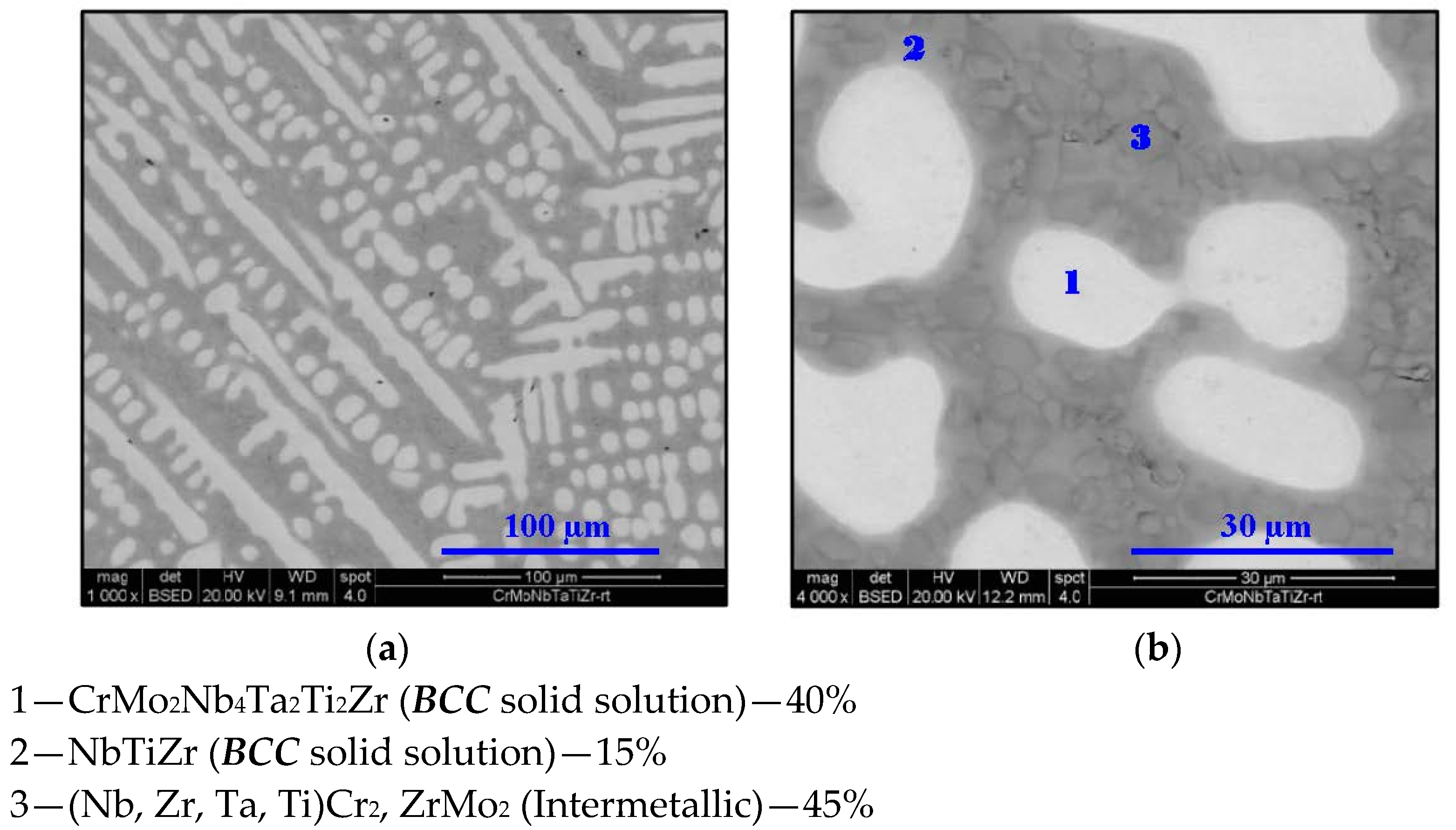
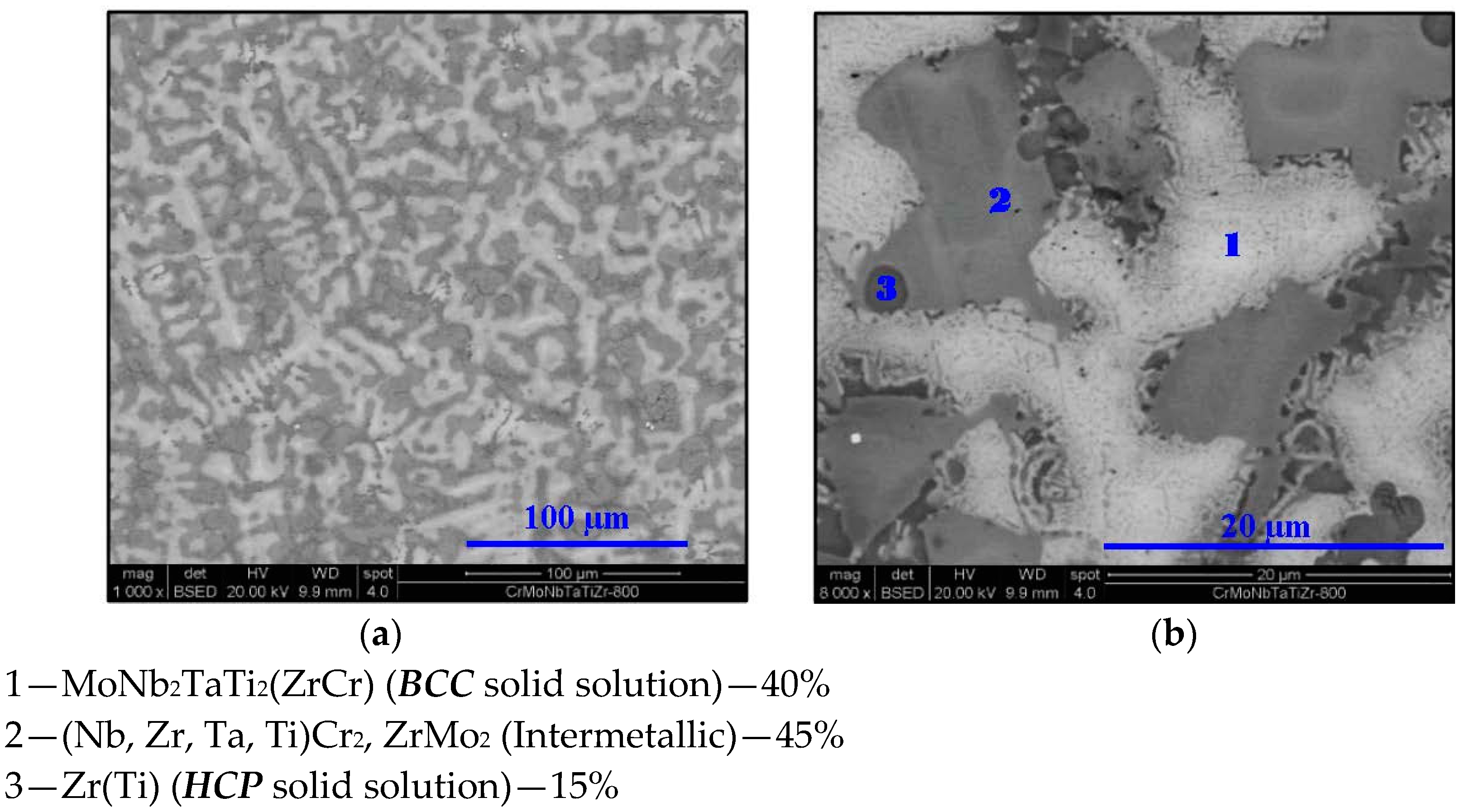
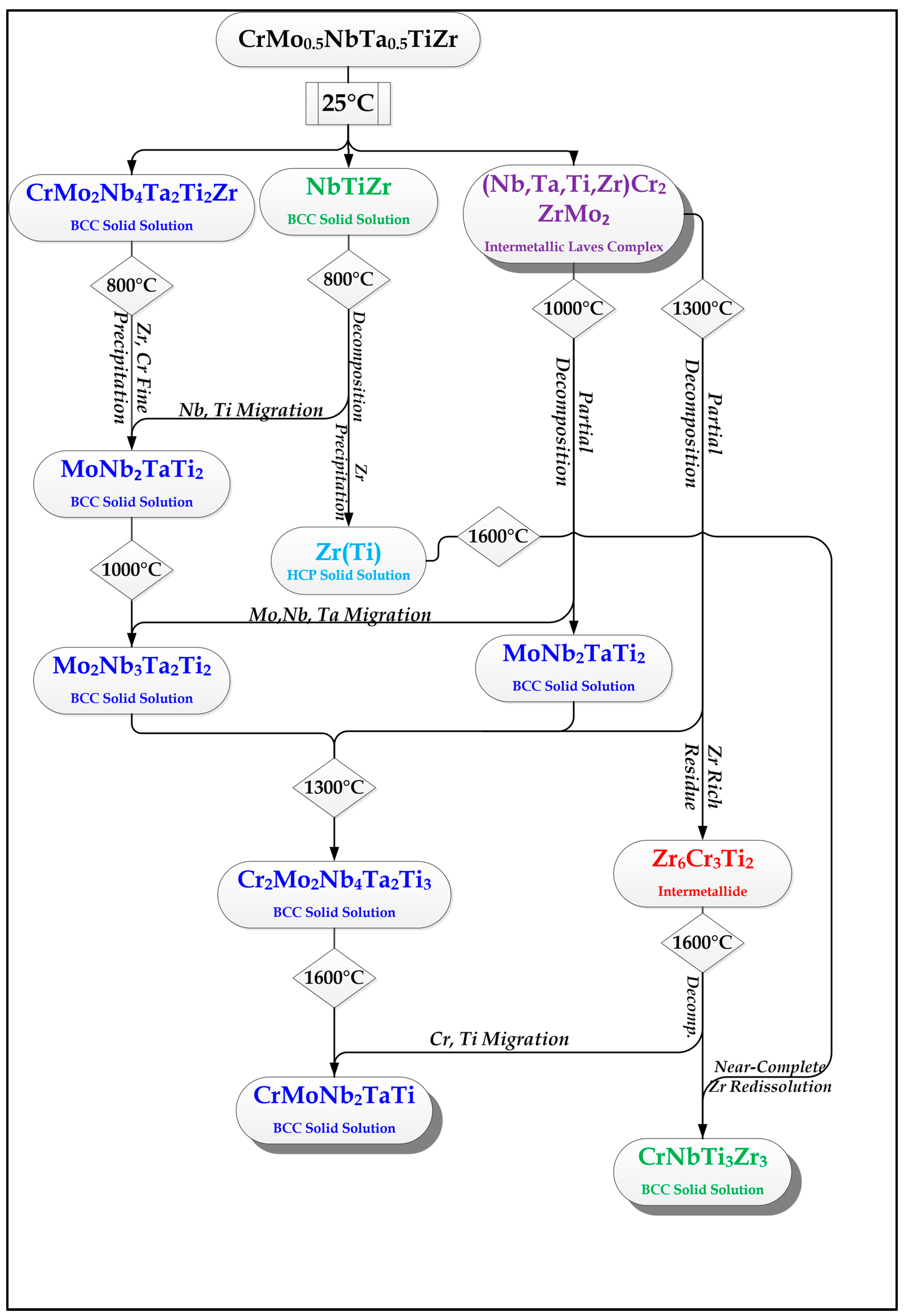
2.2. Cr20Mo10Nb20Ta10Ti20Zr20 Alloy
3. Discussion
4. Thermodynamic Modelling
5. Materials and Methods
6. Conclusions
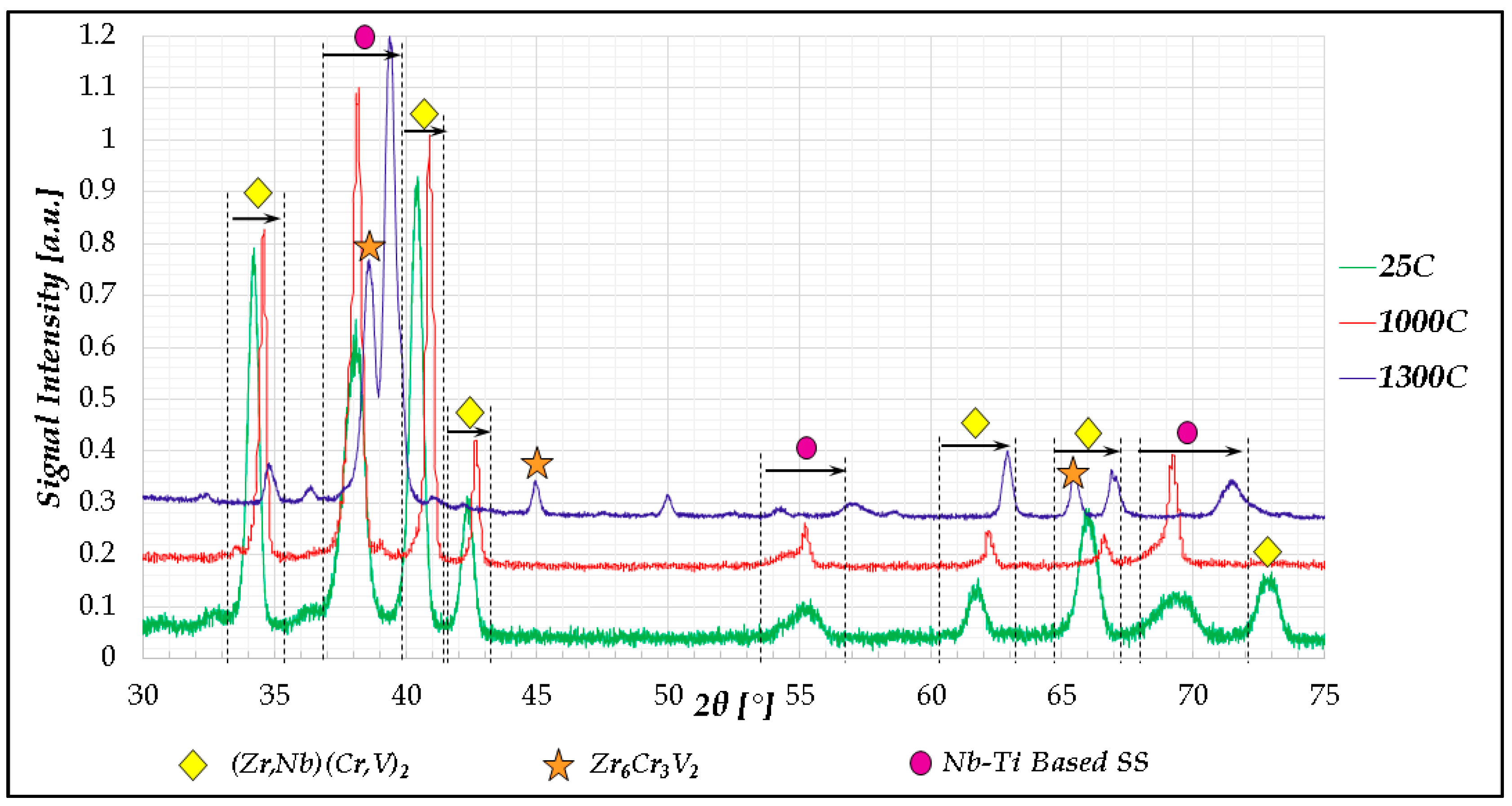
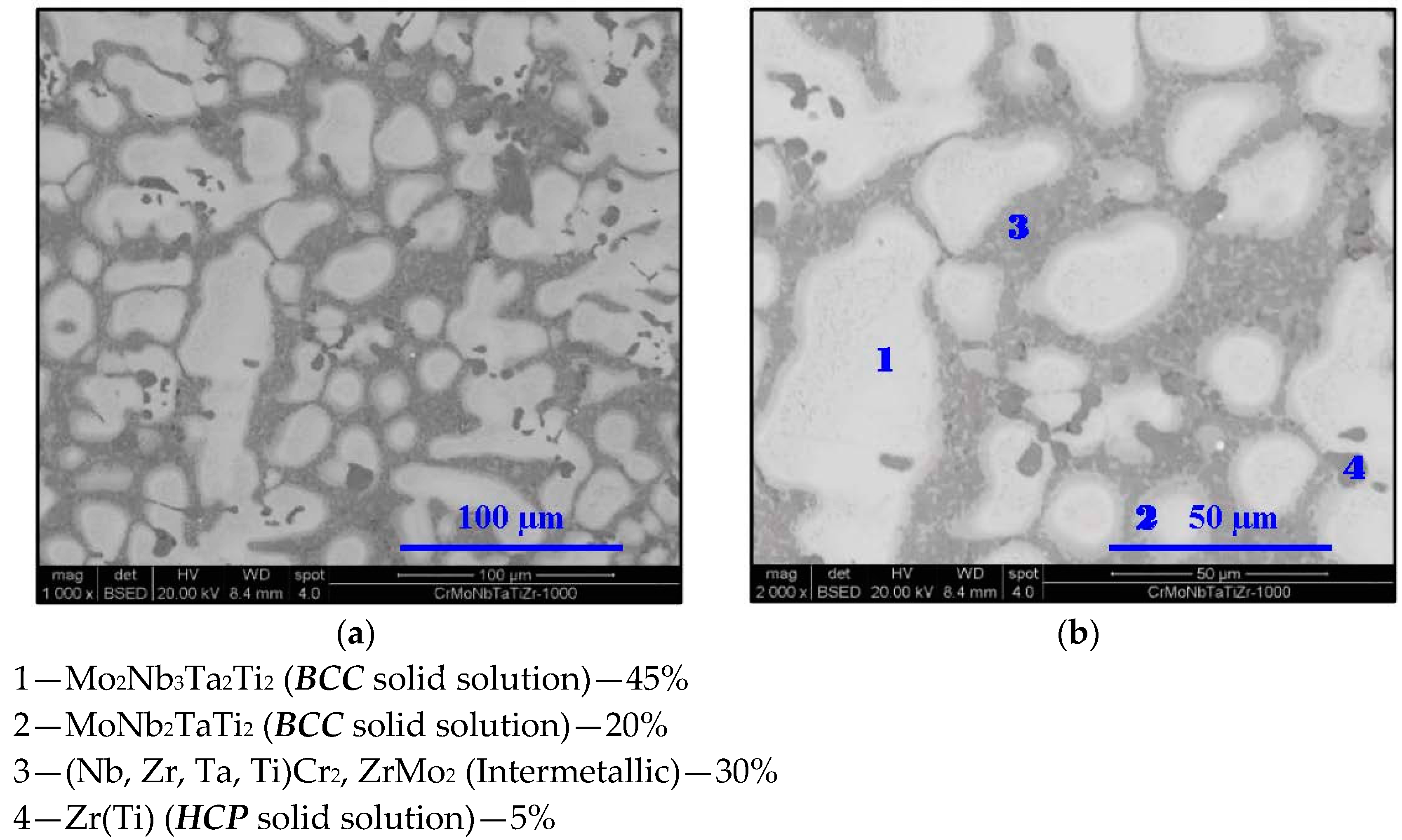
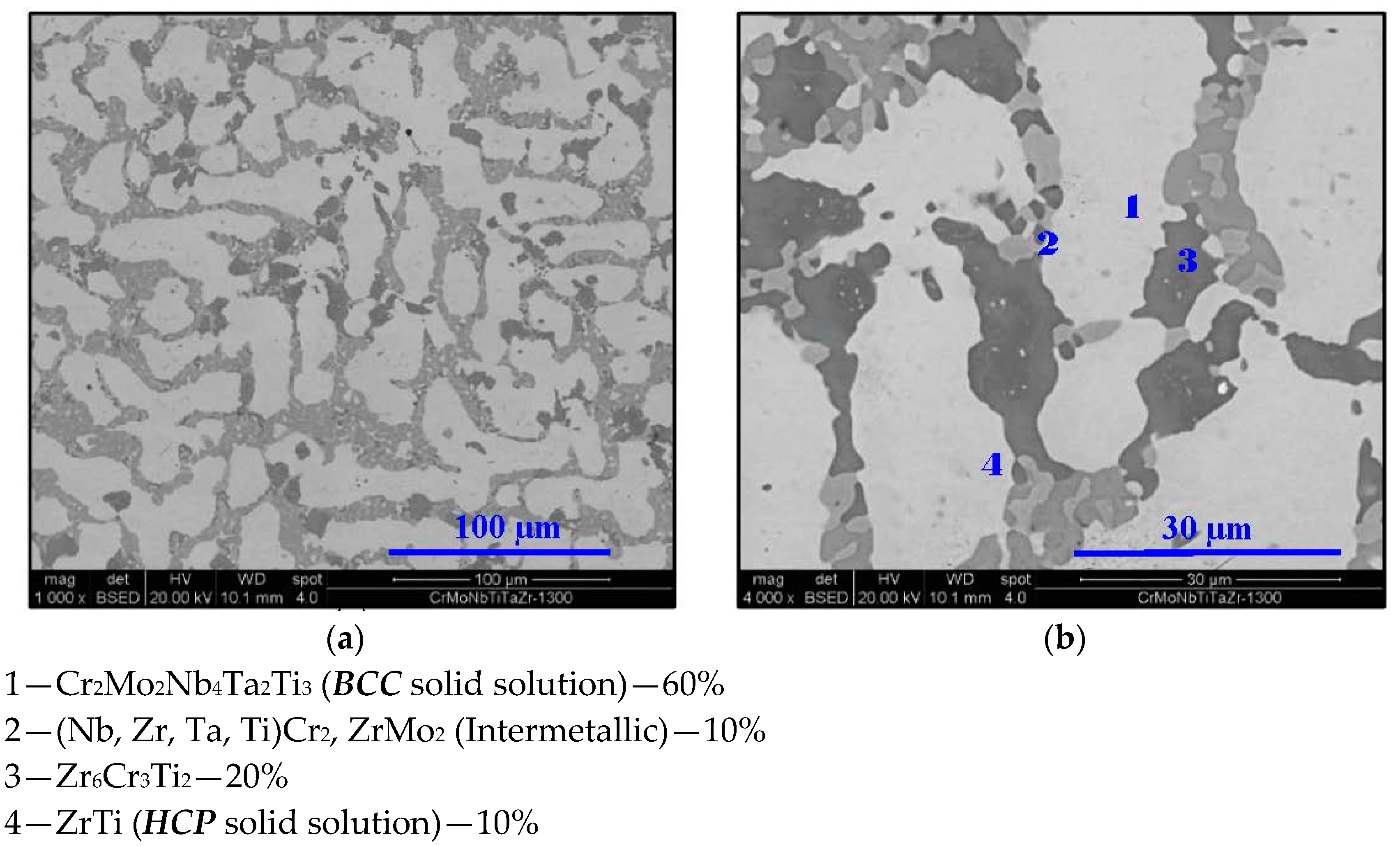
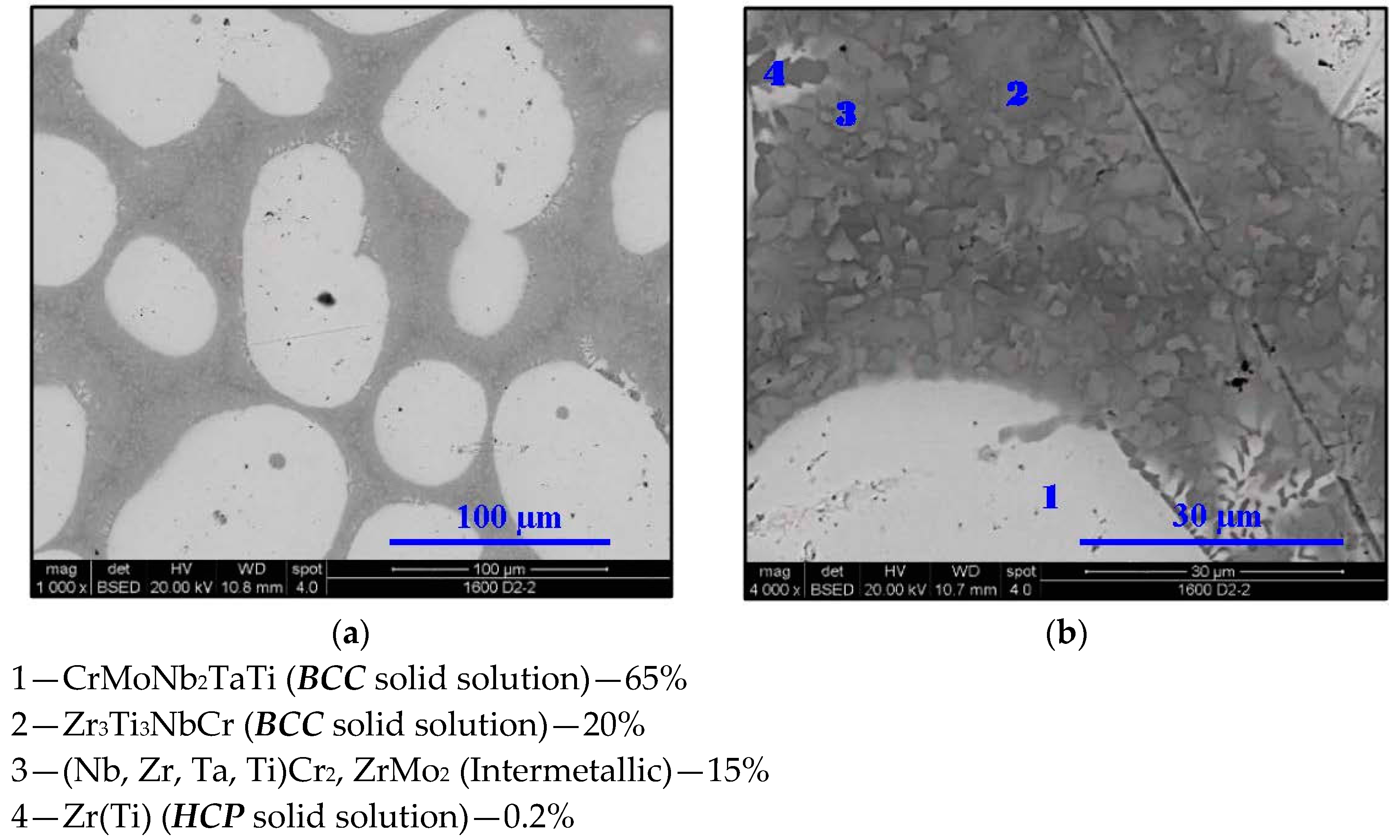
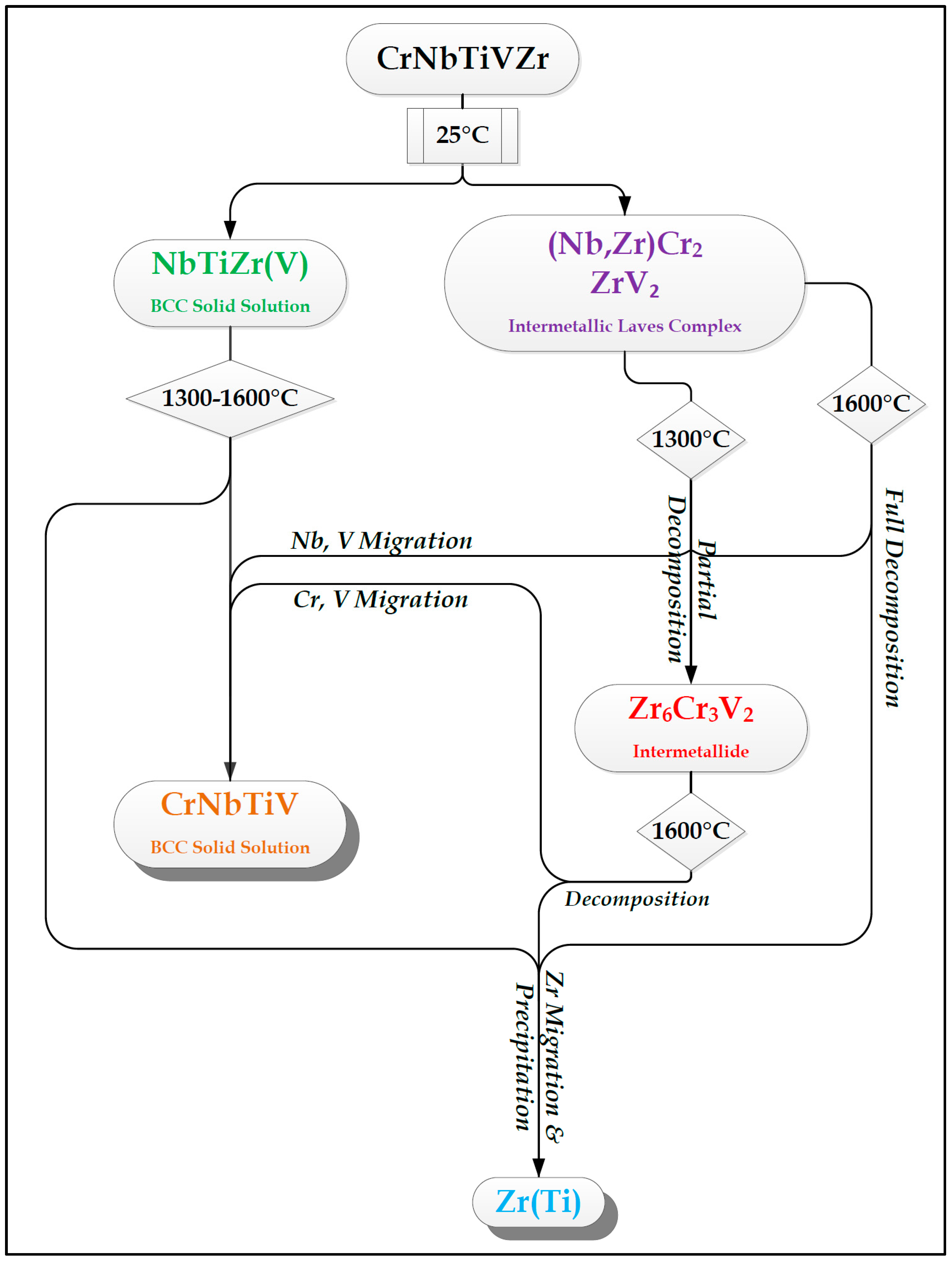
- In high-entropy alloys based on Zr and BCC elements, such as Cr, Ti, and Nb (the first two falling under the wider definition of refractory elements and Nb being a refractory element), a unique phenomenon of gradual Zr segregation as the temperature rises can be observed. This phenomenon is apparently related to the contrasting crystal structures of the HCP Zr solid solution and the BCC Cr-Nb based solid solution. It is more than likely that this phenomenon is not in equilibrium since Zr is expected to have a BCC structure at high temperatures as well.Some Ti is also incorporated in the secluded Zr solid solution regions and, to a small degree, it behaves in the same manner, being an HCP element itself.
- The Zr segregation is initiated by the onset of Laves phase decomposition at around 1000 °C, which leaves behind a Zr-rich phase. The other elements are then incorporated in the increasingly Zr-depleted BCC solid solution phase, which gradually grows in volume as the temperature rises further.
- The thermodynamic simulation successfully predicts the general trend of increasing volume percentage of the Cr-inclusive solid solution phase, as well as the increasing Cr incorporation in it, as a function of temperature (around 1000 °C and higher), however, Zr segregation is not accounted for, due to the fact that it may not be an equilibrium phenomenon. The Nb-Ti-Zr based solid solution phase appearing at low to moderate temperatures in both alloys forms as a result of the relatively rapid cooling associated with arc melting and is not likely to be the equilibrium solid solution phase predicted at those temperatures by the model.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Fu, H.Z. Solid solution or intermetallics in a high-entropy alloy. Adv. Eng. Mater. 2009, 11, 641–644. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy design and properties optimization of high-entropy alloys. J. Miner. Met. Mater. Soc. 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V. Microstructure and room temperature properties of a high entropy TaNbHfZrTi alloy. J. Alloys Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure of Aluminum-containing refractory high-entropy alloys. J. Miner. Met. Mater. Soc. 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Li, C.; Xue, Y.; Hua, M.; Cao, T.; Ma, L.; Wang, L. Microstructure and mechanical properties of AlxSi0.2CrFeCoNiCu1-x high entropy alloys. Mater. Des. 2016, 90, 601–609. [Google Scholar] [CrossRef]
- Klimova, M.; Stepanov, N.; Shaysultanov, D.; Chernichenko, R.; Yurchenko, N.; Sanin, V.; Zherebtsov, S. Microstructure and Mechanical Properties Evolution of the Al, C-containing CoCrFeNiMn-type High-Entropy Alloy during Cold Rolling. Materials 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, H.; Xu, Y. Microstructure, MAchanical Properties and Tribological Properties of Oxide Dispersion Strengthened High-Entropy Alloys. Materials 2017, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Qi, Y.; Wang, S. Effects of Short-Range Order on the mAgnetic and Mechanical Properties of FeCoNo(AlSi)x High Entropy Alloys. Metals 2017, 7, 482. [Google Scholar] [CrossRef]
- Popov, V.V.; Gorbachev, I.I. Analysis of solubility of carbides, nitrides, and carbonitrides is steels using methods of computer thermodynamics: I. Description of thermodynamic properties. Computation procedure. Phys. Met. Metallogr. 2004, 98, 344–354. [Google Scholar]
- Popov, V.V.; Gorbachev, I.I. Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: II. Solubility of carbides, nitrides, and carbonitrides in the Fe–V–C, Fe–V–N, and Fe–V–C–N systems. Phys. Met. Metallogr. 2005, 99, 286–299. [Google Scholar]
- Popov, V.V.; Gorbachev, I.I. Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: II. Solubility of carbides, nitrides, and carbonitrides in the Fe–Ti–C, Fe–Ti–N, and Fe–Ti–C–N systems. Phys. Met. Metallogr. 2009, 108, 484–495. [Google Scholar]
- Gorbachev, I.I.; Popov, V.V. Analysis of the solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: IV. Solubility of carbides, nitrides, and carbonitrides in the Fe-Nb-C, Fe-Nb-N, and Ft-Nb-C-N systems. Phys. Met. Metallogr. 2010, 110, 52–61. [Google Scholar] [CrossRef]
- Gorbachev, I.I.; Popov, V.V. Thermodynamic simulation of the Fe-V-Nb-C-N system using the CALPHAD method. Phys. Met. Metallogr. 2011, 111, 495–502. [Google Scholar] [CrossRef]
- Gorbachev, I.I.; Popov, V.V.; Pasynkov, A.Y. Thermodynamic modeling of carbonitride formation in steels with V and Ti. Phys. Met. Metallogr. 2012, 113, 974–981. [Google Scholar] [CrossRef]
- Hillert, M.; Staffonsson, L.-I. The regular solution model for stoichiometric phases and ionic melts. Acta Chem. Scand. 1970, 24, 3618–3626. [Google Scholar] [CrossRef]
- Sundman, B.; Agren, J. A regular solution model for phase with several components and sublattices, suitable for computer applications. J. Phys. Chem. Sol. 1981, 42, 297–301. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Lu, H.-J.; Wang, W.-B.; Zou, N.; Shen, J.-Y.; Lu, X.-G.; He, Y.-L. Thermodynamic modeling of Cr-Nb and Zr-Cr with extension to the ternary Zr-Nb-Cr system. Calphad 2015, 50, 134–143. [Google Scholar] [CrossRef]
- Cui, J.; Guo, C.; Zou, L.; Li, C.; Du, Z. Experimental investigation and thermodynamic modeling of the Ti-V-Zr system. Calphad 2016, 55 Pt 2, 189–198. [Google Scholar] [CrossRef]
- Ghosh, G. Thermodynamic and kinetic modeling of the Cr-Ti-V system. J. Phase Equilib. 2002, 23, 310–328. [Google Scholar] [CrossRef]
- Lee, B.-J. Thermodynamic assessment of the Fe-Nb-Ti-C-N system. Metall. Mater. Trans. A 2001, 32, 2423–2439. [Google Scholar] [CrossRef]
- Hari Kumar, K.C.; Wollants, P.; Delaey, L. Thermodynamic calculation of Nb-Ti-V phase diagram. Calphad 1994, 18, 71–79. [Google Scholar] [CrossRef]



| Temperature (°C) | Phase | Weight Fraction (%) | Chemical Composition (at%) | ||||
|---|---|---|---|---|---|---|---|
| Ti | V | Cr | Zr | Nb | |||
| 800 °C | BCC_A2 | 72.68 | 23.86 | 28.29 | 2.04 | 26.52 | 19.29 |
| Laves_C15 | 27.32 | 10.74 | 0.14 | 63.02 | 4.39 | 21.71 | |
| 1000 °C | BCC_A2 | 74.88 | 23.24 | 27.30 | 4.73 | 24.90 | 19.83 |
| Laves_C15 | 25.12 | 11.21 | 0.19 | 61.46 | 6.68 | 20.46 | |
| 1300 °C | BCC_A2 | 86.14 | 21.34 | 23.42 | 13.23 | 21.69 | 20.32 |
| Laves_C15 | 13.86 | 12.25 | 0.26 | 59.13 | 10.22 | 18.14 | |
| 1600 °C | BCC_A2 | 100.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshed, E.; Larianovsky, N.; Kovalevsky, A.; Popov Jr., V.; Gorbachev, I.; Popov, V.; Katz-Demyanetz, A. Microstructural Evolution and Phase Formation in 2nd-Generation Refractory-Based High Entropy Alloys. Materials 2018, 11, 175. https://doi.org/10.3390/ma11020175
Eshed E, Larianovsky N, Kovalevsky A, Popov Jr. V, Gorbachev I, Popov V, Katz-Demyanetz A. Microstructural Evolution and Phase Formation in 2nd-Generation Refractory-Based High Entropy Alloys. Materials. 2018; 11(2):175. https://doi.org/10.3390/ma11020175
Chicago/Turabian StyleEshed, Eyal, Natalya Larianovsky, Alexey Kovalevsky, Vladimir Popov Jr., Igor Gorbachev, Vladimir Popov, and Alexander Katz-Demyanetz. 2018. "Microstructural Evolution and Phase Formation in 2nd-Generation Refractory-Based High Entropy Alloys" Materials 11, no. 2: 175. https://doi.org/10.3390/ma11020175