A Novel Ruthenium-Decorating Polyoxomolybdate Cs3Na6H[MoVI14RuIV2O50(OH)2]·24H2O: An Active Heterogeneous Oxidation Catalyst for Alcohols
Abstract
:1. Introduction
2. Results and Discussion
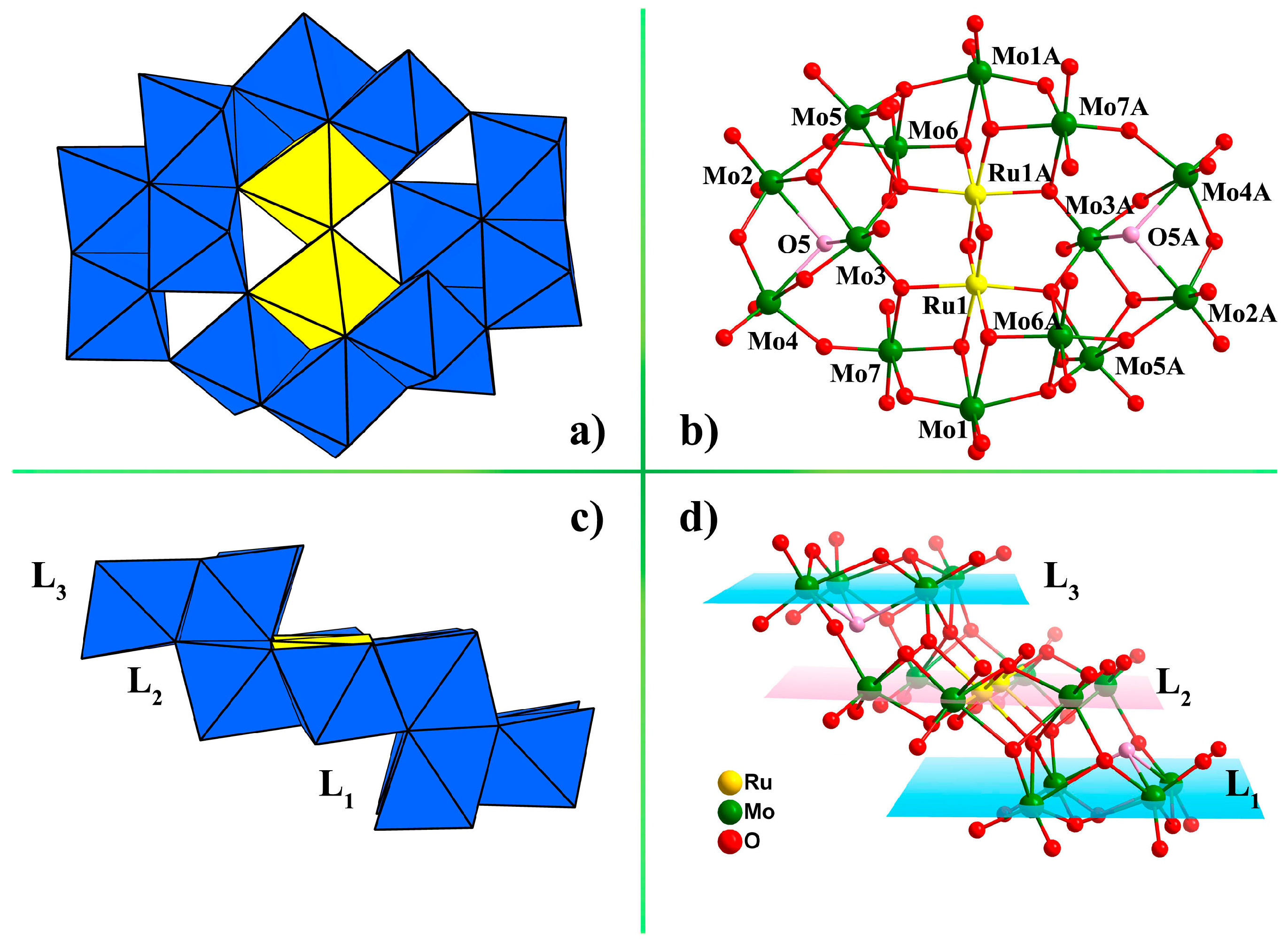
2.1. Structure Description
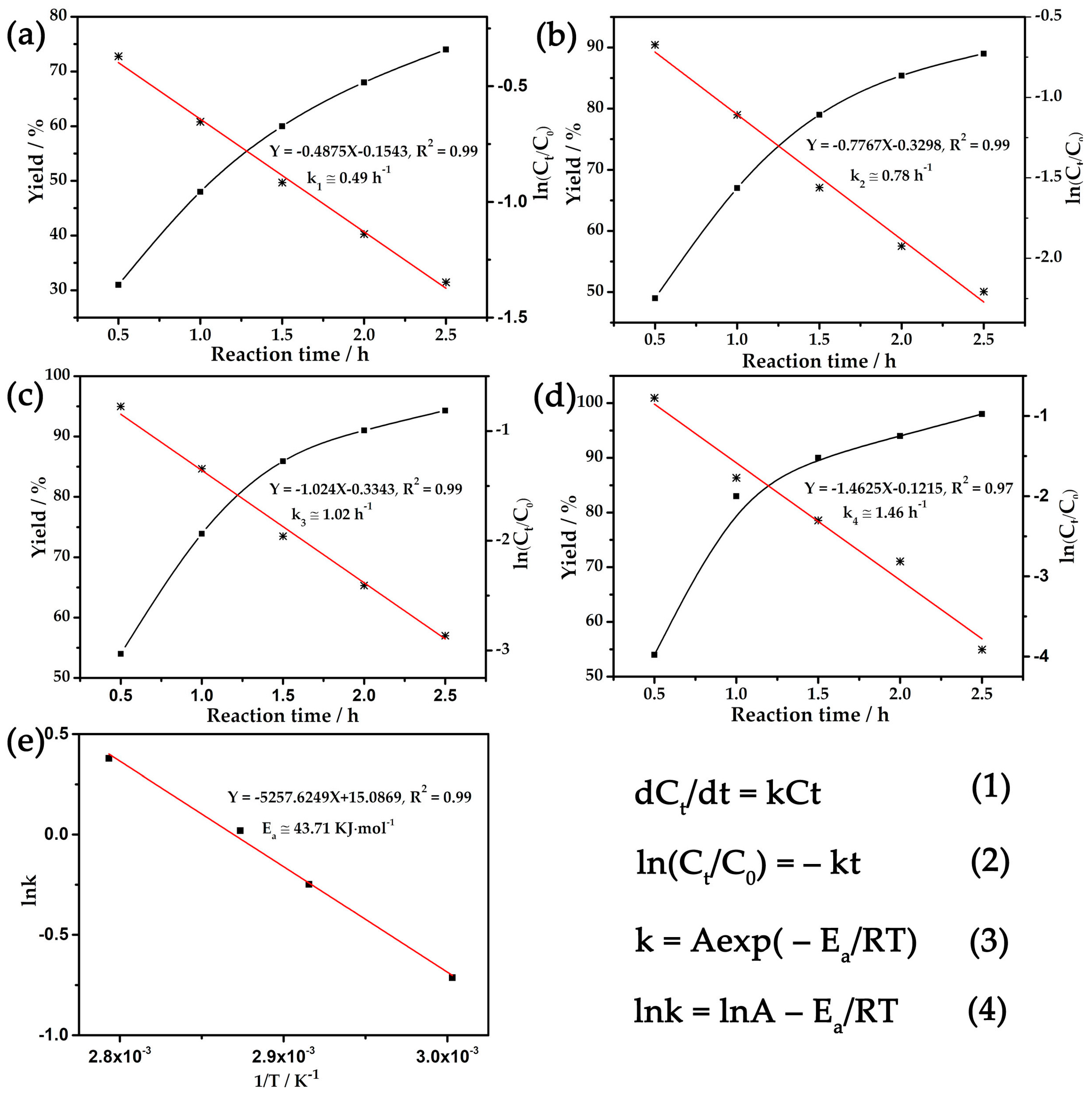
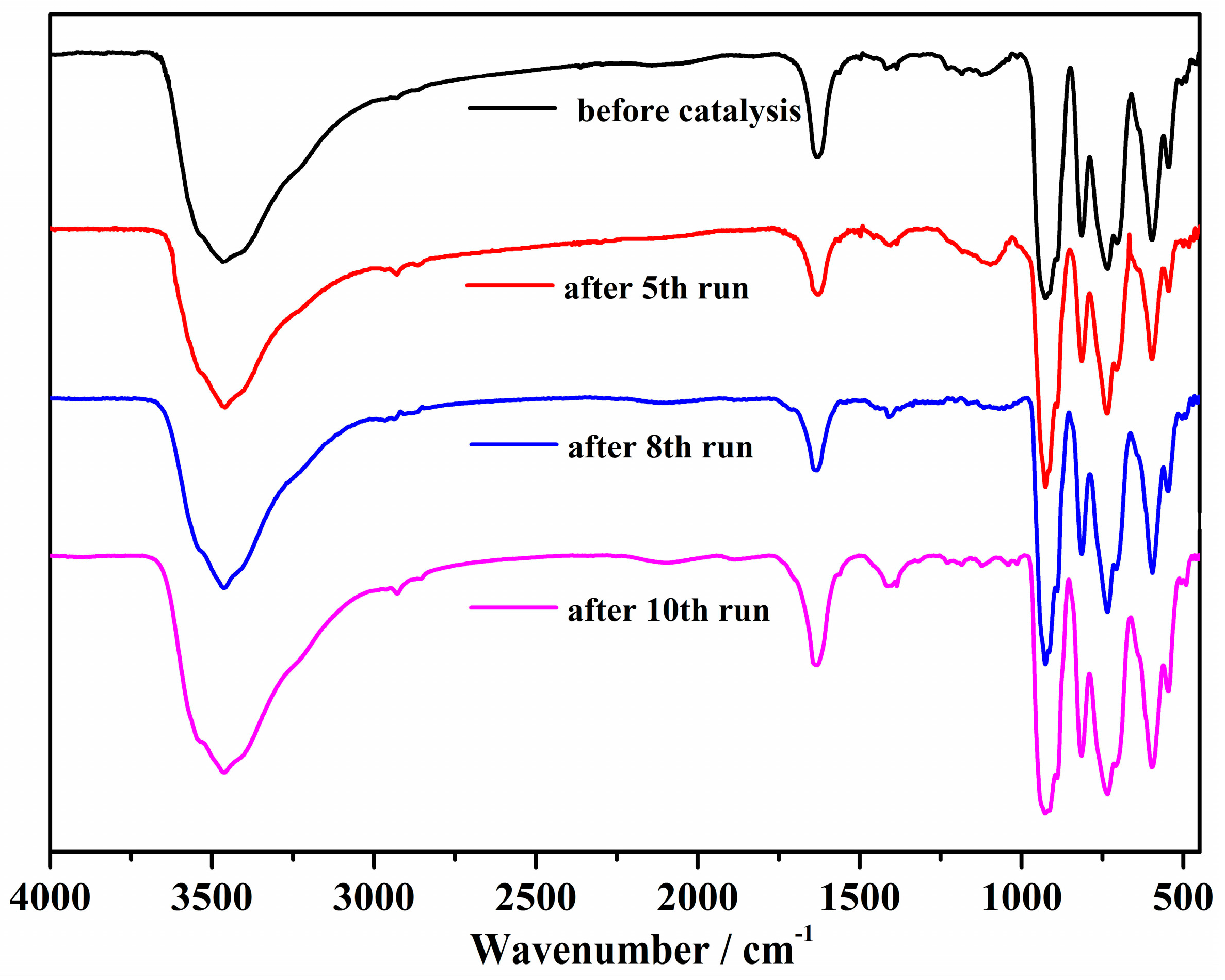
2.2. Catalytic Performance
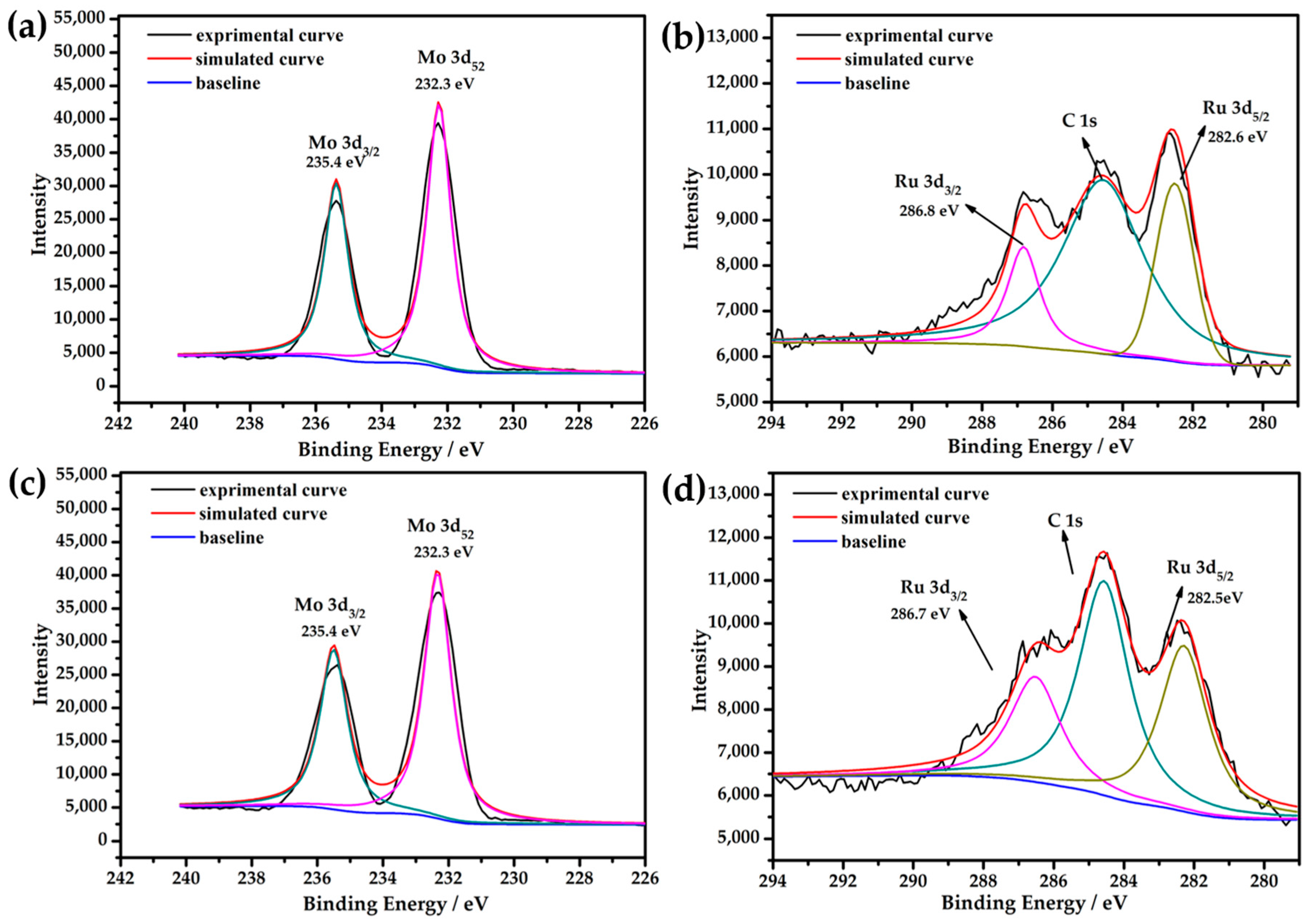
2.3. X-ray Photoelectron Spectroscopy (XPS)
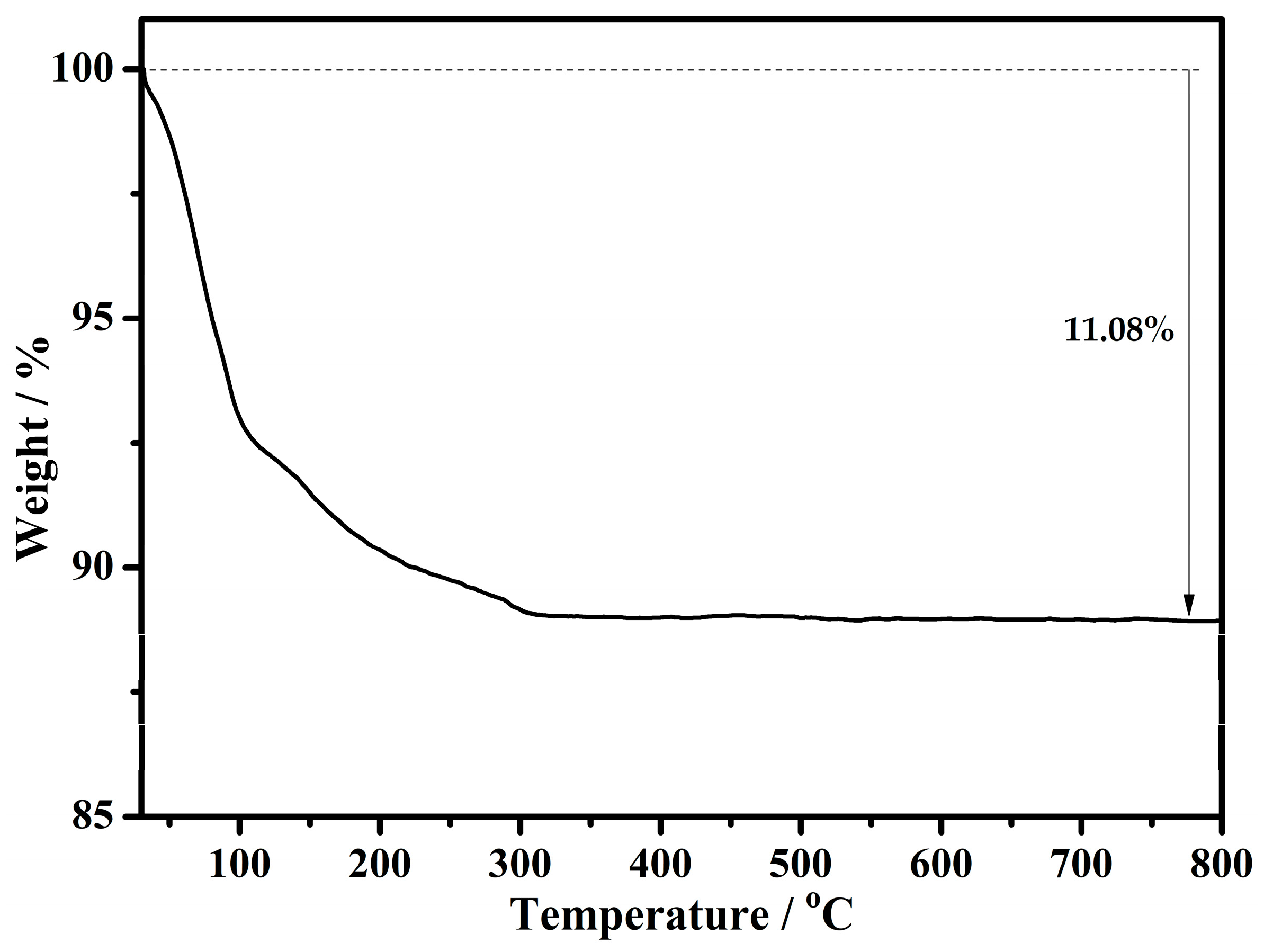
2.4. Thermogravimetric Analysis (TGA)
3. Materials and Methods
3.1. Synthesis of Compound 1
3.2. Characterization
3.3. Crystallography
3.4. General Procedure for Catalysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble Metals in Polyoxometalates. Angew. Chem. Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.; Abu-Gnim, C. A Ruthenium Heteropolyanion as Catalyst for Alkane and Alkene Oxidation. J. Chem. Soc. Chem. Commun. 1989, 18, 1324–1325. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Asami, M.; Hashimoto, M.; Misono, M. Alkane oxidation with mixed addenda heteropoly catalysts containing Ru(III) and Rh(III). J. Mol. Catal. A-Chem. 1996, 114, 161–168. [Google Scholar] [CrossRef]
- Lin, X.; Xu, J.; Liu, H.; Yue, B.; Jin, S.; Xie, G. Studies on styrene oxidation reaction catalyzed by ruthenium substituted polyoxotungstates: Kinetics and phase transfer effect. J. Mol. Catal. A-Chem. 2000, 161, 163–169. [Google Scholar]
- Yamaguchi, K.; Mizuno, N. Heterogeneously catalyzed liquid-phase oxidation of alkanes and alcohols with molecular oxygen. New J. Chem. 2002, 26, 972–974. [Google Scholar] [CrossRef]
- Khenkin, A.M.; Shimon, L.J.W.; Neumann, R. Preparation and Characterization of New Ruthenium and Osmium Containing Polyoxometalates, [M(DMSO)3Mo7O24]4− (M = Ru(II), Os(II)), and Their Use as Catalysts for the Aerobic Oxidation of Alcohols. Inorg. Chem. 2003, 42, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Shringarpure, P.; Patel, A. Undecatungstophospho(aqua)ruthenate(II): One pot synthesis, characterization and non-solvent liquid phase aerobic oxidation of alkenes. Inorg. Chim. Acta 2009, 362, 3796–3800. [Google Scholar] [CrossRef]
- Yokoyama, A.; Ohkubo, K.; Ishizuka, T.; Kojima, T.; Fukuzumi, S. Remarkable enhancement of catalytic activity of a 2:1 complex between a non-planar Mo(V)-porphyrin and a ruthenium-substituted Keggin-type heteropolyoxometalate in catalytic oxidation of benzyl alcohols. Dalton Trans. 2012, 41, 10006–10013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, R.-Q.; Suo, L.; Hou, G.-F.; Liang, J.; Bi, L.-H.; Li, H.-L.; Wu, L.-X. Organo-Ru supported sandwich-type tungstoarsenates: Synthesis, structure and catalytic properties. CrystEngComm 2013, 15, 5867–5876. [Google Scholar] [CrossRef]
- Meng, R.-Q.; Wang, B.; Sui, H.-M.; Li, B.; Song, W.; Wu, L.-X.; Zhao, B.; Bi, L.-H. Organoruthenium-Supported Polyoxotungstate—Synthesis, Structure and Oxidation of n-Hexadecane with Air. Eur. J. Inorg. Chem. 2013, 2013, 1935–1942. [Google Scholar] [CrossRef]
- Sartorel, A.; Miró, P.; Carraro, M.; Berardi, S.; Bortolini, O.; Bagno, A.; Bo, C.; Bonchio, M. Oxygenation by Ruthenium Monosubstituted Polyoxotungstates in Aqueous Solution: Experimental and Computational Dissection of a Ru(III)-Ru(V) Catalytic Cycle. Chem. Eur. J. 2014, 20, 10932–10943. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; He, C.; Qi, B.; Chen, C.; Niu, J.; Duan, C. Merging of the photocatalysis and copper catalysis in metal–organic frameworks for oxidative C–C bond formation. Chem. Sci. 2015, 6, 1035–1042. [Google Scholar] [CrossRef]
- Zheng, D.-M.; Wang, R.-Q.; Du, Y.; Hou, G.-F.; Wu, L.-X.; Bi, L.-H. A new organo-ruthenium substituted tungstotellurate: Synthesis, structural characterization and catalytic properties. New J. Chem. 2016, 40, 8829–8836. [Google Scholar] [CrossRef]
- Murakami, M.; Hong, D.; Suenobu, T.; Yamaguchi, S.; Ogura, T.; Fukuzumi, S. Catalytic Mechanism of Water Oxidation with Single-Site Ruthenium–Heteropolytungstate Complexes. J. Am. Chem. Soc. 2011, 133, 11605–11613. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, M.; Rinn, N.; Moroi, S.; Kitatomi, H.; Ozeki, T.; Kurasawa, M.; Itakura, M.; Hayakawa, S.; Kato, K.; Miyamoto, M.; et al. Preparation and StructuralCharacterization of RuII-DMSO and RuIII-DMSO-substituted α-Keggin-type Phosphotungstates, [PW11O39RuIIDMSO]5− and [PW11O39RuIIIDMSO]4−, and Catalytic Activity for Water Oxidation. Z. Anorg. Allg. Chem. 2011, 637, 1467–1474. [Google Scholar] [CrossRef]
- Miras, H.N.; Yan, J.; Long, D.-L.; Cronin, L. Engineering polyoxometalates with emergent properties. Chem. Soc. Rev. 2012, 41, 7403–7430. [Google Scholar] [CrossRef] [PubMed]
- Nishiki, K.; Umehara, N.; Kadota, Y.; López, X.; Poblet, J.M.; Mezui, C.A.; Teillout, A.-L.; Mbomekalle, I.M.; de Oliveira, P.; Miyamoto, M.; et al. Preparation of α1- and α2-isomers of mono-Ru-substituted Dawson-type phosphotungstates with an aqua ligand and comparison of their redox potentials, catalytic activities, and thermal stabilities with Keggin-type derivatives. Dalton Trans. 2016, 45, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-J.; Lin, Z.; Zhang, Z.-M.; Zhang, T.; Lin, W. Hierarchical Integration of Photosensitizing Metal-Organic Frameworks and Nickel-Containing Polyoxometalates for Efficient Visible-Light-Driven Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 6411–6416. [Google Scholar] [CrossRef] [PubMed]
- Putaj, P.; Lefebvre, F. Polyoxometalates containing late transition and noble metal atoms. Coord. Chem. Rev. 2011, 255, 1642–1685. [Google Scholar] [CrossRef]
- Neumann, R.; Abu-Gnim, C. Alkene oxidation catalyzed by a ruthenium-substituted heteropolyanion, SiRu(L)W11O39: The mechanism of the periodate mediated oxidative cleavage. J. Am. Chem. Soc. 1990, 112, 6025–6031. [Google Scholar] [CrossRef]
- Liu, H.; Yue, B.; Sun, W.; Chen, Z.; Jin, S.; Deng, J.; Xie, G. Synthesis and characterization of noble-metal-substituted Dawson-type polyoxometalates. Transit. Met. Chem. 1997, 22, 321–325. [Google Scholar] [CrossRef]
- Sadakane, M.; Higashijima, M. Synthesis and electrochemical behavior of [SiW11O39RuIII(H2O)]5− and its oxo-bridged dimeric complex [SiW11O39RuIVORuIIISiW11O39]11−. Dalton Trans. 2003, 4, 659–664. [Google Scholar] [CrossRef]
- Sadakane, M.; Tsukuma, D.; Dickman, M.H.; Bassil, B.; Kortz, U.; Higashijima, M.; Ueda, W. Structural characterization of mono-ruthenium substituted Keggin-type silicotungstates. Dalton Trans. 2006, 35, 4271–4276. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, M.; Tsukuma, D.; Dickman, M.H.; Bassil, B.S.; Kortz, U.; Capron, M.; Ueda, W. Dimerization of mono-ruthenium substituted a-Keggin-type tungstosilicate [a-SiW11O39RuIII(H2O)]5− to μ-oxo-bridged dimer in aqueous solution: Synthesis, structure, and redox studies. Dalton Trans. 2007, 26, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Besson, C.; Chen, S.-W.; Villanneau, R.; Izzet, G.; Proust, A. A new synthetic route towards a Ru(III) substituted heteropolytungstate anion. Inorg. Chem. Commun. 2009, 12, 1042–1044. [Google Scholar] [CrossRef]
- Rong, C.; Pope, M.T. Lacunary polyoxometalate anions are π-acceptor ligands. Characterization of some tungstoruthenate(II,III,IV,V) heteropolyanions and their atom-transfer reactivity. J. Am. Chem. Soc. 1992, 114, 2932–2938. [Google Scholar] [CrossRef]
- Randall, W.J.; Weakley, T.J.R.; Finke, R.G. Oxidation Resistant Inorganic-Porphyrin Analog Polyoxometalates. 3. The Synthesis and X-ray Crystallographic Characterization of a New Heteropolyoxoanion Structural Type, the Diruthenium-Oxo-Bridged “Rimetallic Inorganic-Porphyrin Analog” KLi5[O{RuCI(α2-P2W17O61)}2]·2KC1·60H2O. Inorg. Chem. 1993, 32, 1068–1071. [Google Scholar]
- Nomiya, K.; Torii, H.; Nomura, K.; Sato, Y. Synthesis and characterization of a monoruthenium(III)-substituted Dawson polyoxotungstate derived by Br2 oxidation of the 1:2 complex of ruthenium(II) and [α2-P2W17O61]10−. The reactivity of cis-[RuCl2(DMSO)4] as a ruthenium source. J. Chem. Soc. Dalton Trans. 2001, 9, 1506–1512. [Google Scholar] [CrossRef]
- Neumann, R.; Khenkin, A.M. Noble Metal (RuIII, PdII, PtII) Substituted “Sandwich” Type Polyoxometalates: Preparation, Characterization, and Catalytic Activity in Oxidations of Alkanes and Alkenes by Peroxides. Inorg. Chem. 1995, 34, 5753–5760. [Google Scholar] [CrossRef]
- Neumann, R.; Khenkin, A.M.; Juwiler, D.; Miller, H.; Gara, M. Catalytic oxidation with hydrogen peroxide catalyzed by ‘sandwich’ type transition metal substituted polyoxometalates. J. Mol. Catal. A-Chem. 1997, 117, 169–183. [Google Scholar] [CrossRef]
- Neumann, R.; Dahan, M. A ruthenium-substituted polyoxometalate as an inorganic dioxygenase for activation of molecular oxygen. Nature 1997, 388, 353–355. [Google Scholar] [CrossRef]
- Neumann, R.; Dahan, M. Molecular Oxygen Activation by a Ruthenium-Substituted “Sandwich” Type Polyoxometalate. J. Am. Chem. Soc. 1998, 120, 11969–11976. [Google Scholar] [CrossRef]
- Howells, A.R.; Sankarraj, A.; Shannon, C. A Diruthenium-Substituted Polyoxometalate as an Electrocatalyst for Oxygen Generation. J. Am. Chem. Soc. 2004, 126, 12258–12259. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.M.; Anderson, O.P.; Finke, R.G. Reinvestigation of a Ru2-Incorporated Polyoxometalate Dioxygenase Precatalyst, “[WZnRu2III(H2O)(OH)(ZnW9O34)2]11−”: Evidence For Marginal, ≤0.2 Equivalents of Ru Incorporation plus Faster Catalysis by Physical Mixtures of [RuII(DMSO)4Cl2] and the Parent Polyoxometalate [WZn3(H2O)2(ZnW9O34)2]12−. Inorg. Chem. 2009, 48, 4411–4420. [Google Scholar] [PubMed]
- Chen, S.-W.; Villanneau, R.; Li, Y.; Chamoreau, L.-M.; Boubekeur, K.; Thouvenot, R.; Gouzerh, P.; Proust, A. Hydrothermal Synthesis and Structural Characterization of the High-Valent Ruthenium-Containing Polyoxoanion [{PW11O39}2{(HO)RuIV–O–RuIV(OH)}]10−. Eur. J. Inorg. Chem. 2008, 2008, 2137–2142. [Google Scholar] [CrossRef]
- Besson, C.; Musaev, D.G.; Lahootun, V.; Cao, R.; Chamoreau, L.-M.; Villanneau, R.; Villain, F.; Thouvenot, R.; Geletii, Y.V.; Hill, C.L.; et al. Vicinal Dinitridoruthenium-Substituted Polyoxometalates γ-[XW10O38{RuN}2]6− (X = Si or Ge). Chem. Eur. J. 2009, 15, 10233–10243. [Google Scholar] [CrossRef] [PubMed]
- Sartorel, A.; Carraro, M.; Scorrano, G.; Zorzi, R.D.; Geremia, S.; McDaniel, N.D.; Bernhard, S.; Bonchio, M. Polyoxometalate Embedding of a Tetraruthenium(IV)-oxo-core by Template-Directed Metalation of [γ-SiW10O36]8− : A Totally Inorganic Oxygen-Evolving Catalyst. J. Am. Chem. Soc. 2008, 130, 5006–5007. [Google Scholar] [CrossRef] [PubMed]
- Geletii, Y.V.; Botar, B.; Kögerler, P.; Hillesheim, D.A.; Musaev, D.G.; Hill, C.L. An all-inorganic, stable, and highly active tetraruthenium homogeneous catalyst for water oxidation. Angew. Chem. Int. Ed. 2008, 47, 3896–3899. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Uehara, K.; Kamata, K.; Yamaguchi, K.; Mizuno, N. A γ-Keggin-type dimeric silicotungstate sandwiching an adamantanoid tetra-nuclear ruthenium-oxygen cluster core. Chem. Lett. 2008, 37, 328–329. [Google Scholar] [CrossRef]
- Besson, C.; Huang, Z.; Geletii, Y.V.; Lense, S.; Hardcastle, K.I.; Musaev, D.G.; Lian, T.; Proust, A.; Hill, C.L. Cs9[(γ-PW10O36)2Ru4O5(OH)(H2O)4], a new all-inorganic, soluble catalyst for the efficient visible-light-driven oxidation of water. Chem. Commun. 2010, 46, 2784–2786. [Google Scholar] [CrossRef] [PubMed]
- Gamelas, J.A.F.; Carapuça, H.M.; Balula, M.S.; Evtuguin, D.V.; Schlindwein, W.; Figueiras, F.G.; Amaral, V.S.; Cavaleiro, A.M.V. Synthesis and characterisation of novel ruthenium multi-substituted polyoxometalates: α,β-[SiW9O37Ru4(H2O)3Cl3]7−. Polyhedron 2010, 29, 3066–3073. [Google Scholar] [CrossRef]
- Car, P.-E.; Guttentag, M.; Baldridge, K.K.; Alberto, R.; Patzke, G.R. Synthesis and characterization of open and sandwich-type polyoxometalates reveals visible-light-driven water oxidation via POM-photosensitizer complexes. Green Chem. 2012, 14, 1680–1688. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.; Wang, Y.-F.; Yi, X.-Y. Redox chemistry of ruthenium ions in mono-substituted Keggin tungstophosphate: A new synthetic extension for ruthenium derivatives based on [PW11O39RuVIN]4−. Dalton Trans. 2015, 44, 16882–16887. [Google Scholar] [CrossRef] [PubMed]
- Oonaka, T.; Hashimoto, K.; Kominami, H.; Matsubara, Y.; Kera, Y. Synthesis of Ruthennium-containing Polyoxomolybdate and Its Catalytic Featurres for Liquid-phase Oxidation Using Peroxo Compounds. J. Jpn. Pet. Inst. 2005, 48, 178–179. [Google Scholar] [CrossRef]
- Oonaka, T.; Hashimoto, K.; Kominami, H.; Kera, Y.; Matsubara, Y. Oxidative dehydrogenation of methanol over a ruthenium-containing polyoxomolybdate supported on metal oxides chemically modified with silane coupling agent. Catal. Today 2006, 111, 354–360. [Google Scholar] [CrossRef]
- Hashikawa, A.; Fujimoto, M.; Hayashi, Y.; Miyasaka, H. Isolation of a stable lacunary Dawson-type polyoxomolybdate cluster. Chem. Commun. 2011, 47, 12361–12363. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sun, X.; Hu, F.; Wan, R.; Singh, V.; Ma, P.; Niu, J.; Wang, J. Two New Sandwich-Type Polyoxomolybdates Functionalized with Diphosphonates: Efficient and Selective Oxidation of Sulfides to Sulfones. Materials 2017, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Gallezot, P.; Bergeret, G. Cinnamaldehyde hydrogenation: Dual catalytic chemistry of iron-rhodium/Grafoil catalysts. J. Mol. Catal. 1993, 78, 295–307. [Google Scholar] [CrossRef]
- Saini, M.K.; Gupta, R.; Parbhakar, S.; Singh, S.; Hussain, F. Lanthano-phosphotungstate: A water soluble and reusable catalyst for oxidation of alcohols using H2O2 as an oxidant. RSC Adv. 2014, 4, 38446–38449. [Google Scholar] [CrossRef]
- Shang, S.; Wang, L.; Dai, W.; Chen, B.; Lv, Y.; Gao, S. High catalytic activity of mesoporous Co–N/C catalysts for aerobic oxidative synthesis of nitriles. Catal. Sci. Technol. 2016, 6, 5746–5753. [Google Scholar] [CrossRef]
- Jia, Y.; Fang, Y.; Zhang, Y.; Miras, H.N.; Song, Y.-F. Classical Keggin Intercalated into Layered Double Hydroxides: Facile Preparation and Catalytic Efficiency in Knoevenagel Condensation Reactions. Chem. Eur. J. 2015, 21, 14862–14870. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.L.; Sulejmanovic, D.; Schiller, J.B.; Turner, E.M.; Hwu, S.-J.; Whitehead, D.C. Room-Temperature Catalytic Oxidation of Alcohols with the Polyoxovanadate Salt Cs5(V14As8O42Cl). Catal. Sci. Technol. 2016, 6, 3208–3213. [Google Scholar] [CrossRef]
- Zhang, Z.; Sadakane, M.; Murayama, T.; Izumi, S.; Yasuda, N.; Sakaguchi, N.; Ueda, W. Tetrahedral Connection of ε-Keggin-type Polyoxometalates to Form an All-Inorganic Octahedral Molecular Sieve with an Intrinsic 3D Pore System. Inorg. Chem. 2014, 53, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.-G.; Chang, X.-Q.; Yan, J.-S.; Gong, K.-N.; Zhao, C.; Zhai, X.-L. An Unusual Metallic Oxygen Cluster Consisting of a {AlMo12O40(MoO2)}. Inorg. Chem. 2014, 53, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, B. ESCA Studies on the Charge Distribution in Some Dinitrogen Complexes of Rhenium, Iridium, Ruthenium, and Osmium. Acta Chem. Scand. 1973, 27, 287–302. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stikle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Kalinina, I.V.; Izarova, N.V.; Kortz, U. Bis[tetraruthenium(IV)]-Containing Polyoxometalates: [{RuIV4O6(H2O)9}2Sb2W20O68(OH)2]4− and [{RuIV4O6(H2O)9}2{Fe(H2O)2}2{β-TeW9O33}2H]−. Inorg. Chem. 2012, 51, 7442–7444. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SADABS‒Bruker AXS Area Detector Scaling and Absorption, Version 2008/2001; University of Göttingen: Göttingen, Germany, 2008. [Google Scholar]

| Entry | Substrate | Product | Yield (%) | Entry | Substrate | Product | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 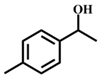 | 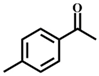 | 99 | 7 |  |  | 58 |
| 2 |  |  | 99 | 8 | 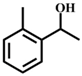 | 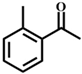 | 52 |
| 3 |  | 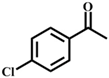 | 97 | 9 |  |  | 70 |
| 4 | 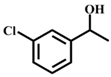 | 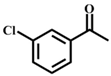 | 100 | 10 | 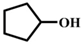 |  | 70 |
| 5 |  | 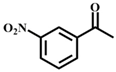 | 97 | 11 | 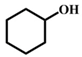 |  | 76 |
| 6 |  |  | 46 | 12 |  |  | 97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, R.; Xu, Q.; Han, M.; Ma, P.; Zhang, C.; Niu, J.; Wang, J. A Novel Ruthenium-Decorating Polyoxomolybdate Cs3Na6H[MoVI14RuIV2O50(OH)2]·24H2O: An Active Heterogeneous Oxidation Catalyst for Alcohols. Materials 2018, 11, 178. https://doi.org/10.3390/ma11020178
Wan R, Xu Q, Han M, Ma P, Zhang C, Niu J, Wang J. A Novel Ruthenium-Decorating Polyoxomolybdate Cs3Na6H[MoVI14RuIV2O50(OH)2]·24H2O: An Active Heterogeneous Oxidation Catalyst for Alcohols. Materials. 2018; 11(2):178. https://doi.org/10.3390/ma11020178
Chicago/Turabian StyleWan, Rong, Qiaofei Xu, Mengdan Han, Pengtao Ma, Chao Zhang, Jingyang Niu, and Jingping Wang. 2018. "A Novel Ruthenium-Decorating Polyoxomolybdate Cs3Na6H[MoVI14RuIV2O50(OH)2]·24H2O: An Active Heterogeneous Oxidation Catalyst for Alcohols" Materials 11, no. 2: 178. https://doi.org/10.3390/ma11020178