The Electronic Structure and Optical Properties of Anatase TiO2 with Rare Earth Metal Dopants from First-Principles Calculations
Abstract
:1. Introduction
2. Method and Calculation
3. Results and Discussions
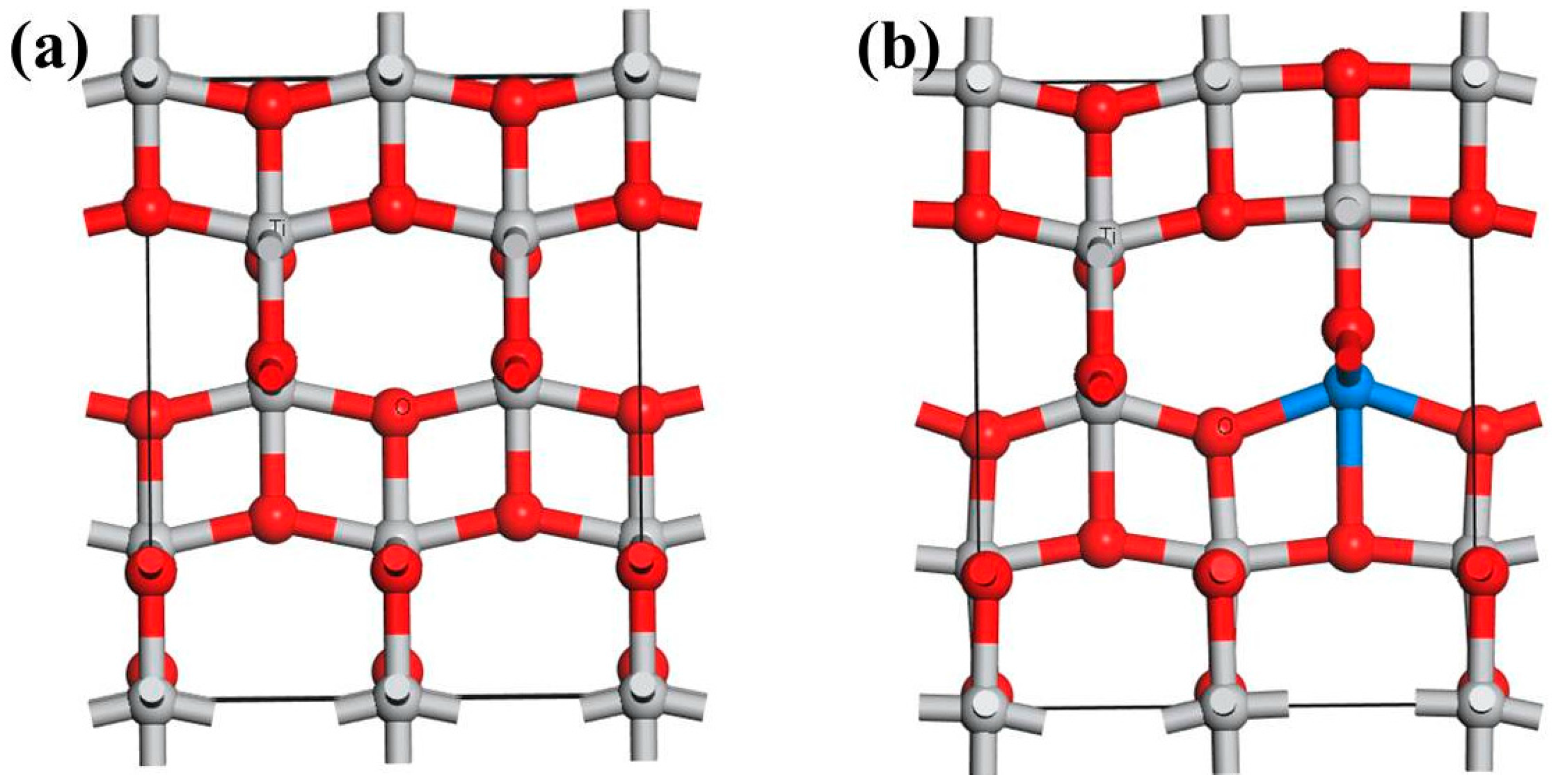
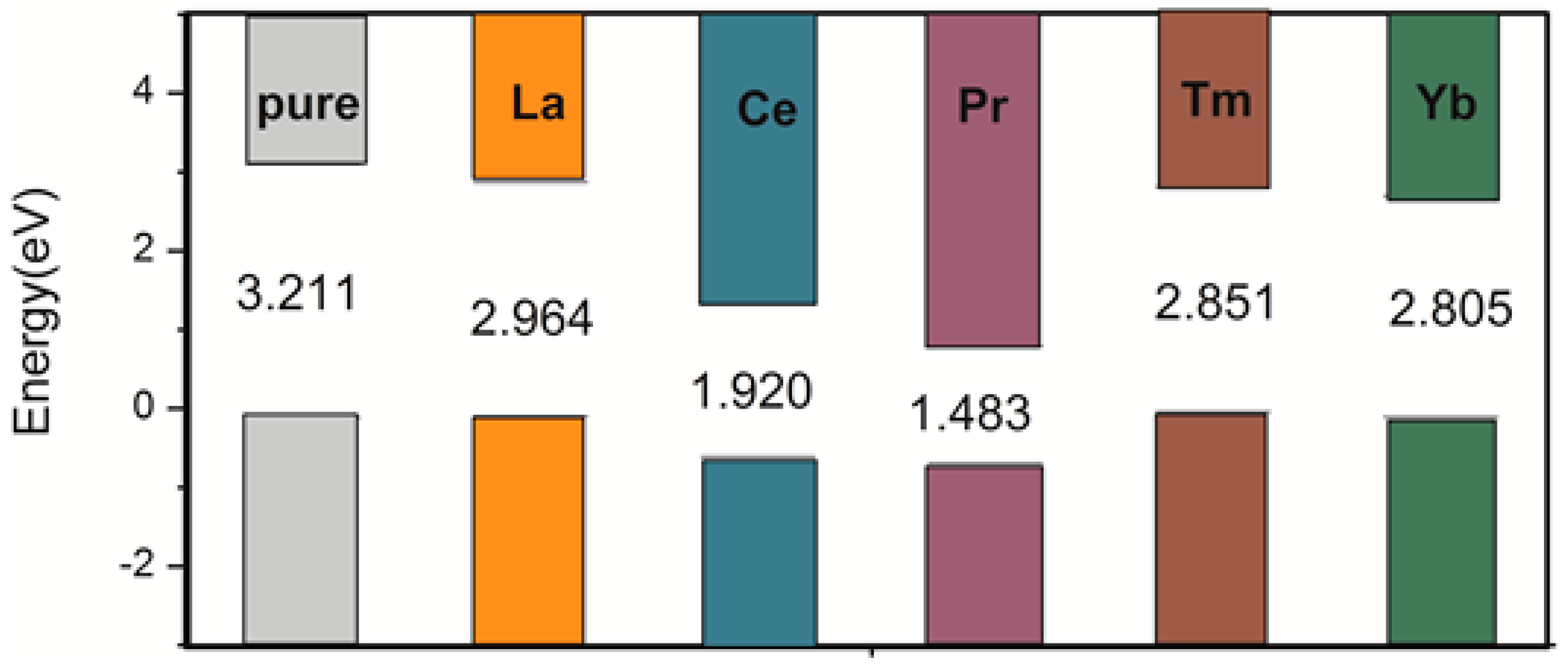
3.1. Defect Configurations and Formation Energy
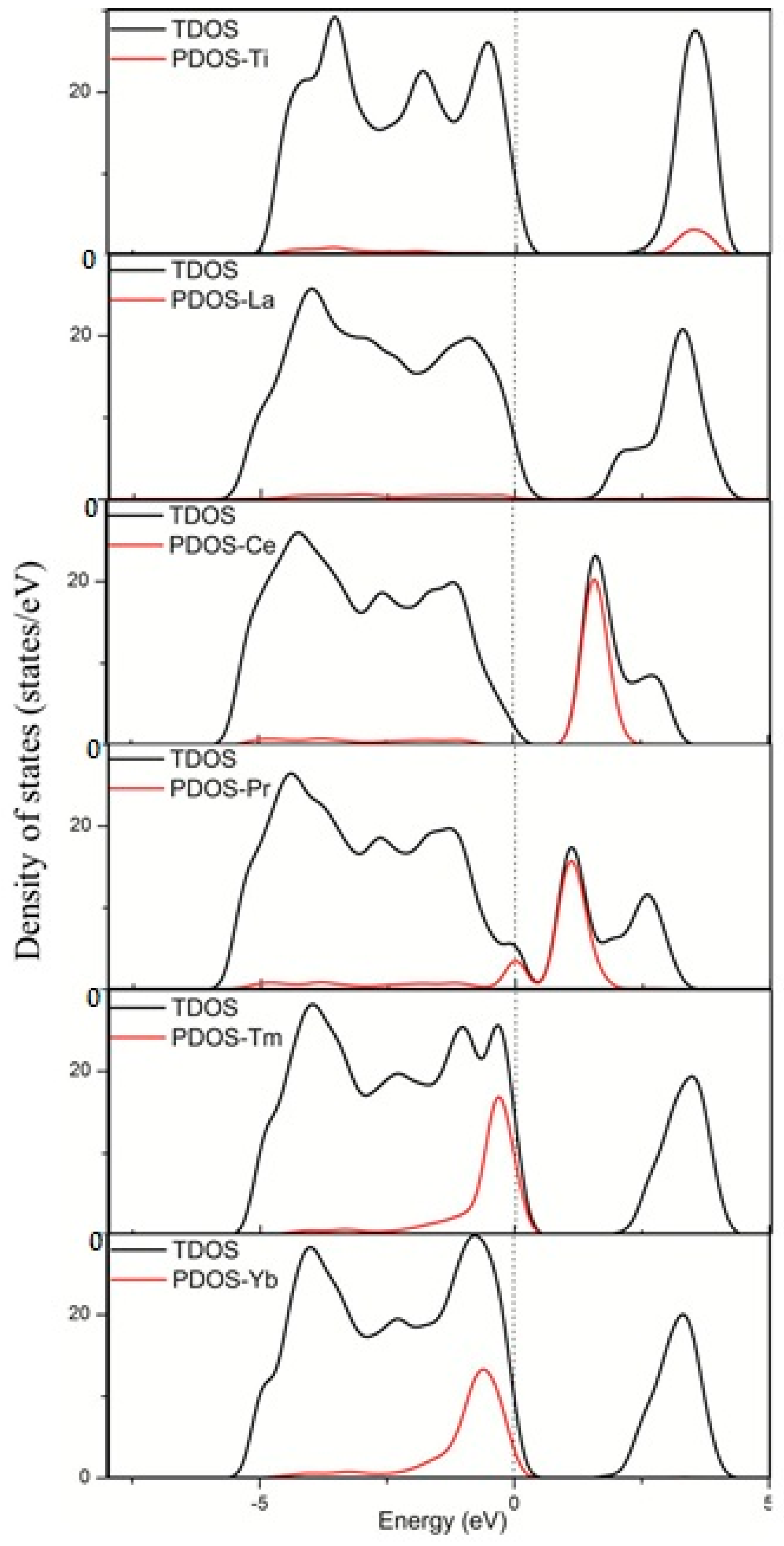
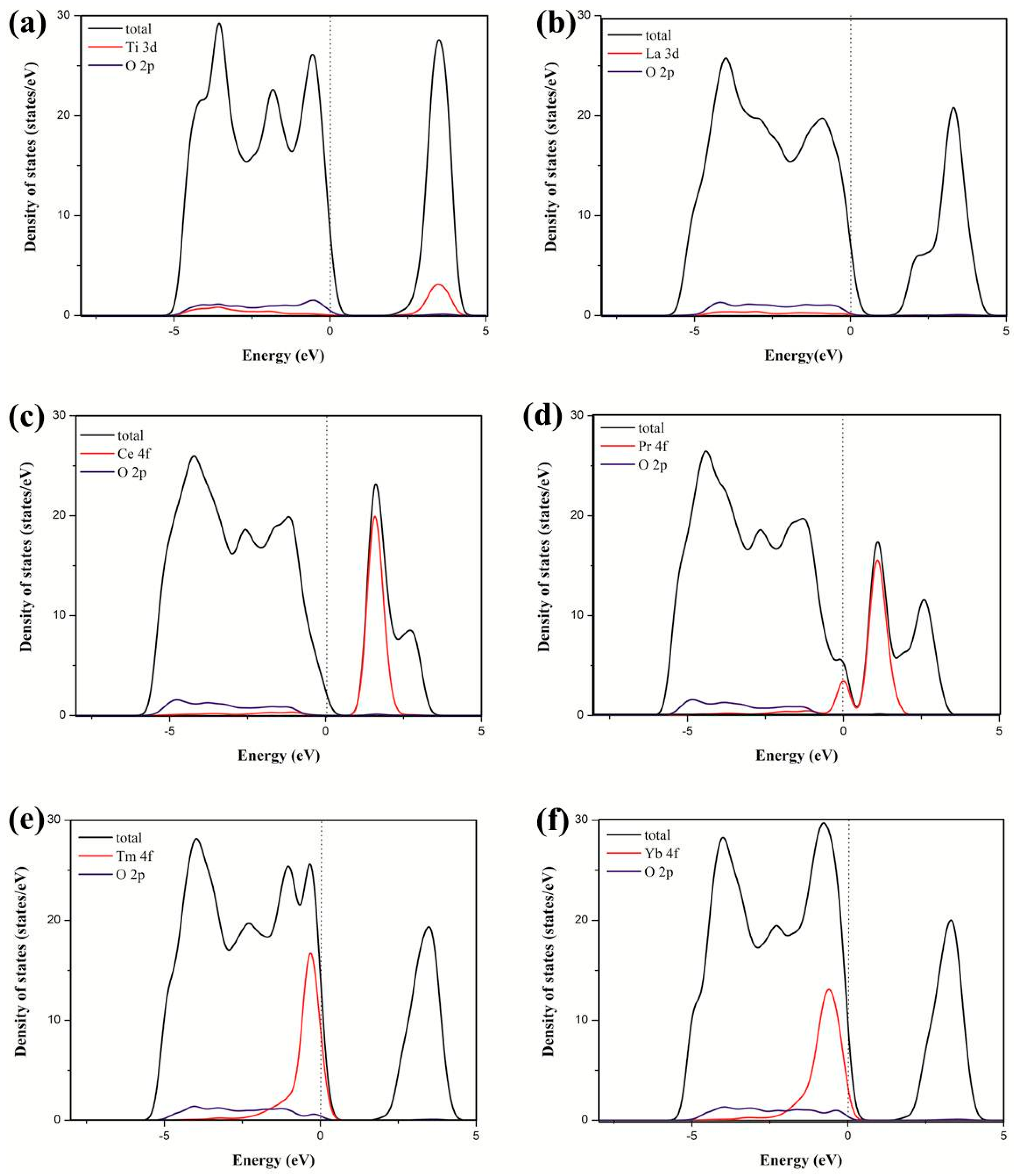
3.2. Electronic Structure and Properties
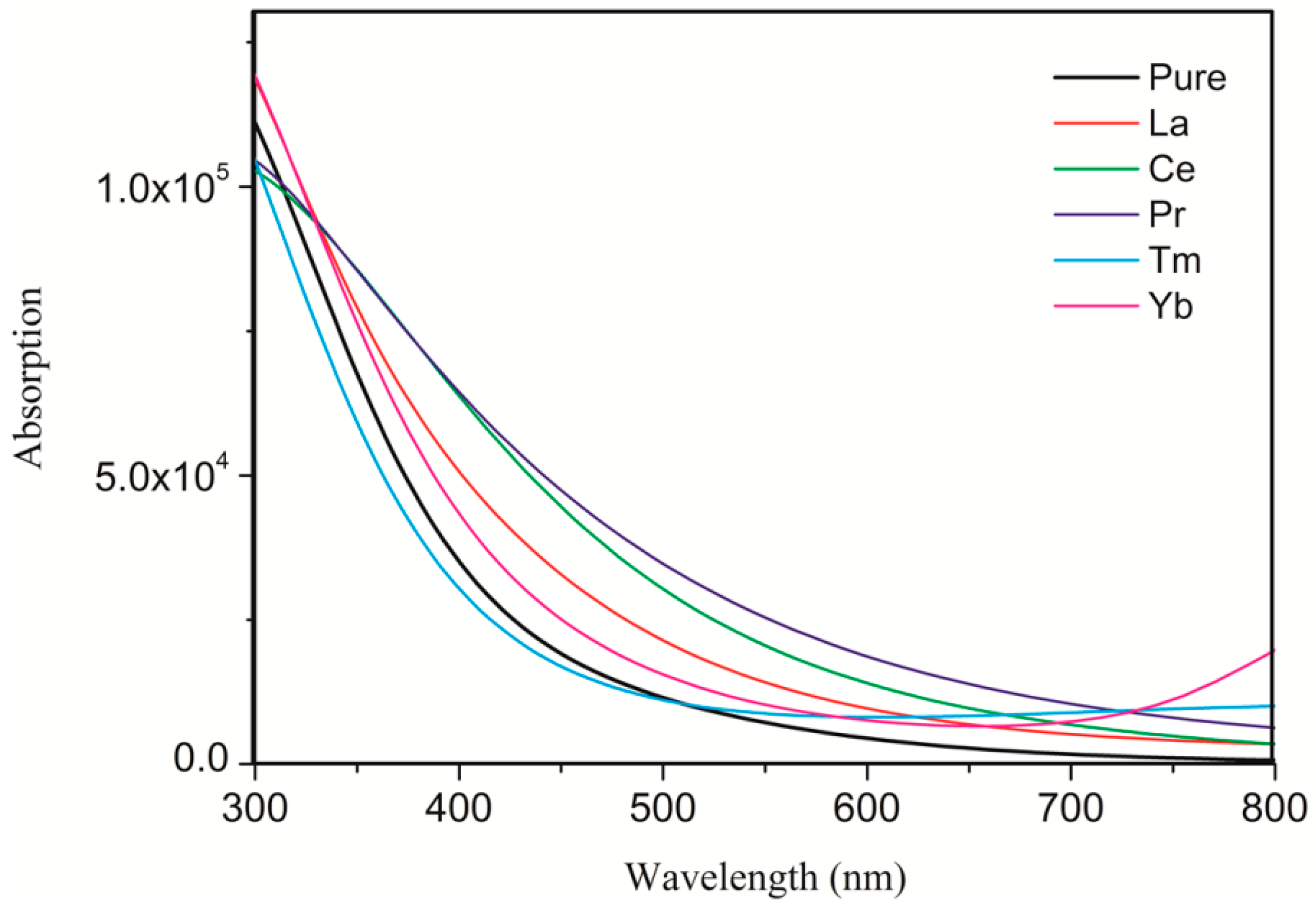
3.3. Optical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at asemiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysisin nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Fujihira, M.; Satoh, Y.; Osa, T. Heterogeneous photocatalytic oxidation ofaromatic compounds on TiO2. Nature 1981, 293, 206–208. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Najme, S.; Reza, M.M. Enhanced electron collection efficiency of nanostructured dye-sensitized solar cells by incorporating TiO2 cubes. J. Am. Ceram. Soc. 2018, 101, 293–306. [Google Scholar]
- Hou, X.; Zhou, J.; Huang, S.; Ou-Yang, W.; Pan, L.; Chen, X. Efficient quasi-mesoscopic perovskite solar cells using Li-doped hierarchical TiO2 as scaffold of scattered distribution. Chem. Eng. J. 2017, 330, 947–955. [Google Scholar] [CrossRef]
- Umeyama, T.; Imahori, H. A chemical approach to perovskite solar cells: Control of electron-transporting mesoporous TiO2 and utilization of nanocarbon materials. Dalton Trans. 2017, 46, 15615–15627. [Google Scholar] [CrossRef] [PubMed]
- El-Bery, H.M.; Matsushita, Y.; Abdel-Moneim, A. Fabrication of efficient TiO2-RGO heterojunction composites for hydrogen generation via water-splitting: Comparison between RGO, Au and Pt reduction sites. Appl. Surf. Sci. 2017, 423, 185–196. [Google Scholar] [CrossRef]
- Morita, K.; Takijiri, K.; Sakai, K.; Ozawa, H. A platinum porphyrin modified TiO2 electrode for photoelectrochemical hydrogen production from neutral water driven by the conduction band edge potential of TiO2. Dalton Trans. 2017, 46, 15181–15185. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, J.J.; Fernández-González, R.; Díaz, L.; Melián, E.P.; Rodríguez, V.D.; Núñez, P. Effect of reaction temperature and sacrificial agent on the photocatalytic H2-production of Pt-TiO2. J. Alloys Compd. 2017, 721, 405–410. [Google Scholar] [CrossRef]
- Díaz-Real, J.A.; Dubed-Bandomo, G.C.; Galindo-de-la-Rosa, J.; Ortiz-Ortega, E.; Ledesma-García, J.; Arriaga, L.G. Evaluation of transferable TiO2 nanotube membranes as electrocatalyst support for methanol photoelectrooxidation. Appl. Catal. B-Environ. 2018, 222, 18–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B-Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Ji, L.; Cao, X.; Lu, S.; Du, C.; Li, X.; Chen, T.; Buekens, A.; Yan, J. Catalytic oxidation of PCDD/F on a V2O5-WO3/TiO2 catalyst: Effect of chlorinated benzenes and chlorinated phenols. J. Hazard. Mater. 2018, 342, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Benjwal, P.; Bibekananda, D.; Kar, K.K. 1-D and 2-D morphology of metal cation co-doped (Zn, Mn) TiO2 and investigation of their photocatalytic activity. Appl. Surf. Sci. 2017, 427, 262–272. [Google Scholar] [CrossRef]
- Baldini, E.; Chiodo, L.; Dominguez, A.; Palummo, M.; Moser, S.; Yazdi-Rizi, M.; Auböck, G.; Mallett, B.P.P.; Berger, H.; Magrez, A.; et al. Strongly bound excitons in anatase TiO2 single crystals and nanoparticles. Nat. Commun. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Longoni, G.; Cabrera, R.L.P.; Polizzi, S.; D’Arienzo, M.; Mari, C.M.; Cui, Y.; Ruffo, R. Shape-Controlled TiO2 Nanocrystals for Na-Ion Battery Electrodes: The Role of Different Exposed Crystal Facets on the Electrochemical Properties. Nano Lett. 2017, 17, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Mazierski, P.; Lisowski, W.; Grzyb, T.; Winiarski, M.J.; Klimczuk, T.; Mikołajczyk, A.; Flisikowski, J.; Hirsch, A.; Kołakowska, A.; Puzyn, T.; et al. Enhanced photocatalytic properties of lanthanide-TiO2 nanotubes: Anexperimental and theoretical study. Appl. Catal. B-Environ. 2017, 205, 376–385. [Google Scholar] [CrossRef]
- Jin, C.; Dai, Y.; Wei, W.; Ma, X.; Li, M.; Huang, B. Effects of single metal atom (Pt, Pd, Rh and Ru) adsorption on the photocatalytic properties of anatase TiO2. Appl. Surf. Sci. 2017, 426, 639–646. [Google Scholar] [CrossRef]
- Lee, Y.; El-Shall, H. Ultra-high aspect ratio Titania nanoflakes for dye-sensitized solar cells. Appl. Surf. Sci. 2017, 426, 1263–1270. [Google Scholar] [CrossRef]
- Vafaeia, M.; Mohammadi, M.R. Impact of chromium doping on physical, optical, electronic and photovoltaic properties of nanoparticle TiO2 photoanodes in dye-sensitized solar cells. New J. Chem. 2017, 41, 14516–14527. [Google Scholar] [CrossRef]
- Wei, K.; Li, K.; Yan, L.; Luo, S.; Guo, H.; Dai, Y.; Luo, X. One-step fabrication of g-C3N4 nanosheets/TiO2 hollow microspheres heterojunctions with atomic level hybridization and their application in the multi-component synergistic photocatalytic systems. Appl. Catal. B-Environ. 2018, 222, 88–98. [Google Scholar] [CrossRef]
- Yang, T.; Peng, J.; Zheng, Y.; He, X.; Hou, Y.; Wu, L.; Fu, X. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites. Appl. Catal. B-Environ. 2018, 221, 223–234. [Google Scholar] [CrossRef]
- Bansal, P.; Verma, A. In-situ dual effect studies using novel Fe-TiO2 composite for the pilot-plant degradation of pentoxifylline. Chem. Eng. J. 2018, 332, 682–694. [Google Scholar] [CrossRef]
- Duan, L.; Jiang, N.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Synergetic effect of TiO2 and Fe3+ as co-catalysts for enhanced phenol degradation in pulsed discharge system. Appl. Catal. B-Environ. 2018, 221, 521–529. [Google Scholar] [CrossRef]
- Salazar-Villanueva, M.; Cruz-López, A.; Zaldívar-Caden, A.A.; Tovar-Coron, A.; Guevara-Romero, M.L.; Vazquez-Cuchillo, O. Effect of the electronic state of Ti on M-doped TiO2 nanoparticles (M = Zn, Ga or Ge) with high photocatalytic activities: An experimental and DFT molecular study. Mater. Sci. Semicond. Proc. 2017, 58, 8–14. [Google Scholar] [CrossRef]
- Neubert, S.; Mitoraj, D.; Shevlin, S.A.; Pulisova, P.; Heimann, M.; Du, Y.; Goh, G.K.L.; Pacia, M.; Kruczala, K.; Turner, S.; et al. Highly efficient rutile TiO2 photocatalysts with single Cu(II) and Fe(III) surface catalytic sites. J. Mater. Chem. A 2016, 4, 3127–3138. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local densityfunctional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Krishna Rao, K.V.; Nagender Naidu, S.V.; Iyengar, L. Thermal expansion of rutile and anatase. J. Am. Ceram. Soc. 1970, 53, 124–126. [Google Scholar]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustra-tions and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Melrose, D.B.; Stoneham, R.J. Generalised Kramers-Kronig formula for spatially dispersive media. J. Phys. A Math. Gen. 1977, 10, L17–L20. [Google Scholar] [CrossRef]
- Baizaee, S.M.; Mousavi, N. First-principles study of the electronic and optical properties of rutile TiO2. Phys. B Condens. Matter 2009, 404, 2111–2116. [Google Scholar] [CrossRef]
- Wang, H.; He, J.; Tian, Y.; Sun, J. Ab initio investigations of optical properties of the high-pressure phases of ZnO. Phys. Rev. B 2005, 71, 125132–125136. [Google Scholar]
- Li, X.; Shi, J.; Chen, H.; Wan, R.; Leng, C.; Chen, S.; Lei, Y. A DFT study on the modification mechanism of (Cr, C) co-doping for the electronic and optical properties of anatase TiO2. Comput. Mater. Sci. 2017, 129, 295–303. [Google Scholar] [CrossRef]
| Model | Electronic Configuration | dTi-O | dre-O | |
|---|---|---|---|---|
| Pure | 1.930 | - | - | |
| La | [Xe]5d16s2 | 1.835 | 2.180 | 9.34 |
| Ce | [Xe]4f15d16s2 | 1.846 | 2.138 | 5.16 |
| Pr | [Xe]4f36s2 | 1.847 | 2.132 | 5.36 |
| Nd | [Xe]4f46s2 | 1.844 | 2.125 | 14.64 |
| Pm | [Xe]4f56s2 | 1.845 | 2.120 | 18.14 |
| Sm | [Xe]4f66s2 | 1.841 | 2.125 | 23.01 |
| Eu | [Xe]4f76s2 | 1.829 | 2.142 | 28.84 |
| Gd | [Xe]4f75d16s2 | 1.848 | 2.100 | 28.70 |
| Tb | [Xe]4f96s2 | 1.850 | 2.103 | 20.96 |
| Dy | [Xe]4f106s2 | 1.844 | 2.106 | 16.20 |
| Ho | [Xe]4f116s2 | 1.845 | 2.101 | 11.26 |
| Er | [Xe]4f126s2 | 1.846 | 2.097 | 10.81 |
| Tm | [Xe]4f136s2 | 1.848 | 2.092 | 8.08 |
| Yb | [Xe]4f146s2 | 1.849 | 2.082 | 7.95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Jia, Q.; Wang, Y.; Zhang, W.; Xu, J. The Electronic Structure and Optical Properties of Anatase TiO2 with Rare Earth Metal Dopants from First-Principles Calculations. Materials 2018, 11, 179. https://doi.org/10.3390/ma11020179
Xie K, Jia Q, Wang Y, Zhang W, Xu J. The Electronic Structure and Optical Properties of Anatase TiO2 with Rare Earth Metal Dopants from First-Principles Calculations. Materials. 2018; 11(2):179. https://doi.org/10.3390/ma11020179
Chicago/Turabian StyleXie, Kefeng, Qiangqiang Jia, Yizhe Wang, Wenxue Zhang, and Jingcheng Xu. 2018. "The Electronic Structure and Optical Properties of Anatase TiO2 with Rare Earth Metal Dopants from First-Principles Calculations" Materials 11, no. 2: 179. https://doi.org/10.3390/ma11020179




