Novel Formulations of Phase Change Materials—Epoxy Composites for Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methodology
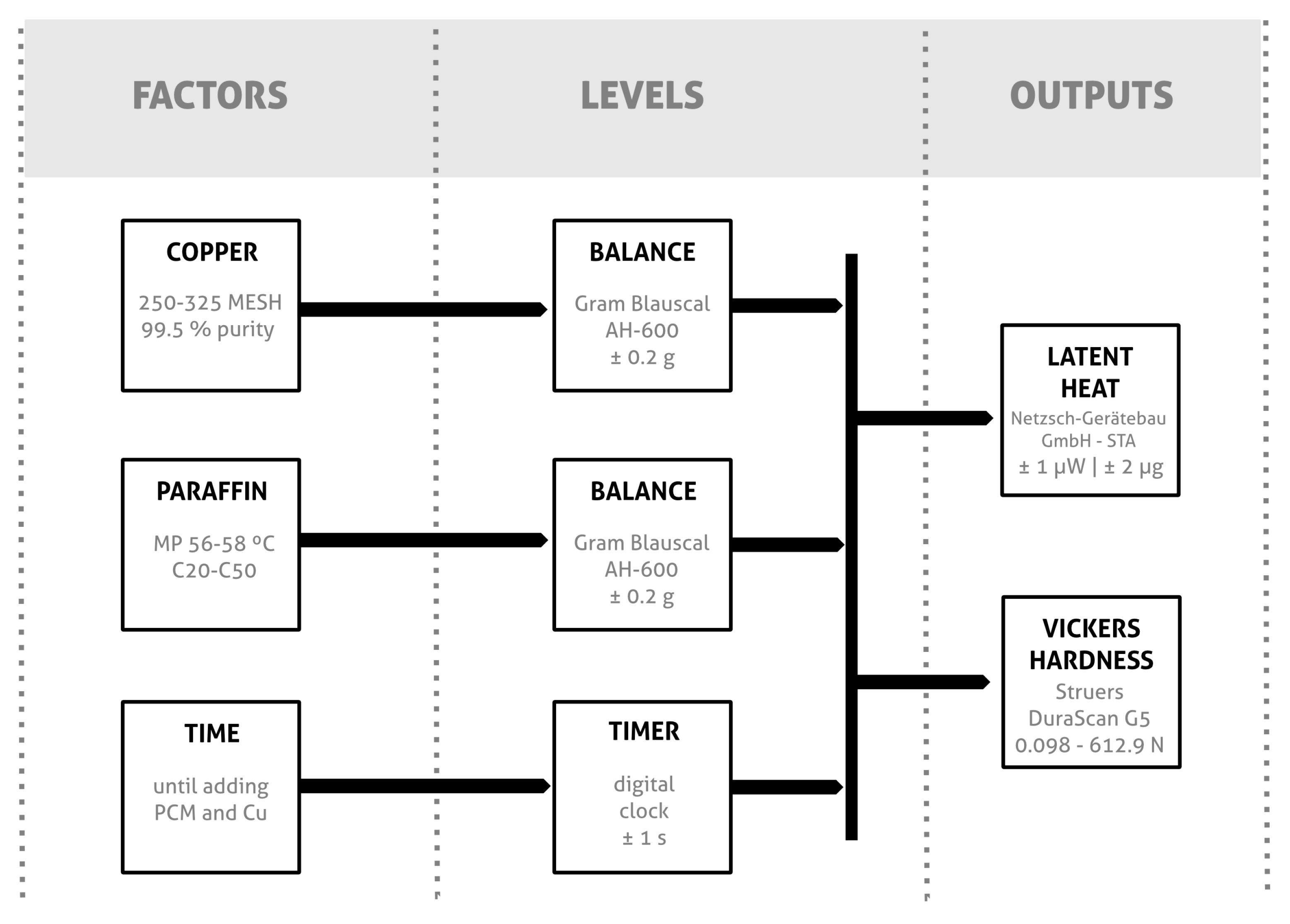
2.1. Materials
- -
- Epoxy resins: For the inorganic PCM-epoxy trials, the resin system employed already contained conductive particles as part of the commercial product formulation. It employed the aluminum-filled epoxy casting system Resoltech® 1450 T Alu 25, acquired from Castro Composites (Pontevedra, Spain).For the organic PCM-epoxy trials, the epoxy base Resolcoat® VLSUV A (density = 1.1 g/cm3, viscosity at 25 °C = 1200 mPa·s) and the hardener Resolcoat® VLSUV B (density = 0.95 g/cm3, viscosity at 25 °C = 1200 mPa·s) were supplied by Castro Composites (Pontevedra, Spain). The mechanical and physical properties of the mixture (epoxy resin + hardener) at 25 °C are: impact resistance = 18 J, viscosity = 1200 mPa s, density = 1.1 g/cm3 and TG of 45 °C. All materials were used as received. The mixture’s A:B ratio by weight was 100:70. The benefit of using this system was the option to acquire it as a bare resin and add diverse loadings of conductive particles as part of our experimental steps.
- -
- Inorganic PCM: Given the known transition temperatures of some salts used in previous studies [39], three different inorganic PCM were used:
- Na2S2O3·5H2O (assay 99.0–101.0%, melting temperature = 48.5 °C, relative density = 1.7)
- Zn(NO3)2·4H2O (assay 96.0–103.0% (ex Zn), melting temperature = 36.4 °C, density = 2.07 g/cm3)
- CH3COONa·3H2O, Acetic Acid Sodium Salt, (assay ≥ 98.0%, melting temperature = 58 °C, density = 1.45 g/cm3). These three laboratory reagents were supplied by Labbox Labware (Barcelona, Spain).
- -
- Organic PCM: PR 5658 CH® Paraffin wax (melting temperature = 56 °C–58 °C (ASTM D 938 method), maximum oil content = 0.5% (ASTM D 721 method), density at 25 °C = 0.8 g/cm3) was supplied by Ceras Martí (Barcelona, Spain). This paraffin wax is composed of straight chain paraffin hydrocarbons having carbon numbers predominantly in the approximate range of C20 through C50.
- -
- Conductive phase: With the aim of improving mechanical and thermal properties of the composites, free-flowing fine Cu powder Amertek® lab grade > 99.5% purity and 250–325 MESH (particle size) was used as an additive.
- -
- Thickening agent: Garamite 1958 powdered rheology additive was used to increase storage stability and sag resistance. This additive is composed of organophilic phyllosilicates (water content <6% and specific weight 1.5–1.7 g/cm³). The optimal loading in the composites (5% of total weight of the epoxy resin + hardener mixture) was determined through a series of preliminary laboratory tests. As with other materials, this additive was acquired from Castro Composites (Pontevedra, Spain).
2.2. Fabrication Steps
2.3. Experimental Design (Plackett-Burman) Followed for Organic PCM-Epoxy Composites
2.4. Characterization Methods
3. Results and Discussion
3.1. Inorganic PCM-Epoxy Composites
3.2. Organic PCM-Epoxy Composites
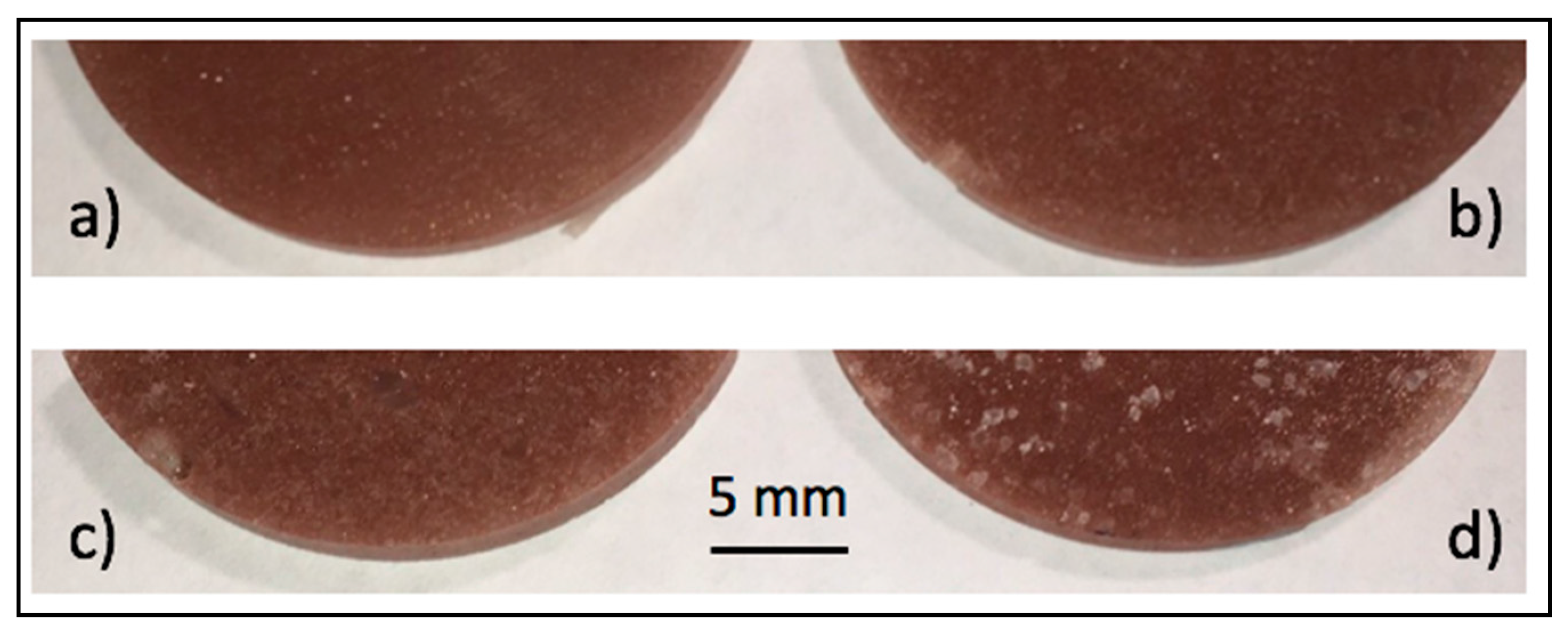
3.2.1. Visual and Microscopic Observations of Phases
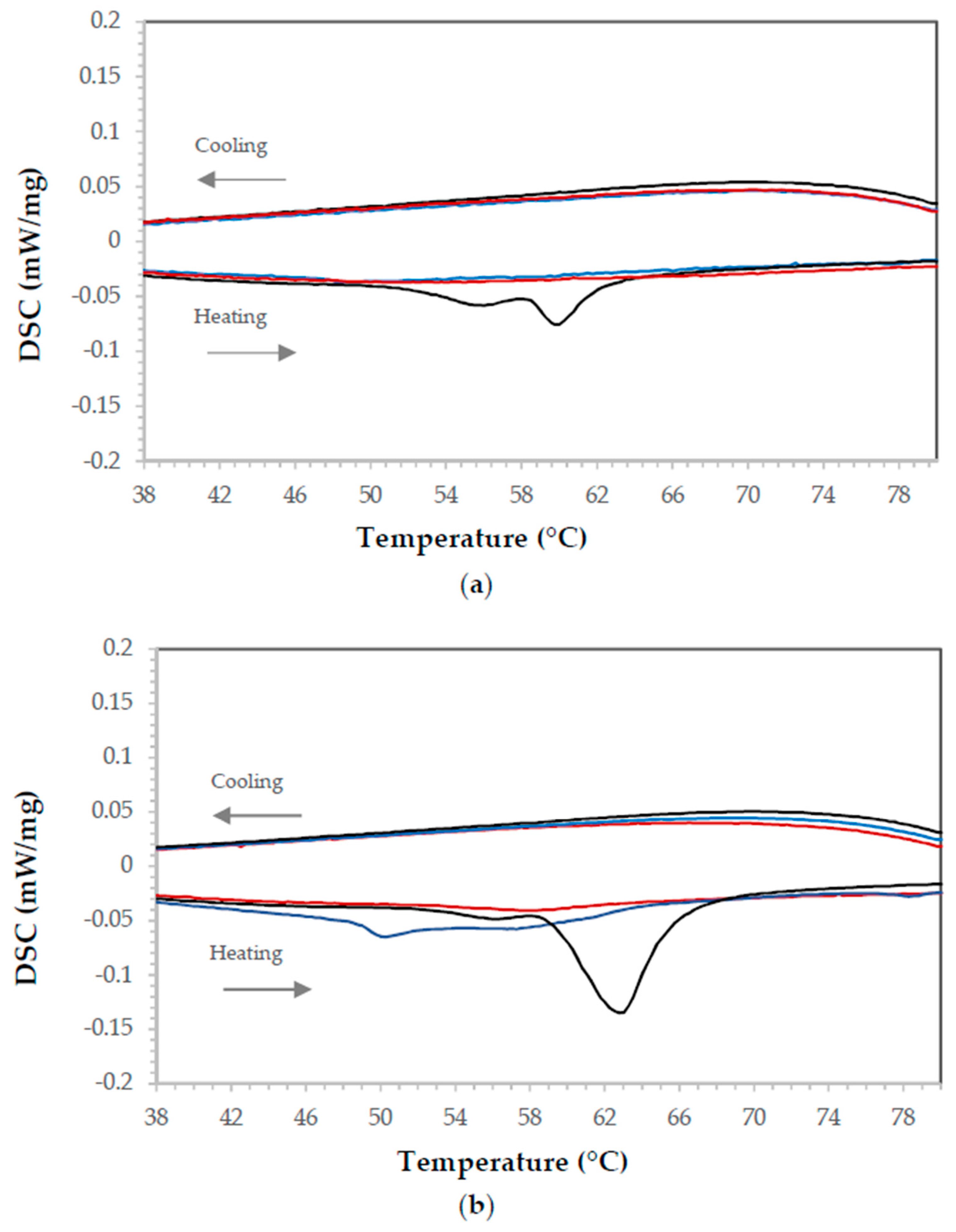
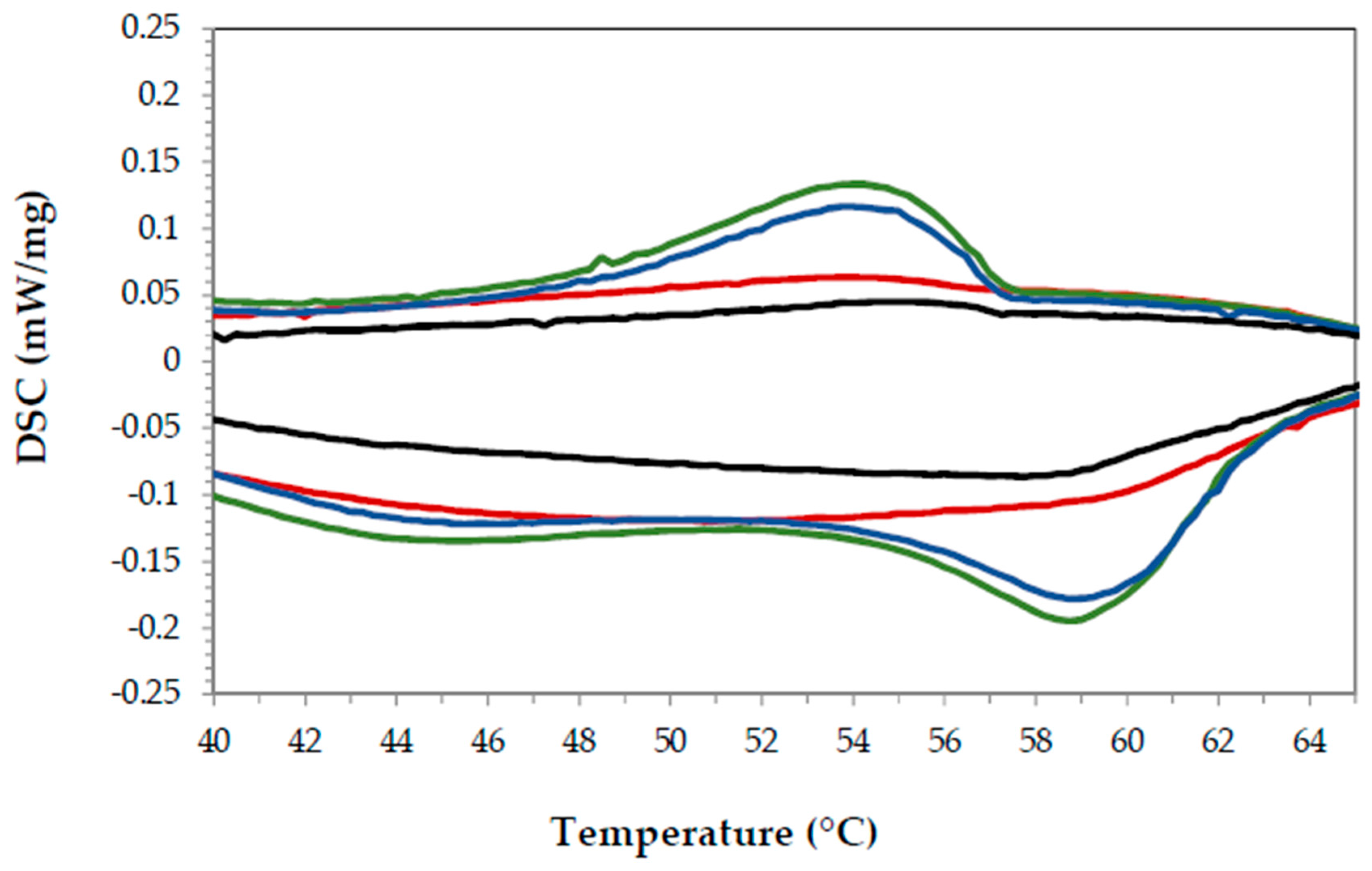
3.2.2. Thermal Analysis
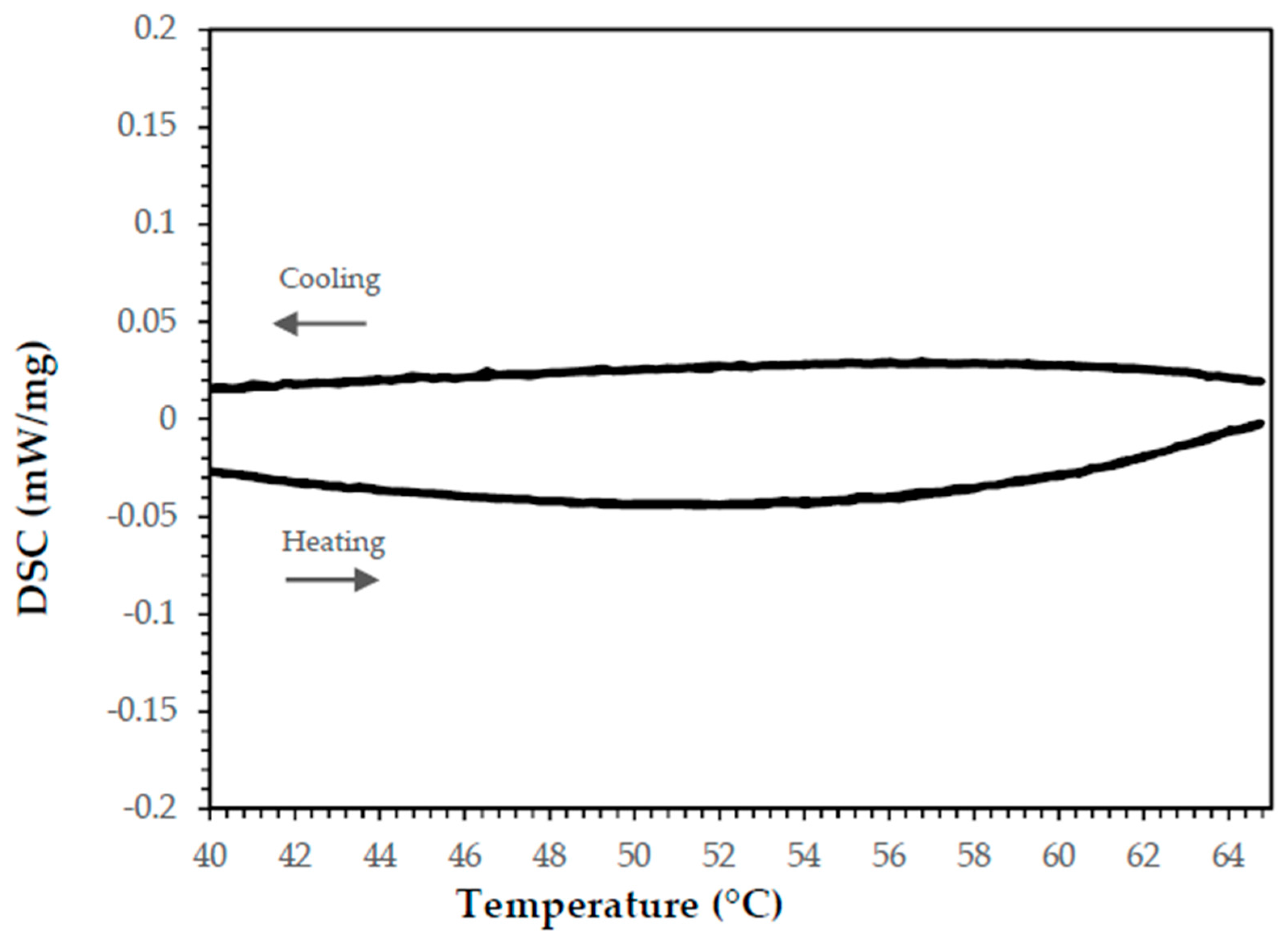
Paraffin
Cured Epoxy Resin
Organic PCM-Epoxy Composites
Performance over Time
3.2.3. Microhardness Test (HV).
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Diakaki, C.; Grigoroudis, E.; Kolokotsa, D. Towards a multi-objective optimization approach for improving energy efficiency in buildings. Energy Build. 2008, 40, 1747–1754. [Google Scholar] [CrossRef]
- Cabeza, L.F. Heat and Cold Storage with PCM: An Up to Date Introduction into Basics and Applications. Heat and Mass Transfer; Springer: Berlin, Germany, 2008. [Google Scholar]
- Dincer, I.; Rosen, M. Thermal Energy Storage: Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Panwar, N.; Kaushik, S.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Vazquez, S.; Lukic, S.M.; Galvan, E.; Franquelo, L.G.; Carrasco, J.M. Energy storage systems for transport and grid applications. IEEE Trans. Ind. Electron. 2010, 57, 3881–3895. [Google Scholar] [CrossRef]
- Khaligh, A.; Li, Z. Battery, ultracapacitor, fuel cell, and hybrid energy storage systems for electric, hybrid electric, fuel cell, and plug-in hybrid electric vehicles: State of the art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Chen, K.; Yu, X.; Tian, C.; Wang, J. Preparation and characterization of form-stable paraffin/polyurethane composites as phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 77, 13–21. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Huang, J.; Lu, S.; Kong, X.; Liu, S. Form-stable phase change materials based on eutectic mixture of tetradecanol and fatty acids for building energy storage: Preparation and performance analysis. Materials 2013, 6, 4758–4775. [Google Scholar] [CrossRef] [PubMed]
- Porteiro, J.; Míguez, J.L.; Crespo, B.; de Lara, J.; Pousada, J.M. On the Behavior of Different PCMs in a Hot Water Storage Tank against Thermal Demands. Materials 2016, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Eames, I.W.; Adref, K.T. Freezing and melting of water in spherical enclosures of the type used in thermal (ice) storage systems. Appl. Therm. Eng. 2002, 22, 733–745. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Lin, K.; Zhang, Q.; Di, H. Application of latent heat thermal energy storage in buildings: State-of-the-art and outlook. Build. Environ. 2007, 42, 2197–2209. [Google Scholar] [CrossRef]
- Pasupathy, A.; Velraj, R.; Seeniraj, R. Phase change material-based building architecture for thermal management in residential and commercial establishments. Renew. Sustain. Energy Rev. 2008, 12, 39–64. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.d.; De Gracia, A.; Fernández, A. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Rodriguez-Ubinas, E.; Ruiz-Valero, L.; Vega, S.; Neila, J. Applications of phase change material in highly energy-efficient houses. Energy Build. 2012, 50, 49–62. [Google Scholar] [CrossRef]
- Höhlein, S.; König-Haagen, A.; Brüggemann, D. Thermophysical Characterization of MgCl2 6H2O, Xylitol and Erythritol as Phase Change Materials (PCM) for Latent Heat Thermal Energy Storage (LHTES). Materials 2017, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhao, C.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Alkan, C.; Sarı, A.; Karaipekli, A.; Uzun, O. Preparation, characterization, and thermal properties of microencapsulated phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 143–147. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Kong, X.; Zhong, Y.; Rong, X.; Min, C.; Qi, C. Building energy storage panel based on paraffin/expanded perlite: Preparation and thermal performance study. Materials 2016, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, S.; Zhou, J.; Huang, Z.; Zhao, Y.; Yuan, X.; Zhang, Y.; Kou, J. Fabrication and interfacial morphologies of methanol–melamine–formaldehyde (MMF) shell microPCMs/epoxy composites. Colloid Polym. Sci. 2011, 289, 169–177. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Fambri, L.; Pegoretti, A. Wax Confinement with Carbon Nanotubes for Phase Changing Epoxy Blends. Polymers 2017, 9, 405. [Google Scholar] [CrossRef]
- Moulahi, C.; Trigui, A.; Karkri, M.; Boudaya, C. Thermal performance of latent heat storage: Phase change material melting in horizontal tube applied to lightweight building envelopes. Compos. Struct. 2016, 149, 69–78. [Google Scholar] [CrossRef]
- Fang, Y.; Kang, H.; Wang, W.; Liu, H.; Gao, X. Study on polyethylene glycol/epoxy resin composite as a form-stable phase change material. Energy Convers. Manag. 2010, 51, 2757–2761. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Li, D.; Sun, Y.; Wang, Z.; Hou, C.; Chen, L.; Cao, Y.; Liu, Y. Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.; Lee, H.; Shin, J. Experimental study on the electrochemical anti-corrosion properties of steel structures applying the arc thermal metal spraying method. Materials 2014, 7, 7722–7736. [Google Scholar] [CrossRef] [PubMed]
- Barreneche, C.; Navarro, H.; Serrano, S.; Cabeza, L.F.; Fernández, A.I. New database on phase change materials for thermal energy storage in buildings to help PCM selection. Energy Procedia 2014, 57, 2408–2415. [Google Scholar] [CrossRef]
- Analytical Methods Committee (AMCTB). Experimental design and optimisation (4): Plackett–Burman designs. Anal. Methods 2013, 5, 1901–1903. [Google Scholar]
- Hu, P.; Lu, D.; Fan, X.; Zhou, X.; Chen, Z. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Solar Energy Mater. Solar Cells 2011, 95, 2645–2649. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Svensson, G.; Hiebler, S.; Mehling, H. Thermal performance of sodium acetate trihydrate thickened with different materials as phase change energy storage material. Appl. Therm. Eng. 2003, 23, 1697–1704. [Google Scholar] [CrossRef]
- Günther, E.; Mehling, H.; Hiebler, S. Modeling of subcooling and solidification of phase change materials. Model. Simul. Mater. Sci. Eng. 2007, 15, 879. [Google Scholar] [CrossRef]
- Naumann, R.; Emons, H. Results of thermal analysis for investigation of salt hydrates as latent heat-storage materials. J. Therm. Anal. Calorim. 1989, 35, 1009–1031. [Google Scholar] [CrossRef]
- Mamunya, Y.P.; Davydenko, V.; Pissis, P.; Lebedev, E. Electrical and thermal conductivity of polymers filled with metal powders. Eur. Polym. J. 2002, 38, 1887–1897. [Google Scholar] [CrossRef]
- Vander Heyden, Y.; Luypaert, K.; Hartmann, C.; Massart, D.; Hoogmartens, J.; De Beer, J. Ruggedness tests on the high-performance liquid chromatography assay of the United States Pharmacopeia XXII for tetracycline hydrochloride. A comparison of experimental designs and statistical interpretations. Anal. Chim. Acta 1995, 312, 245–262. [Google Scholar] [CrossRef]
- Dejaegher, B.; Capron, X.; Smeyers-Verbeke, J.; Vander Heyden, Y. Randomization tests to identify significant effects in experimental designs for robustness testing. Anal. Chim. Acta 2006, 564, 184–200. [Google Scholar] [CrossRef]
- Liu, L.; Su, D.; Tang, Y.; Fang, G. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2016, 62, 305–317. [Google Scholar] [CrossRef]
- Jegadheeswaran, S.; Pohekar, S.D. Performance enhancement in latent heat thermal storage system: A review. Renew. Sustain. Energy Rev. 2009, 13, 2225–2244. [Google Scholar] [CrossRef]
- Yavari, F.; Fard, H.R.; Pashayi, K.; Rafiee, M.A.; Zamiri, A.; Yu, Z.; Ozisik, R.; Borca-Tasciuc, T.; Koratkar, N. Enhanced thermal conductivity in a nanostructured phase change composite due to low concentration graphene additives. J. Phys. Chem. C 2011, 115, 8753–8758. [Google Scholar] [CrossRef]
- Xiang, J.; Drzal, L.T. Investigation of exfoliated graphite nanoplatelets (xGnP) in improving thermal conductivity of paraffin wax-based phase change material. Solar Energy Mater. Solar Cells 2011, 95, 1811–1818. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Zhou, W.; Wang, J.; Wang, Y. Thermal and electrical conductivity enhancement of graphite nanoplatelets on form-stable polyethylene glycol/polymethyl methacrylate composite phase change materials. Energy 2012, 39, 294–302. [Google Scholar] [CrossRef]





| Type of PCM | Advantages | Disadvantages | Literature |
|---|---|---|---|
| Organic: Paraffins and non-paraffins |
|
| [13,18,19,20,21,22,23,24,25,26,27] |
| Inorganic: Hydrated salts |
|
| [13,18,19,20,22,23,24,25,26,27,28] |
| Eutectic |
|
| [13,18,24,29] |
| Parameter | Description | Levels | ||
|---|---|---|---|---|
| − | 0 | + | ||
| A | Amount of Cu * | 5% | 7.5% | 10% |
| B | Amount of paraffin * | 5% | 7.5% | 10% |
| C | Time to wait to add paraffin and Cu | 10 min | 15 min | 20 min |
| d1 | Dummy variable #1 | |||
| d2 | Dummy variable #2 | |||
| d3 | Dummy variable #3 | |||
| d4 | Dummy variable #4 | |||
| PCM | Exothermic Peak Temperature (°C) | Heat of Fusion (J/g) |
|---|---|---|
| PCM1 [Zn(NO3)2·4H2O] | 40.50 | 70.96 |
| PCM2 [Na2S2O3·5H2O] | 70.20 | 251.10 |
| PCM3 [NaCH3COO·3H2O] | 68.50 | 356.50 |
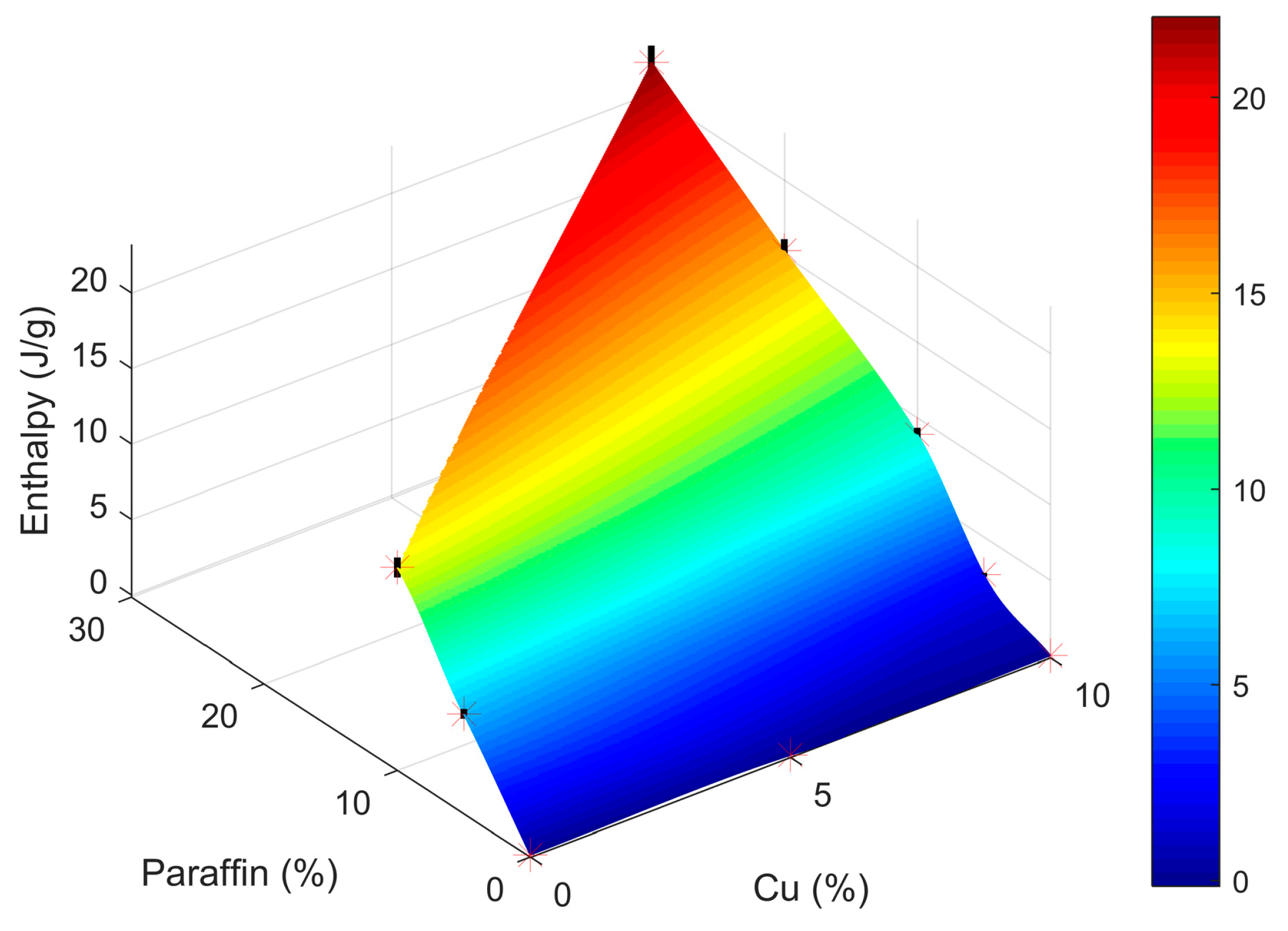
| Sample | A (% Cu) | d1 | B (% Paraffin) | d2 | C (Time 2) | d3 | d4 | Enthalpy |
|---|---|---|---|---|---|---|---|---|
| (J/g) | ||||||||
| #1 | + | - | - | + | - | + | + | 2.47 |
| #2 | + | + | - | - | + | - | + | 4.88 |
| #3 | + | + | + | - | - | + | - | 8.89 |
| #4 | - | + | + | + | - | - | + | 8.31 |
| #5 | + | - | + | + | + | - | - | 14.31 |
| #6 | - | + | - | + | + | + | - | 3.82 |
| #7 | - | - | + | - | + | + | + | 11.37 |
| #8 | - | - | - | - | - | - | - | 2.76 |
| #9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.75 |
| Effect | 1.07 | −1.25 | 7.24 | 0.25 | 2.99 | −0.93 | −0.69 | * |
| SS | 2.30 | 3.14 | 104.76 | 0.13 | 17.85 | 1.72 | 0.95 | * |
| F-value ** | 1.55 | * | 70.66 | * | 12.04 | * | * | * |
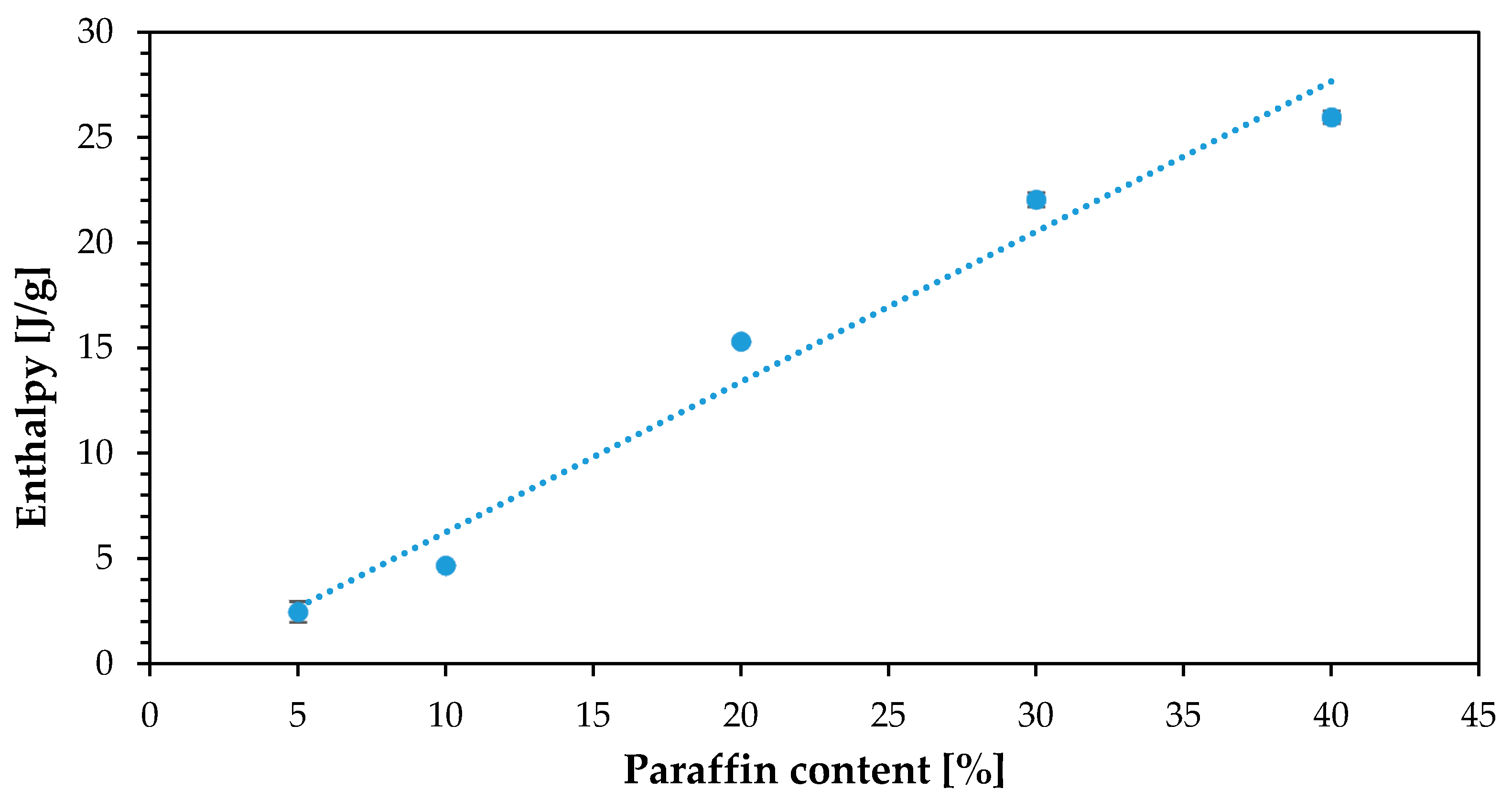
| % Cu | % Paraffin | Enthalpy (J/g) |
|---|---|---|
| 10 | 5 | 2.47 |
| 10 | 10 | 8.89 |
| 10 | 20 | 15.32 |
| 10 | 30 | 22.06 |
| 10 | 40 | 25.98 |
| 20 | 40 | 24.60 |
| 30 | 40 | 23.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arce, M.E.; Alvarez Feijoo, M.A.; Suarez Garcia, A.; Luhrs, C.C. Novel Formulations of Phase Change Materials—Epoxy Composites for Thermal Energy Storage. Materials 2018, 11, 195. https://doi.org/10.3390/ma11020195
Arce ME, Alvarez Feijoo MA, Suarez Garcia A, Luhrs CC. Novel Formulations of Phase Change Materials—Epoxy Composites for Thermal Energy Storage. Materials. 2018; 11(2):195. https://doi.org/10.3390/ma11020195
Chicago/Turabian StyleArce, Maria Elena, Miguel Angel Alvarez Feijoo, Andres Suarez Garcia, and Claudia C. Luhrs. 2018. "Novel Formulations of Phase Change Materials—Epoxy Composites for Thermal Energy Storage" Materials 11, no. 2: 195. https://doi.org/10.3390/ma11020195
APA StyleArce, M. E., Alvarez Feijoo, M. A., Suarez Garcia, A., & Luhrs, C. C. (2018). Novel Formulations of Phase Change Materials—Epoxy Composites for Thermal Energy Storage. Materials, 11(2), 195. https://doi.org/10.3390/ma11020195







