Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials
Abstract
:1. Introduction
1.1. Alkaline-Earth Oxyfluoride Glass-Ceramics, M1F2 (M1 = Mg, Pb, Sr, Ca, Ba), M1M2F4 (BaMgF4)
1.2. Lanthanides Oxyfluoride Glass-Ceramics, M1F3 (M1 = La, Y, Gd) and M1M2F4, M1M2F5 (M1 = Na, K, Li; M2 = Gd, Y)
1.2.1. M1F3
1.2.2. M1M2F4/F5
1.3. Other Glass-Ceramics (Oxides and Oxyclorides)
1.4. Prospects and Perspectives
2. Experimental
2.1. Synthesis of (100 − x)SiO2-xLaF3
2.2. Synthesis of (100 − x)SiO2−xGdF3/NaGdF4
2.3. Thermal and Structural Characterization
3. Results and Discussion
3.1. Materials
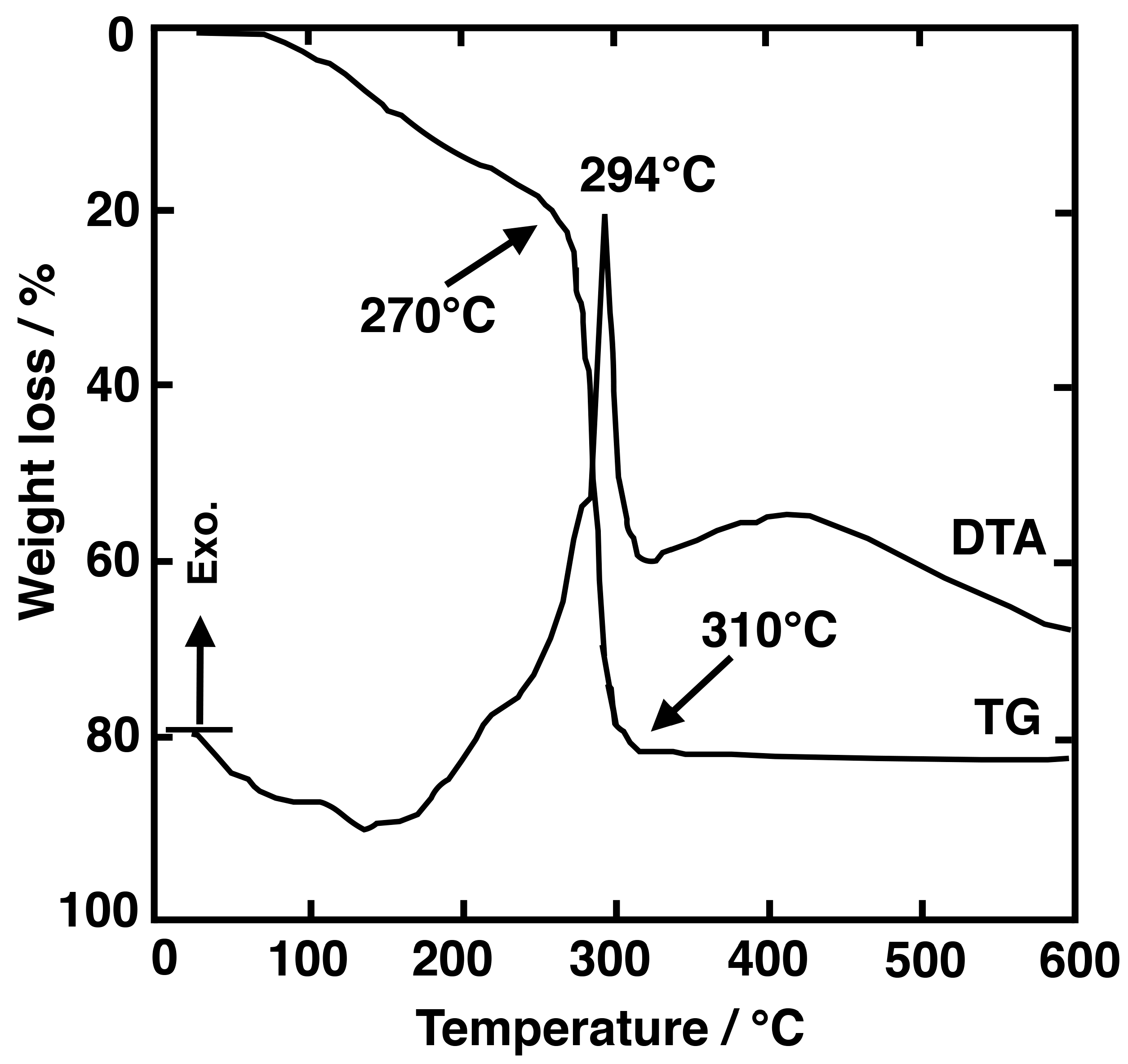
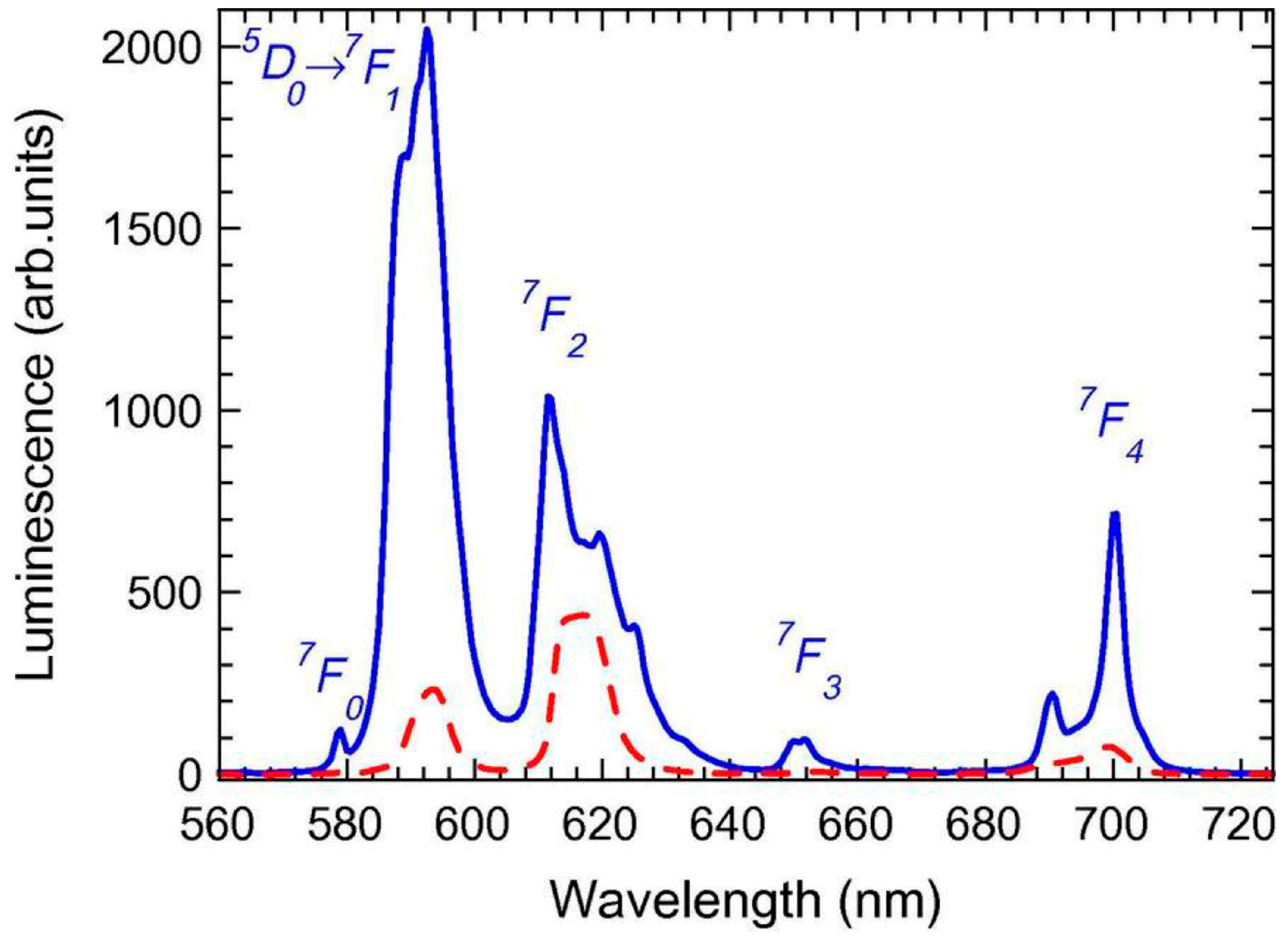
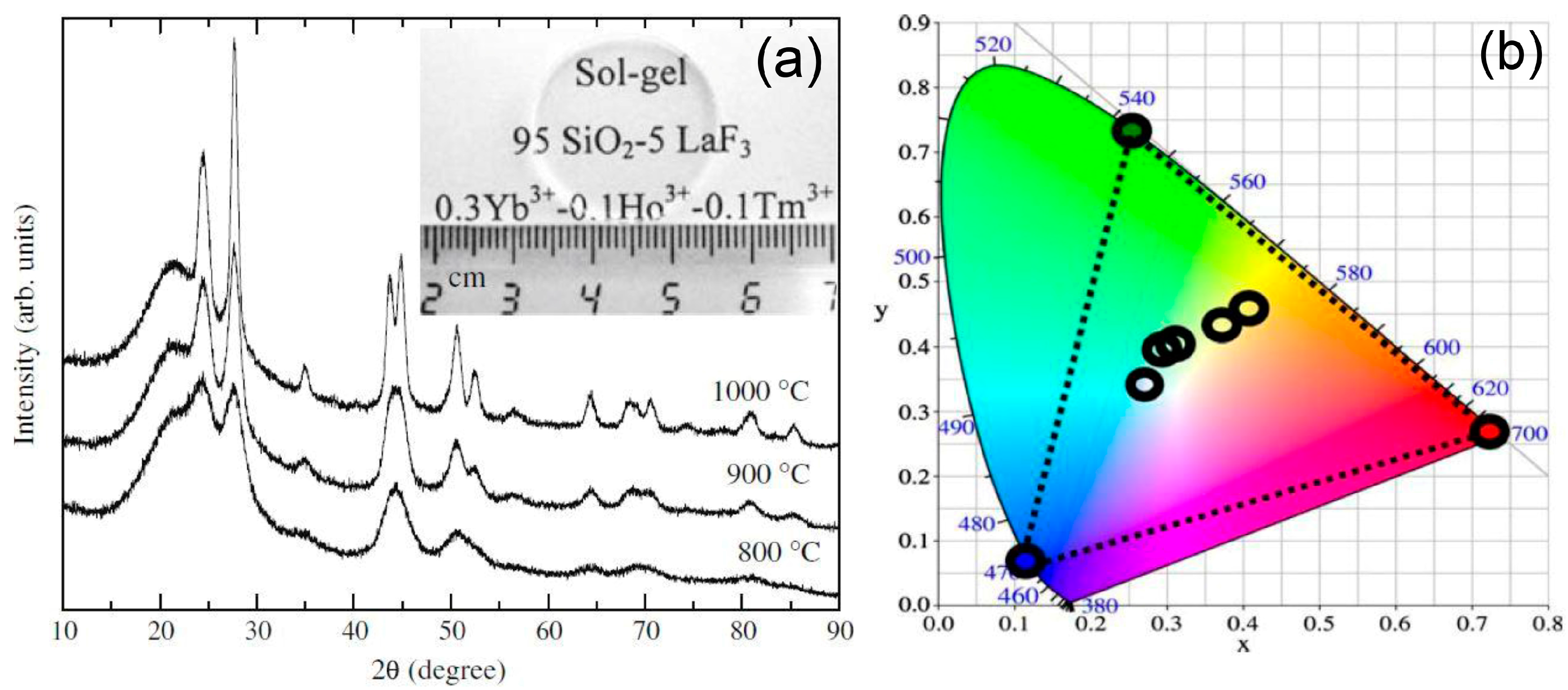
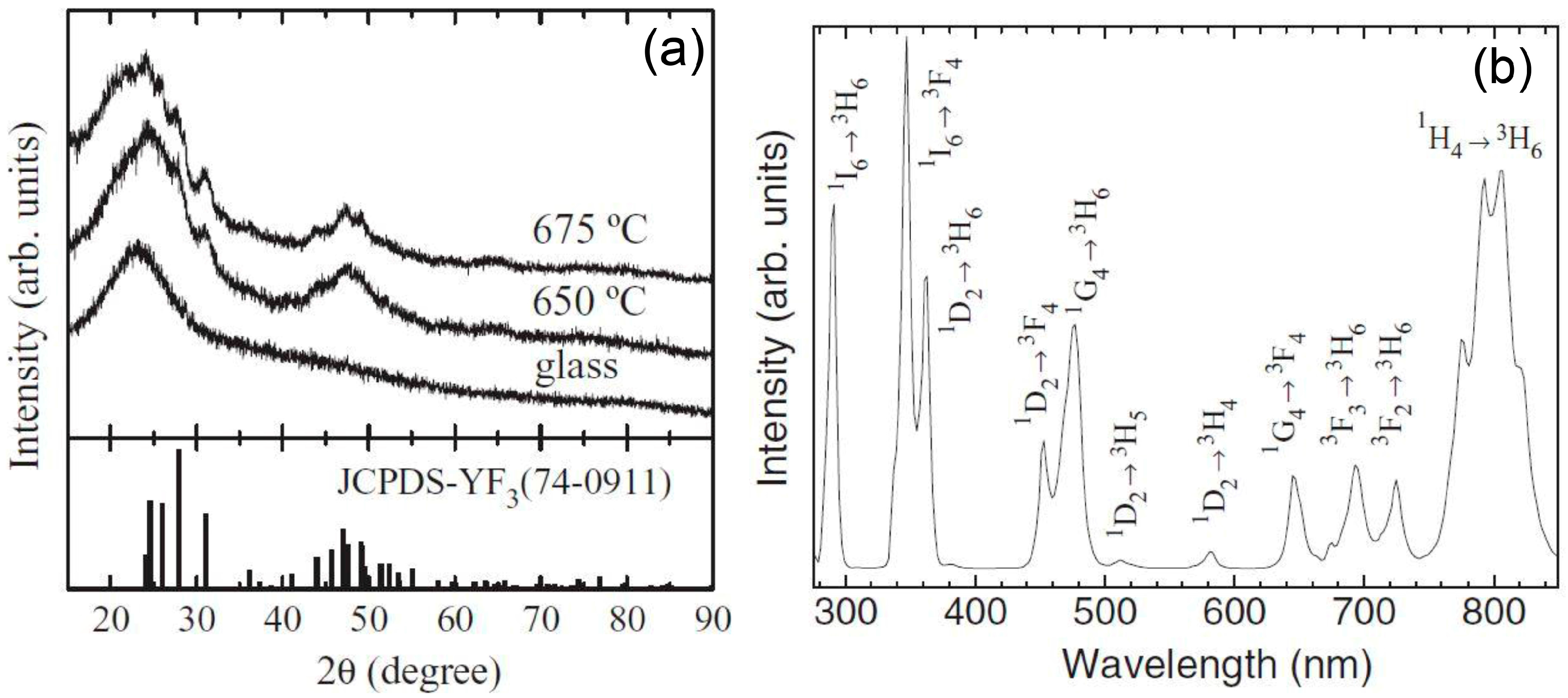
3.2. Thermal and Structural Characterization
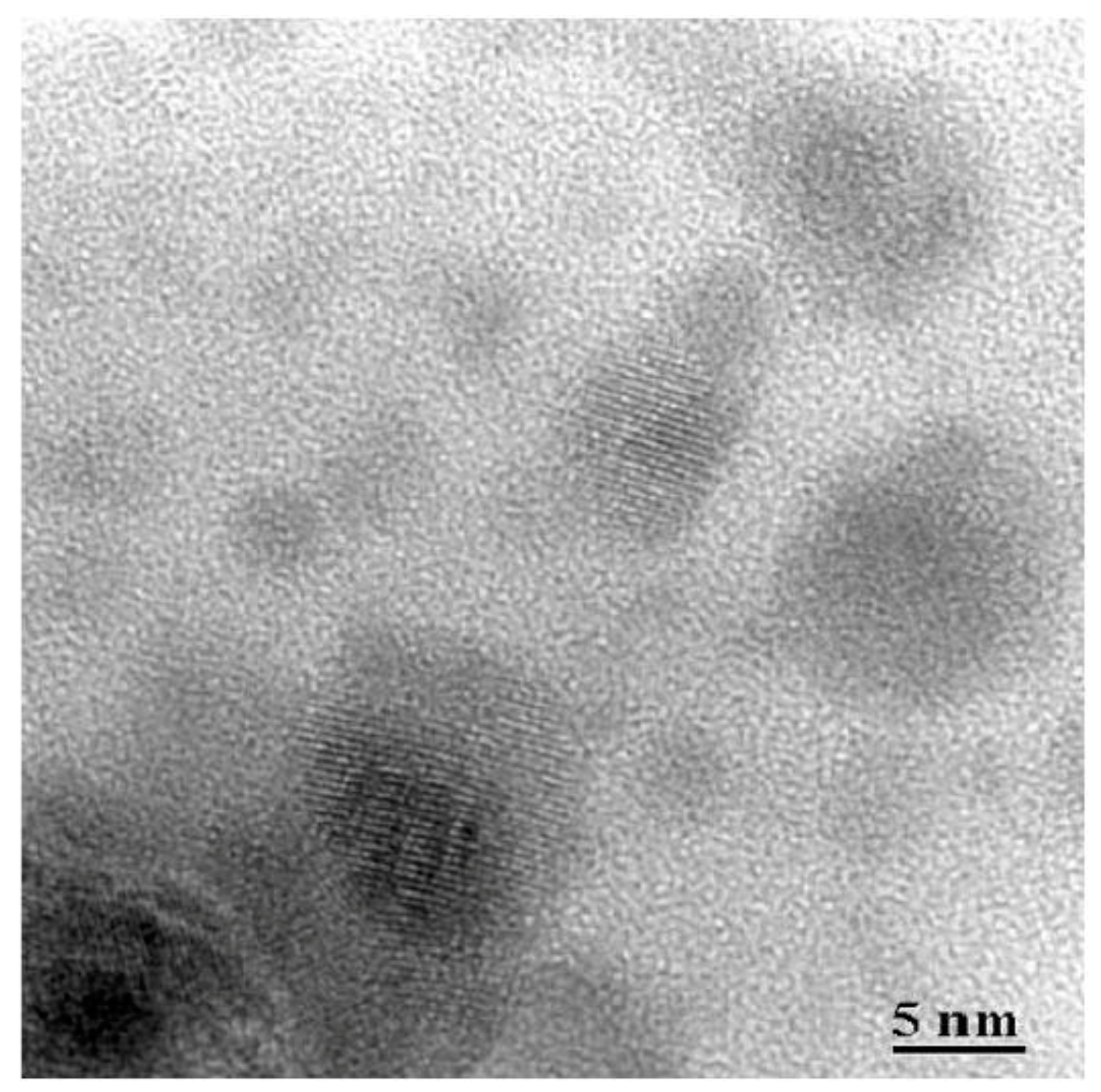
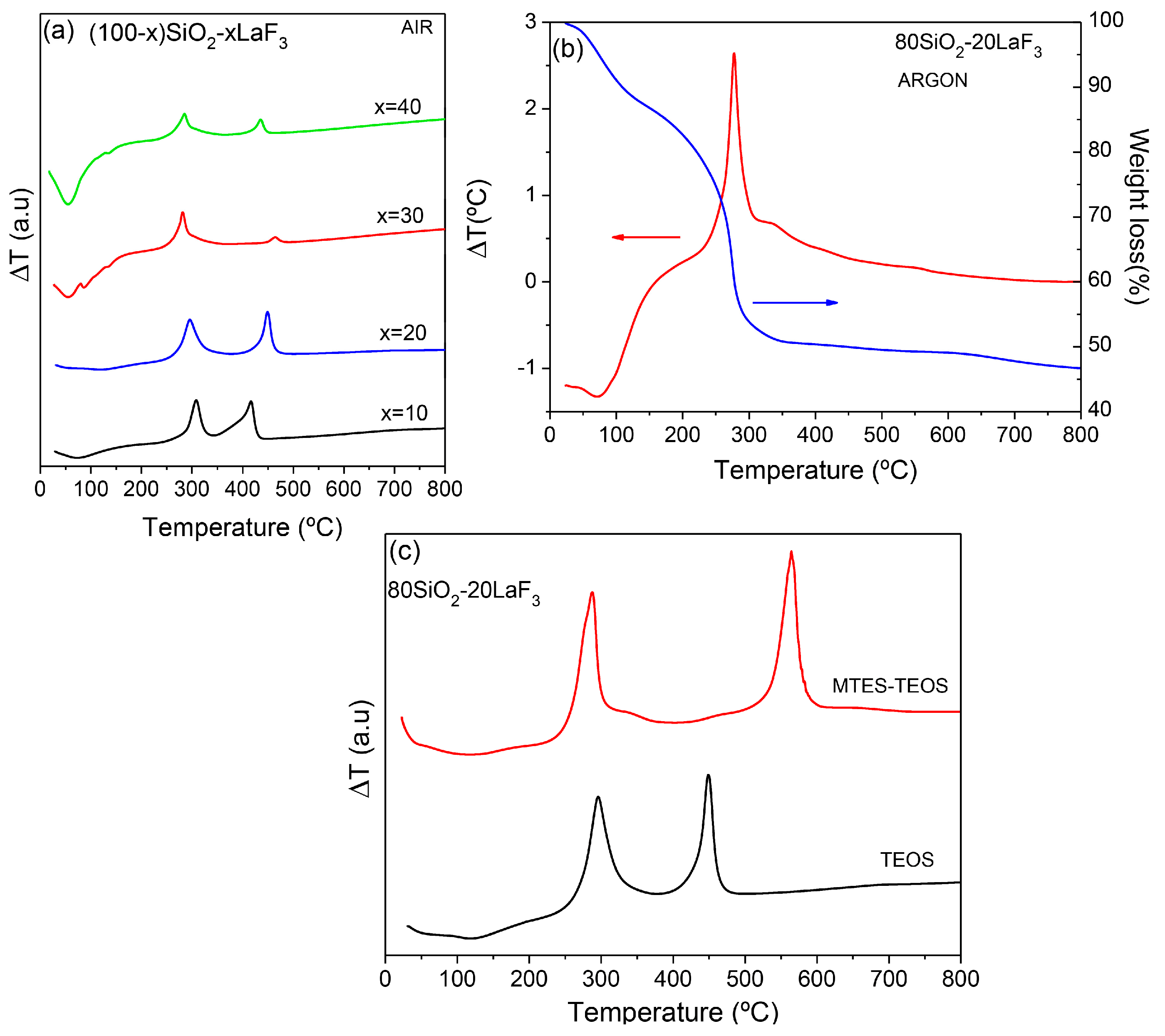
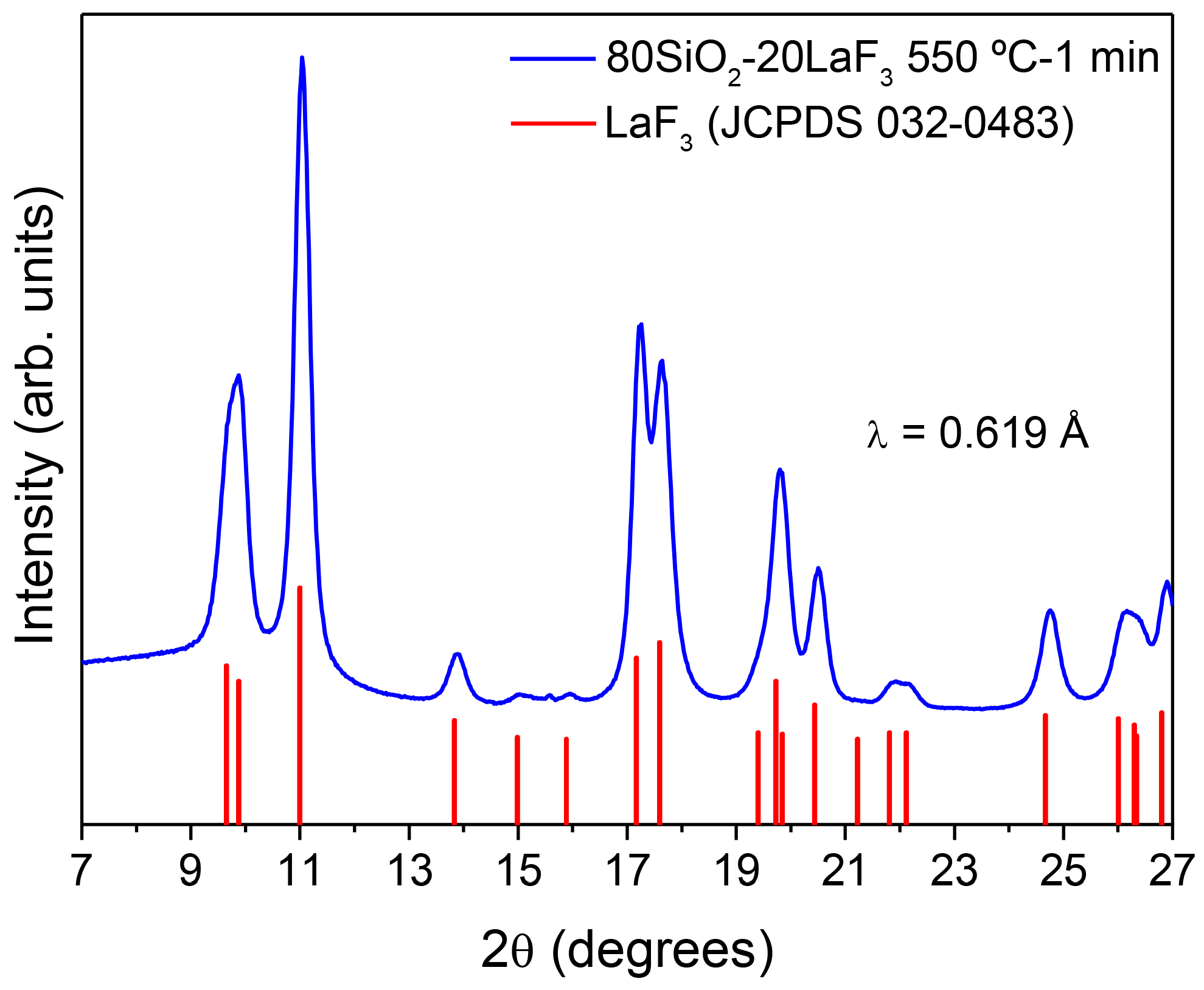
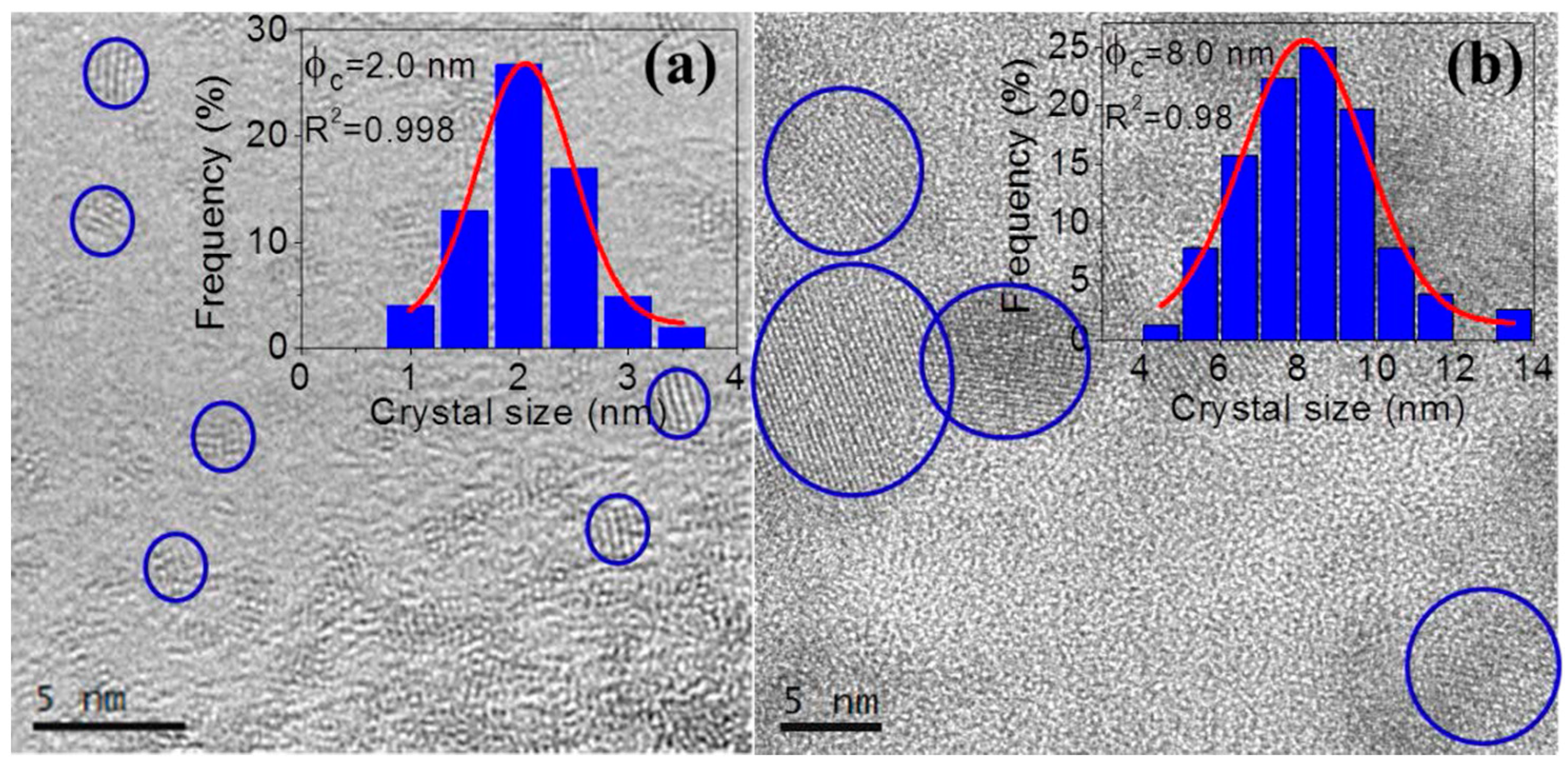
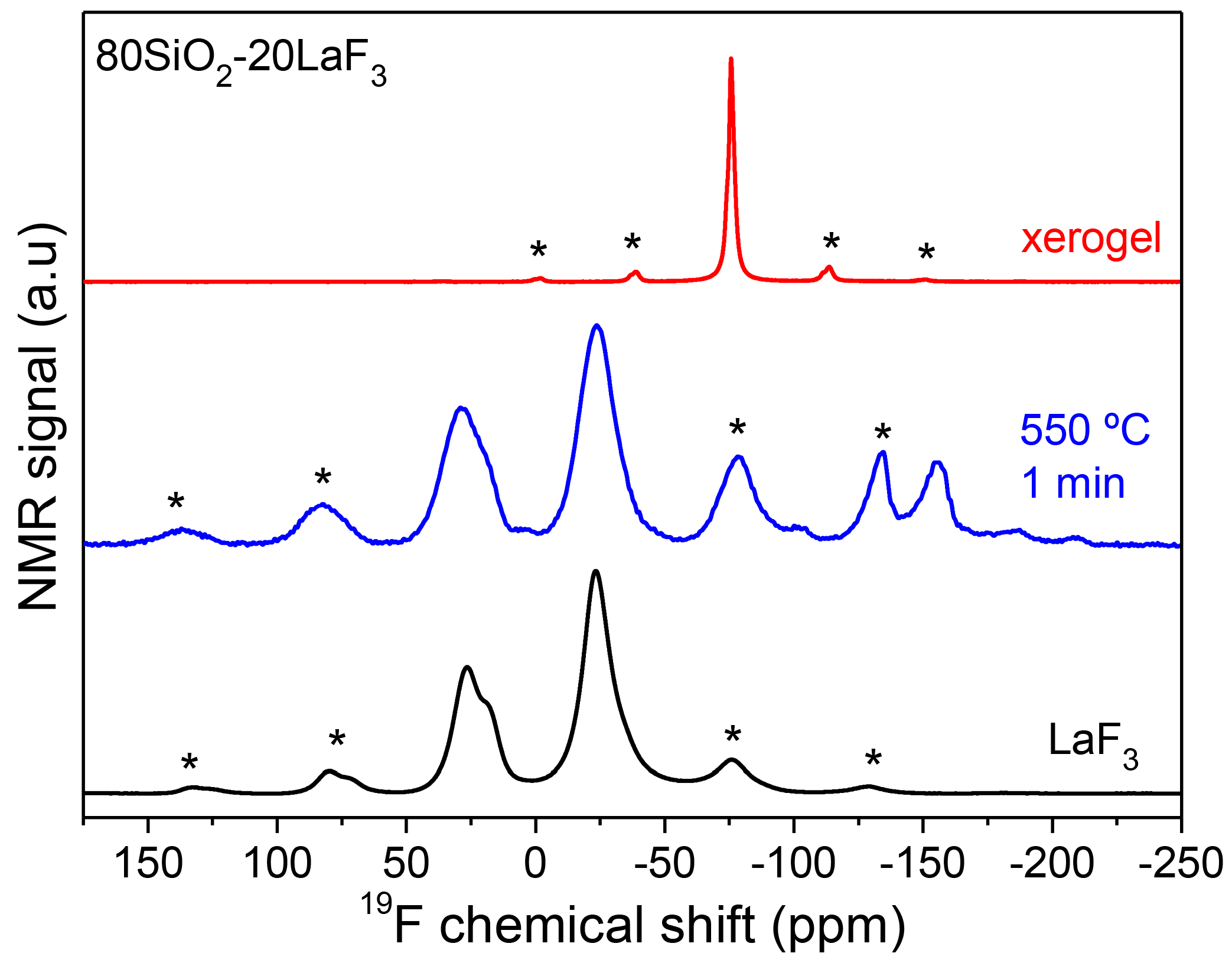
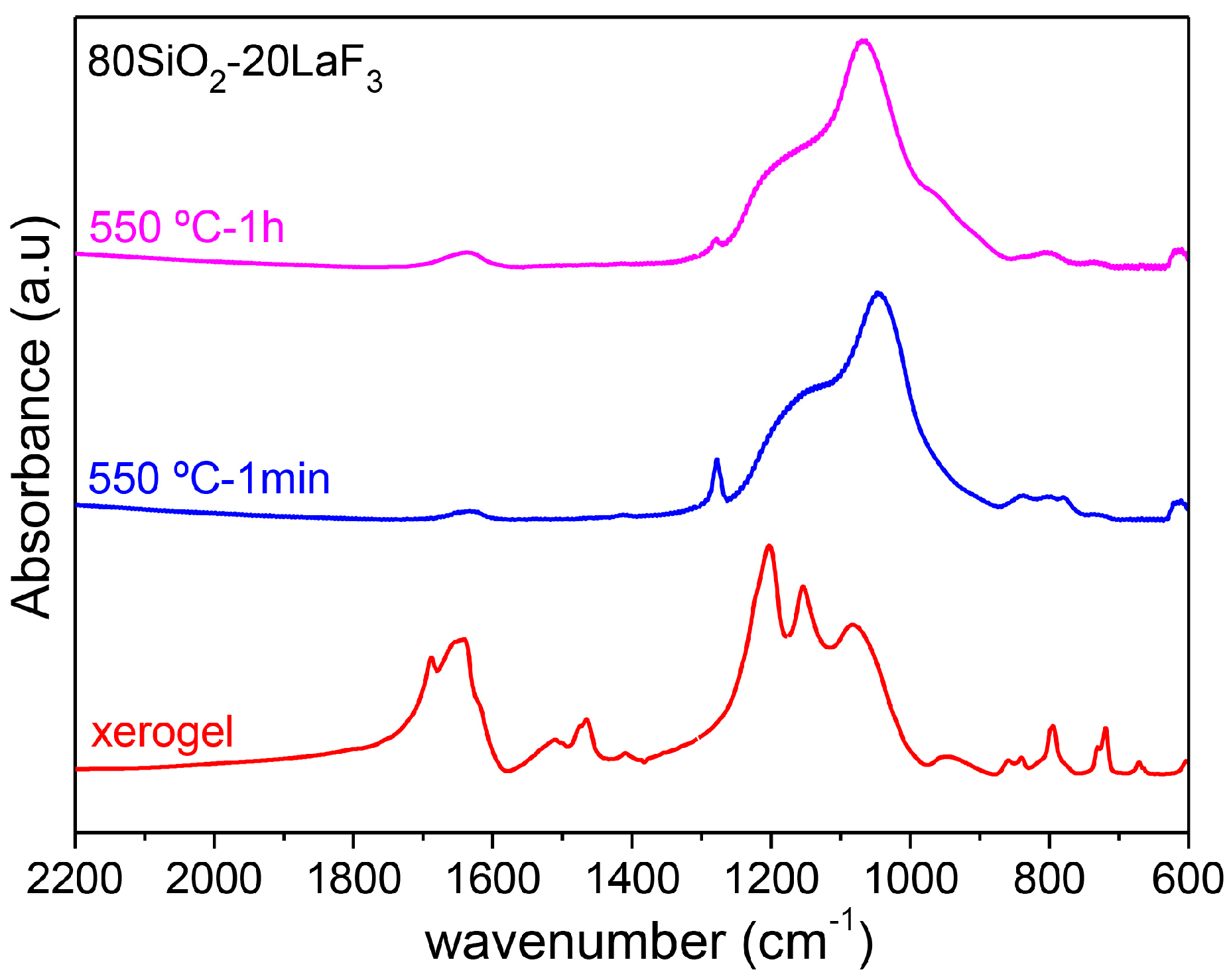
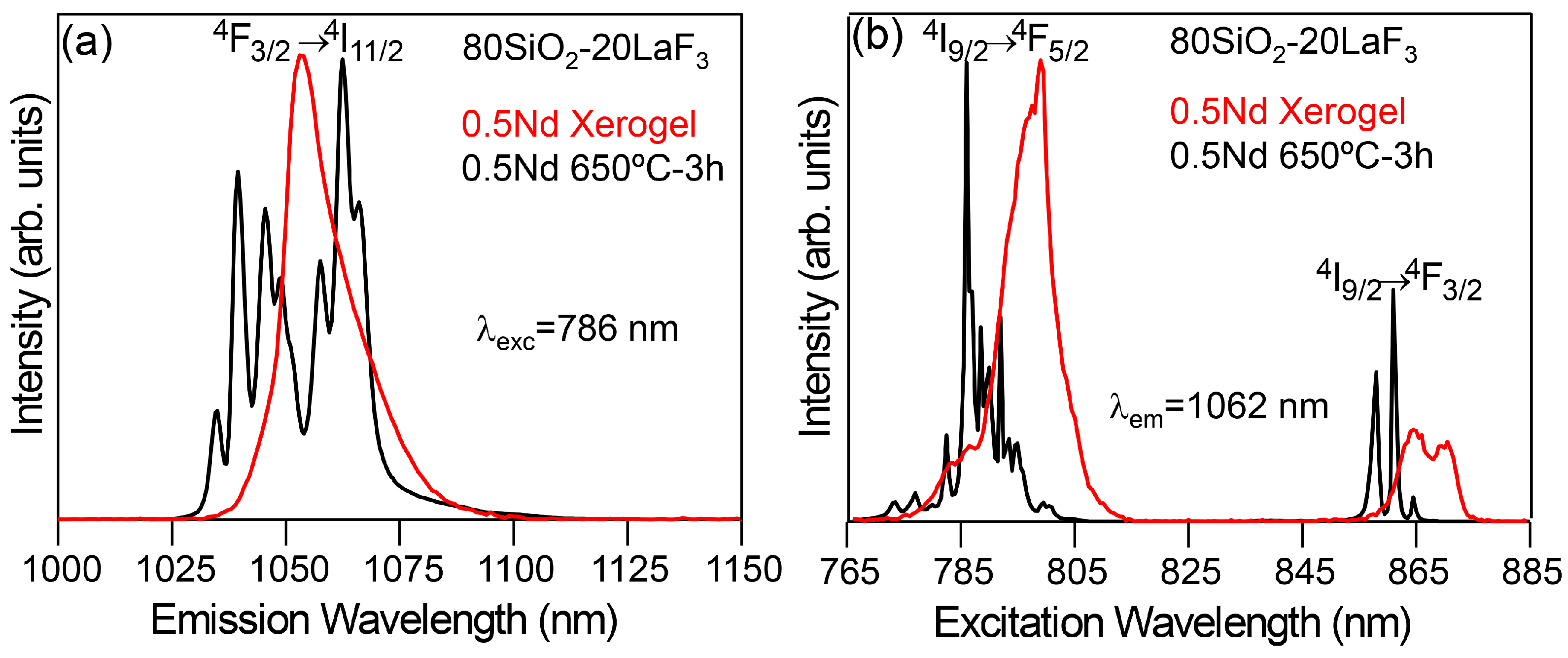
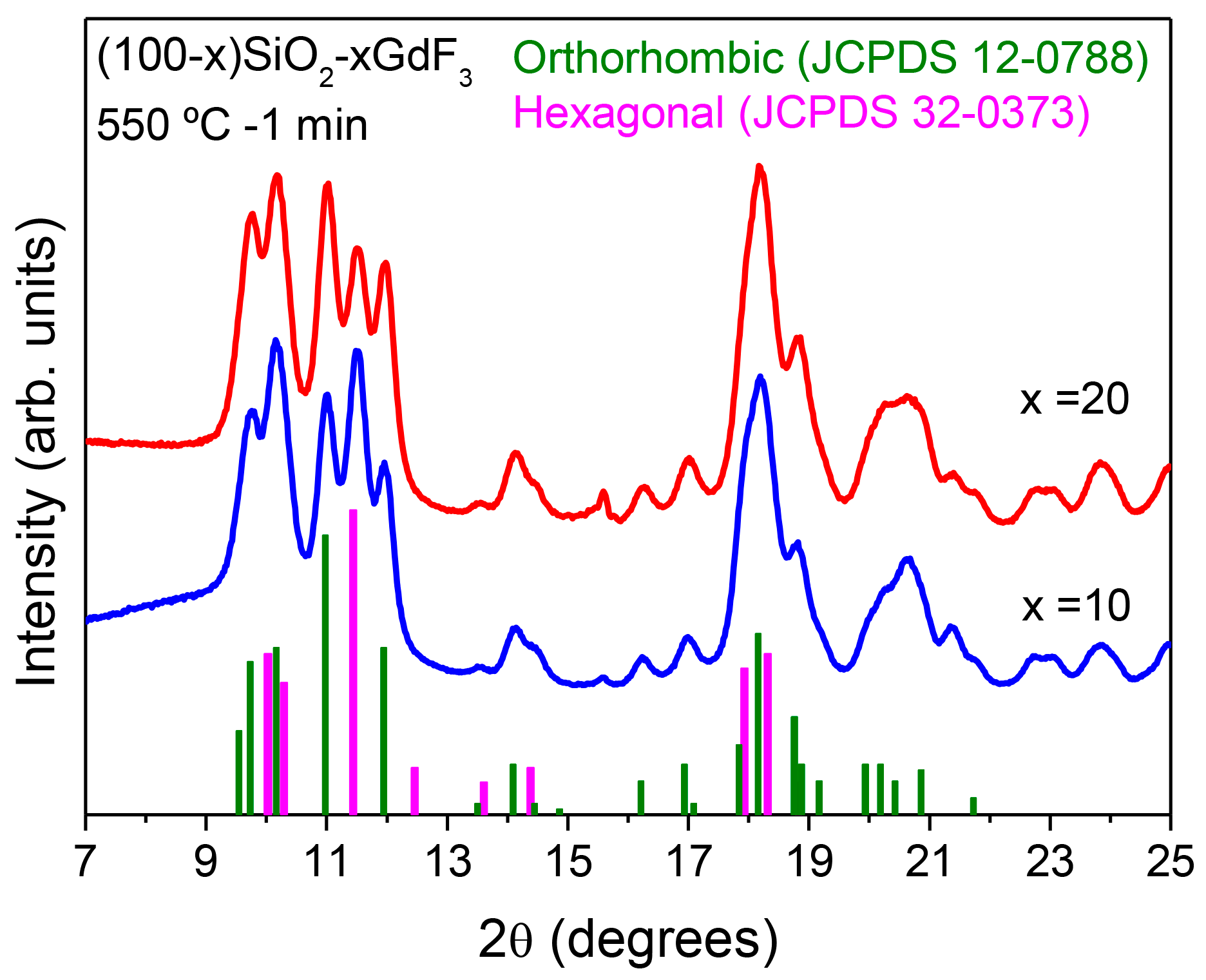
3.2.1. SiO2–LaF3
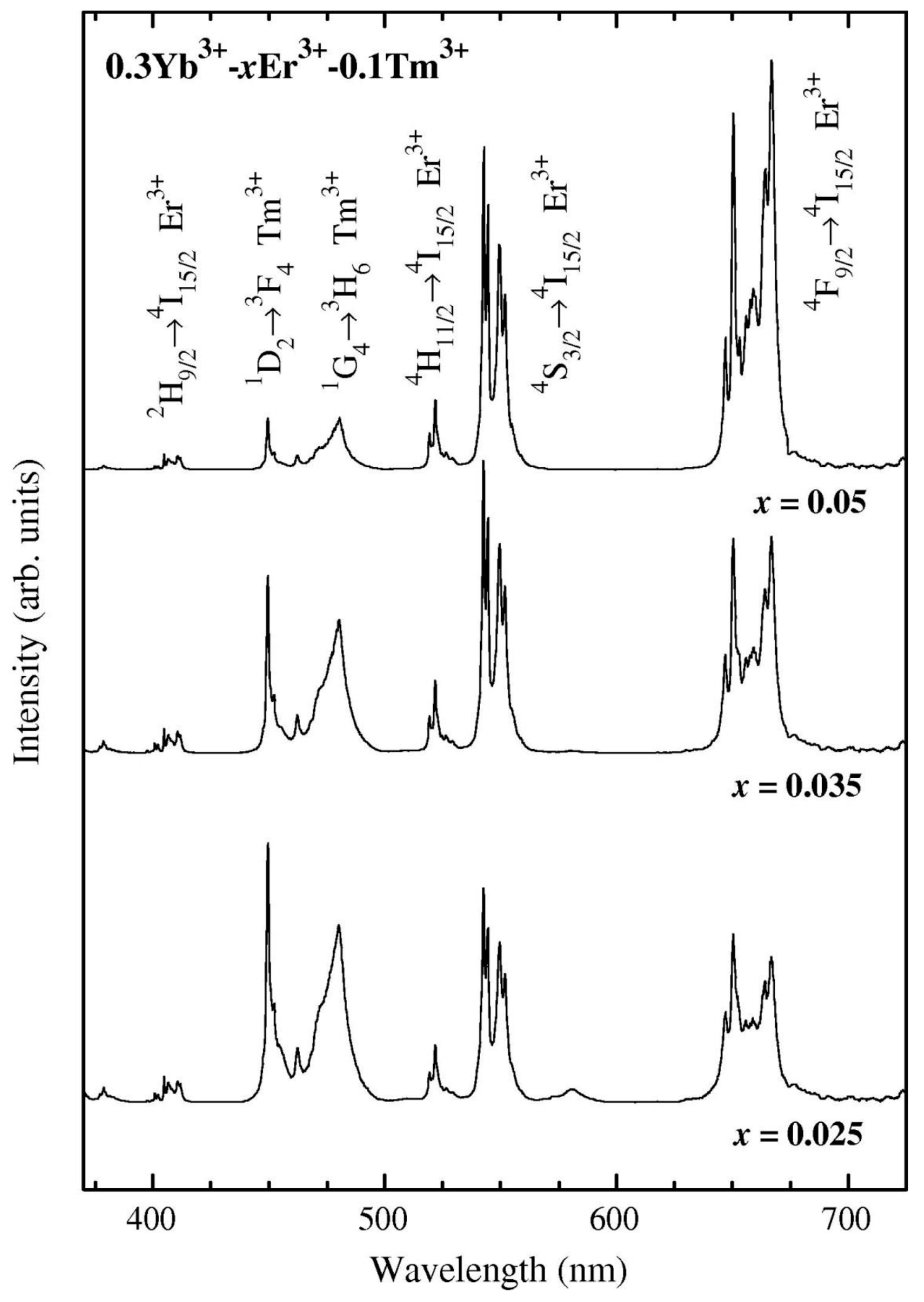
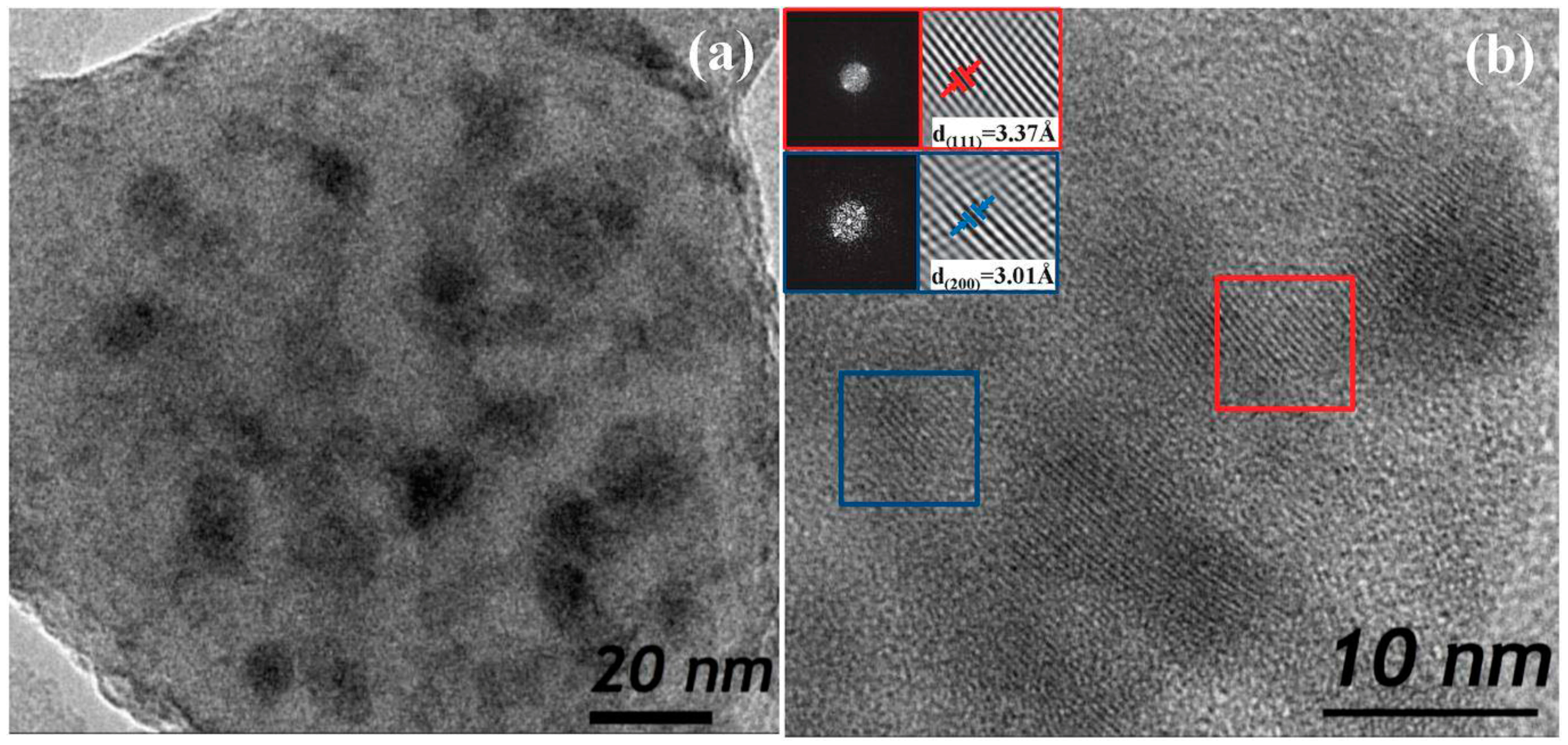
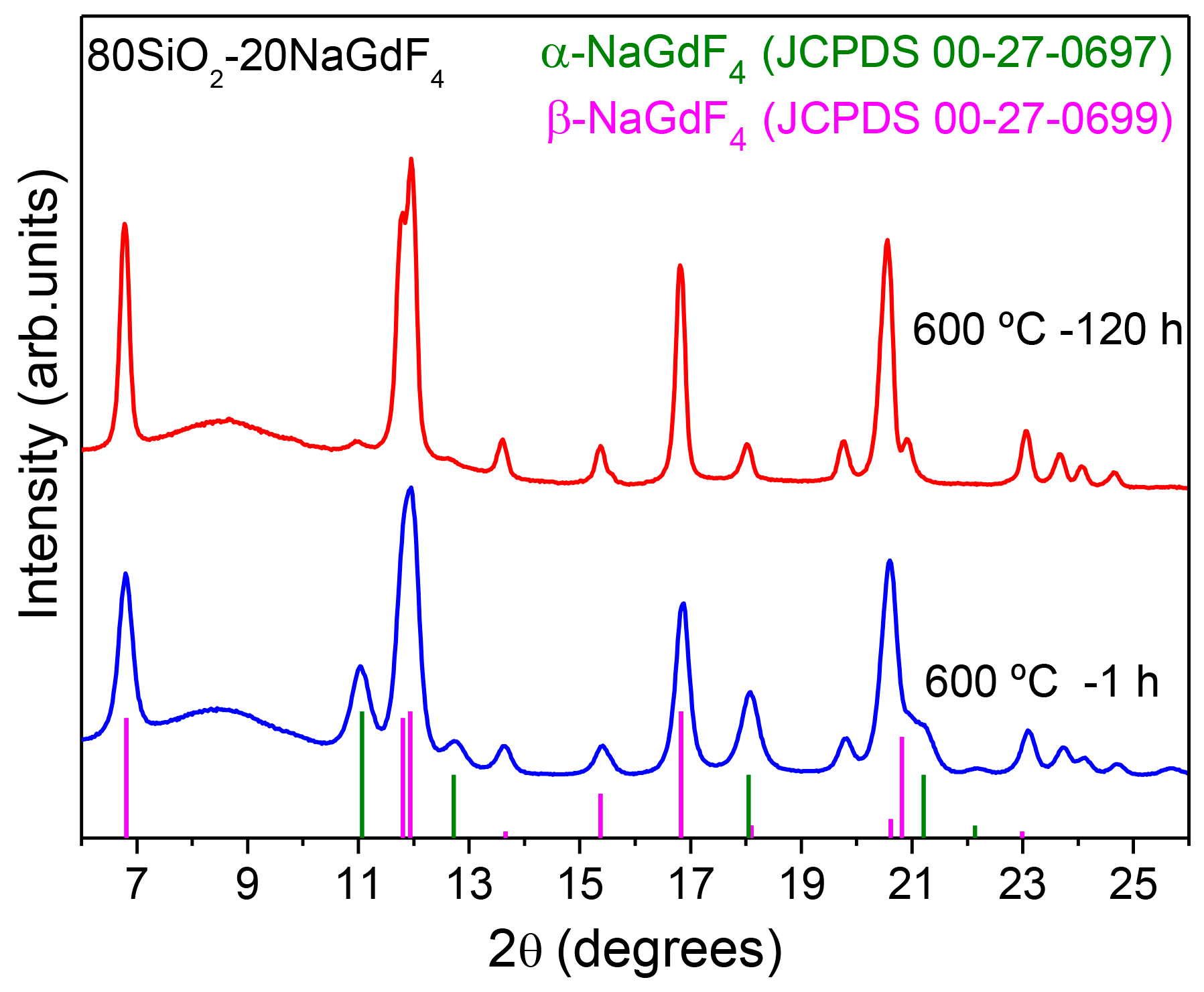
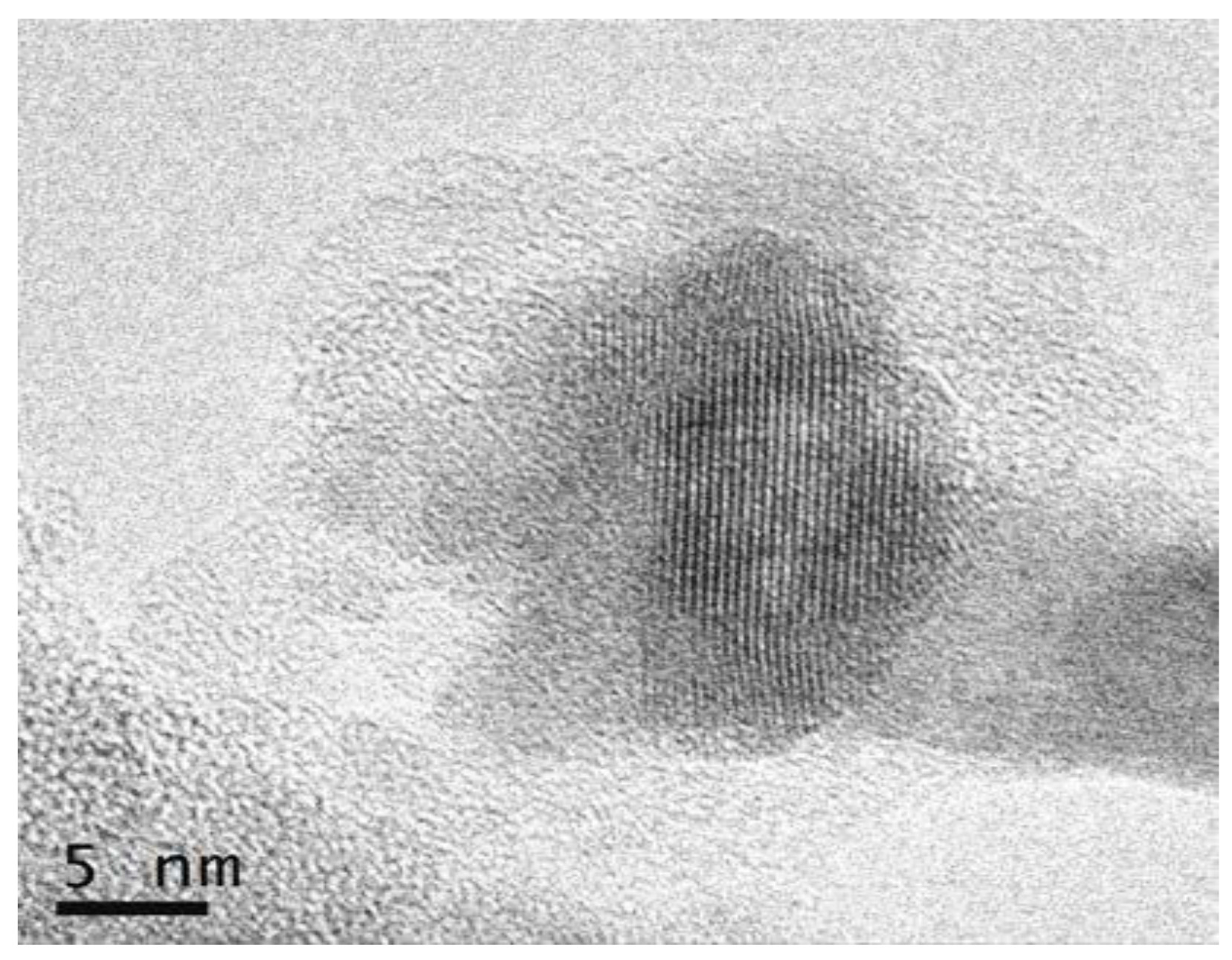
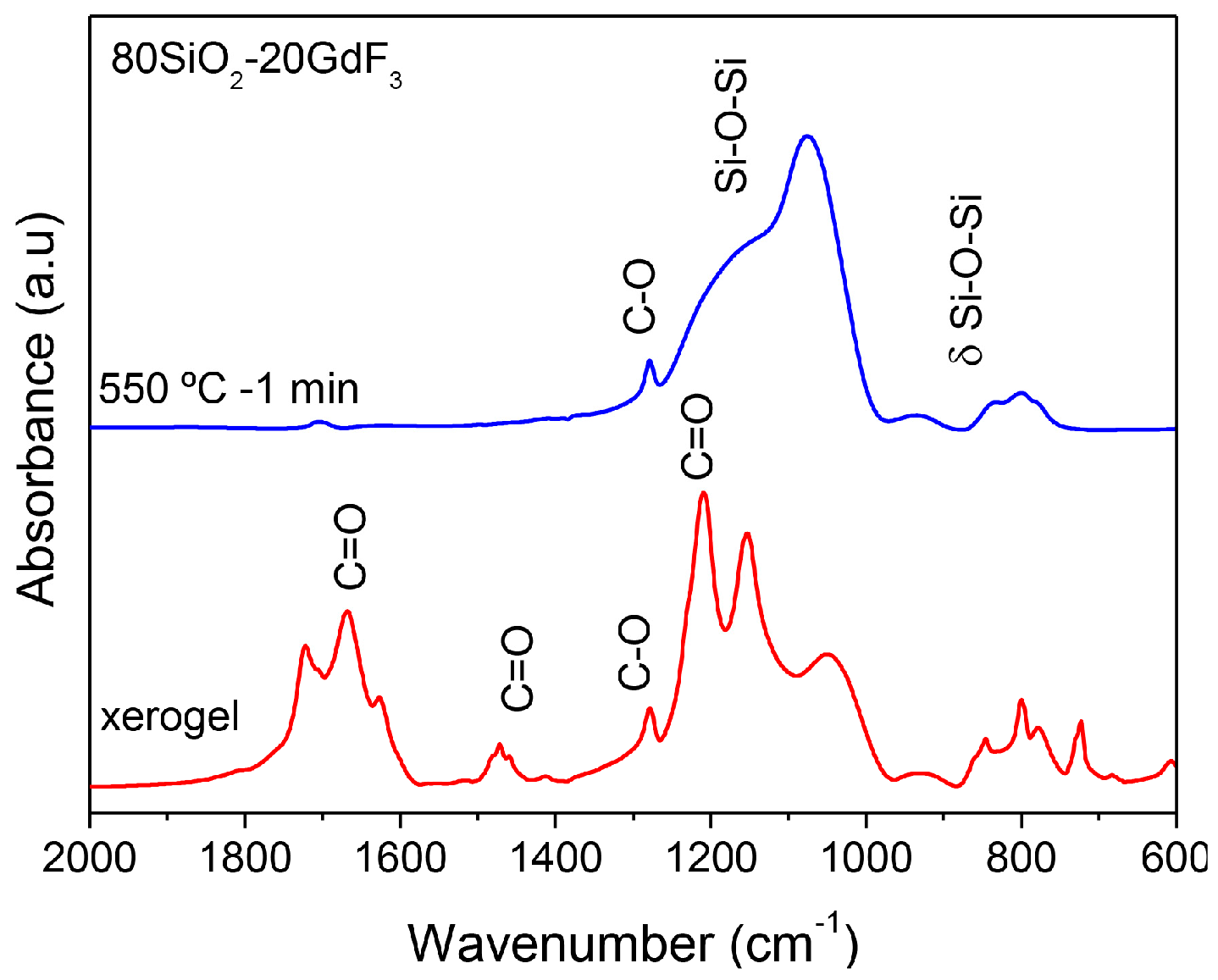
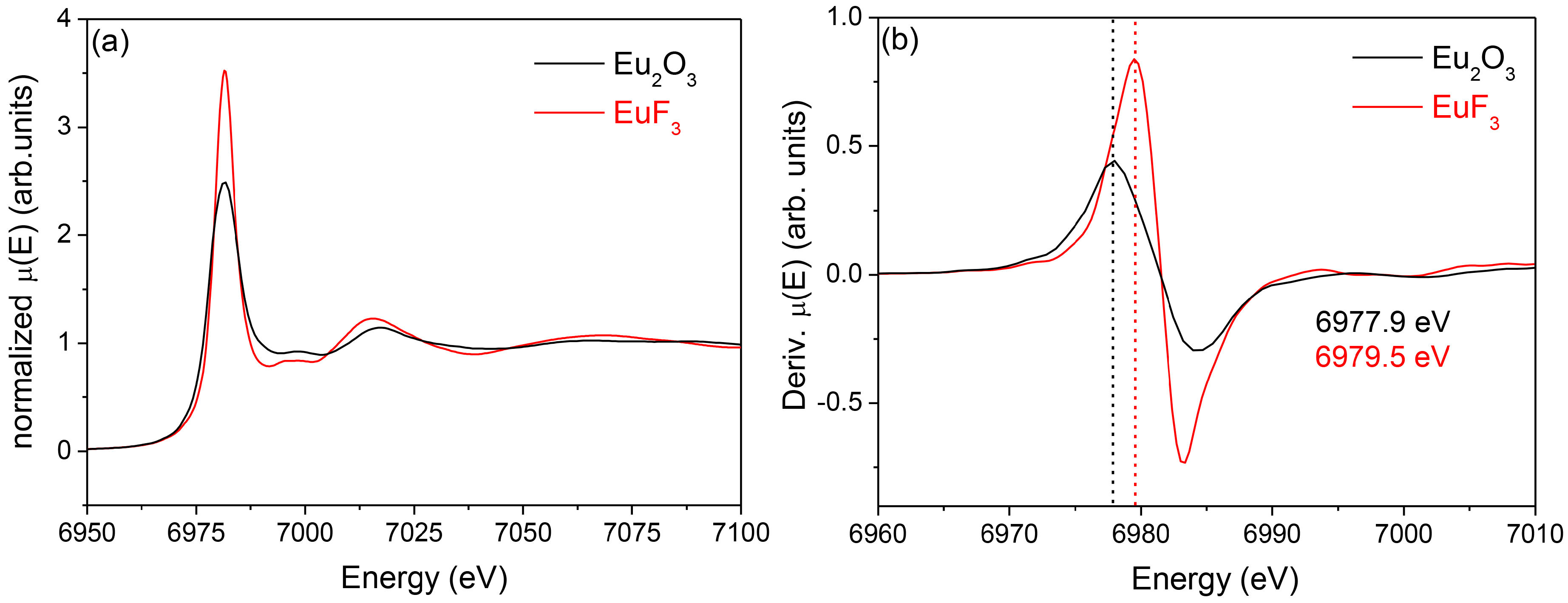
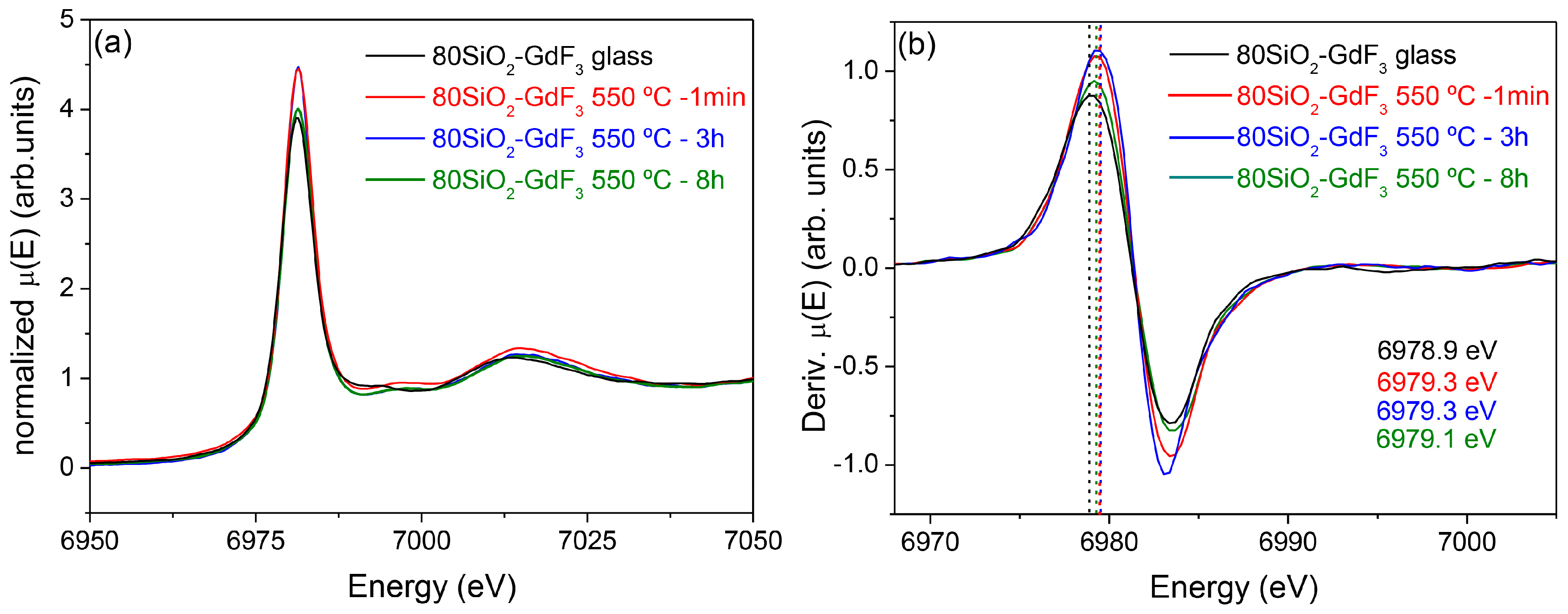
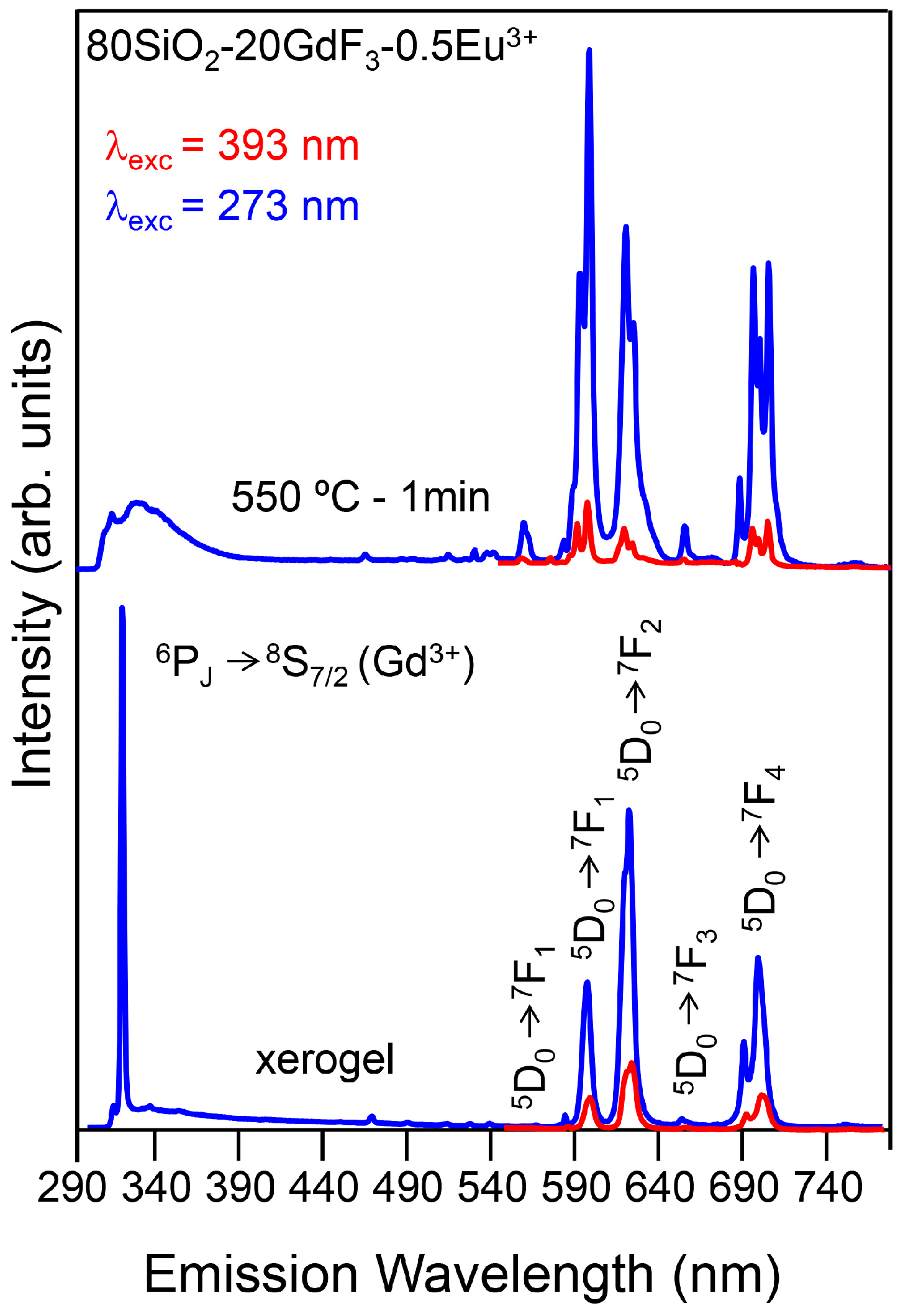
3.2.2. SiO2–GdF3/NaGdF4
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feldmann, C.; Jüstel, T.; Ronda, C.R.; Schmidt, P.J. Inorganic luminescent materials: 100 years of research and application. Adv. Funct. Mater. 2003, 13, 511–516. [Google Scholar] [CrossRef]
- George, N.C.; Denault, K.A.; Seshadri, R. Phosphors for solid-state white lightening. Annu. Rev. Mater. Res. 2013, 43, 481–501. [Google Scholar] [CrossRef]
- Huang, X. Solid-state lighting: Red phosphor converts white LEDs. Nat. Photonics 2014, 8, 748–749. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bunzli, J.C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.S.; Park, H.G.; et al. Black Phosphorus (BP) nanodots for potential biomedical applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef] [PubMed]
- De Pablos-Martín, A.; Durán, A.; Pascual, M.J. Nanocrystallisation in oxyfluoride systems: Mechanisms of crystallisation and photonic properties. Int. Mater. Rev. 2012, 57, 165–186. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Luginina, A.A.; Popov, A.I. Transparent oxyfluoride glass ceramics. J. Fluor. Chem. 2015, 172, 22–50. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Ferrari, M.; Pascual, M.J.; Righini, G.C. Glass-ceramics: A class of nanostructured materials for photonics. La Rivista del Nuovo Cimento 2015, 38, 311–369. [Google Scholar] [CrossRef]
- Wang, Y.; Ohwaki, J. New transparent vitroceramics codoped with Er3+ and Yb3+ for efficient frequency upconversion. Appl. Phys. Lett. 1993, 63, 3268–3270. [Google Scholar] [CrossRef]
- Stevenson, A.J.; Serier-Brault, H.; Gredin, P.; Mortier, M. Fluoride materials for optical applications: Single crystals, ceramics, glasses, and glass–ceramics. J. Fluor. Chem. 2011, 132, 1165–1173. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Salminen, T.; Hakkarainen, T.; Petit, L.; Janner, D.; Boetti, N.; Lastusaari, M.; Pugliese, D.; Paturi, P.; Milanese, D. Effect of partial crystallization on the structural and luminescence properties of Er3+-doped phosphate glasses. Materials 2017, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Höland, W.; Beall, G. Glass-Ceramic Technology, 3rd ed.; The American ceramic society: Westerville, OH, USA, 2002; pp. 15–18. [Google Scholar]
- Augustyn, E.; Żelechower, M.; Stróż, D.; Chrapoński, J. The microstructure of erbium-ytterbium co-doped oxyfluoride glass-ceramic optical fibers. Opt. Mater. 2012, 34, 944–950. [Google Scholar] [CrossRef]
- Reben, M.; Dorosz, D.; Wasylak, J.; Burtan-Gwizdala, B.; Jaglarz, J.; Zontek, J. Nd3+-doped oxyfluoride glass ceramics optical fibre with SrF2 nanocrystals. Opt. Appl. 2012, 42, 353–364. [Google Scholar] [CrossRef]
- Krishnaiah, K.V.; Ledemi, Y.; Genevois, C.; Veron, E.; Sauvage, X.; Morency, S.; Soares de Lima Filho, E.; Nemova, G.; Allix, M.; Messaddeq, Y. Ytterbium-doped oxyfluoride nano-glass-ceramic fibers for laser cooling. Opt. Mater. Express 2017, 7, 1980–1994. [Google Scholar] [CrossRef]
- Gorni, G.; Balda, R.; Fernández, J.; Ipparraguirre, I.; Velázquez, J.J.; Castro, Y.; Chen, G.; Sundararayan, M.; Pascual, M.J.; Durán, A. Oxyfluoride glass-ceramic fibers doped with Nd3+: Structural and optical characterization. CrystEngComm 2017, 19, 6620–6629. [Google Scholar] [CrossRef]
- Roberts, R.B.; Tainsh, R.J.; White, G.K. Thermal properties of Zerodur at low temperatures. Cryogenics 1982, 22, 566–568. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Rywak, A.A.; Burlitch, J.M. Sol-gel synthesis of nanocrystalline magnesium fluoride: Its use in the preparation of MgF2 films and MgF2-SiO2 composites. Chem. Mater. 1996, 8, 60–67. [Google Scholar] [CrossRef]
- Rywak, A.A.; Burlitch, J.M. The crystal chemistry and thermal stability of sol-gel prepared fluoride-substituted talc. Phys. Chem. Miner. 1996, 23, 418–431. [Google Scholar] [CrossRef]
- Fujihara, S.; Tada, M.; Kimura, T. Preparation and characterization of MgF2 thin film by a trifluoroacetic acid method. Thin Solid Films 1997, 304, 252–255. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Bao, F.; Zhou, L.; Wang, X. Crystallization behavior of PbF2-SiO2 based bulk xerogels. J. Non-Cryst. Solids 2004, 347, 31–38. [Google Scholar] [CrossRef]
- Del-Castillo, J.; Yanes, A.C.; Méndez-Ramos, J.; Tikhomirov, V.K.; Rodríguez, V.D. Structure and up-conversion luminescence in sol–gel derived Er3+-Yb3+ co-doped SiO2:PbF2 nano-glass-ceramics. Opt. Mater. 2009, 32, 104–107. [Google Scholar] [CrossRef]
- Del-Castillo, J.; Yanes, A.C.; Méndez-Ramos, J.; Tikhomirov, V.K.; Moshchalkov, V.V.; Rodríguez, V.D. Sol-gel preparation and white up-conversion luminescence in rare-earth doped PbF2 nanocrystals dissolved in silica glass. J. Sol-Gel Sci. Technol. 2010, 53, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Szpikowska-Sroka, B.; Zur, L.; Czoik, R.; Goryczka, T.; Swinarew, A.S.; Żadło, M.; Pisarski, W.A. Long-lived emission from Eu3+:PbF2 nanocrystals distributed into sol-gel silica glass. J. Sol-Gel Sci. Technol. 2013, 68, 278–283. [Google Scholar] [CrossRef]
- Szpikowska-Sroka, B.; Pawlik, N.; Zur, L.; Czoik, R.; Goryczka, T.M.; Pisarski, W.A. Effect of fluoride ions on the optical properties of Eu3+:PbF2 nanocrystals embedded into sol-gel host materials. Mater. Chem. Phys. 2016, 174, 138–142. [Google Scholar] [CrossRef]
- Szpikowska-Sroka, B.; Pawlik, N.; Goryczka, T.; Pietrasik, E.; Bańczyk, M.; Pisarski, W.A. Lead fluoride β-PbF2 nanocrystals containing Eu3+ and Tb3+ ions embedded in sol-gel materials: Thermal, structural and optical investigations. Ceram. Int. 2017, 43, 8424–8432. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Wang, Y.; Luo, W.; Zheng, Y.; Cheng, Y.; Zhou, L. Structural evolution and its influence on luminescence of SiO2-SrF2-ErF3 glass ceramics prepared by sol-gel method. Mater. Chem. Phys. 2006, 100, 241–245. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Chen, D.; Liu, F. Efficient upconversion luminescence of Er3+:SrF2-SiO2-Al2O3 sol-gel glass ceramics. Ceram. Int. 2008, 34, 2143–2146. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, D.; Luo, W.; Wang, Y.; Yu, Y.; Liu, F. Transparent glass ceramic containing Er3+:CaF2 nano-crystals prepared by sol-gel method. Mater. Lett. 2007, 61, 3988–3990. [Google Scholar] [CrossRef]
- Georgescu, S.; Voiculescu, A.M.; Matei, C.; Secu, C.E.; Negre, R.F.; Secu, M. Ultraviolet and visible up-conversion luminescence of Er3+/Yb3+ co-doped CaF2 nanocrystals in sol-gel derived glass-ceramics. J. Lumin. 2013, 143, 150–156. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, J.; Jiang, B.; Mao, X.; Zhou, C.; Zhang, L. Structure and optical properties of transparent Er3+-doped CaF2-silica glass ceramic prepared by controllable sol-gel method. Ceram. Int. 2016, 42, 9571–9576. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Yu, Y.; Ma, E.; Zhou, L. Microstructure and luminescence of transparent glass ceramic containing Er3+:BaF2 nano-crystals. J. Solid State Chem. 2006, 179, 532–537. [Google Scholar] [CrossRef]
- Secu, C.E.; Secu, M.; Ghica, C.; Mihut, L. Rare-earth doped sol-gel derived oxyfluoride glass-ceramics: Structural and optical characterization. Opt. Mater. 2011, 33, 1770–1774. [Google Scholar] [CrossRef]
- Secu, C.E.; Bartha, C.; Polosan, S.; Secu, M. Thermally activated conversion of a silicate gel to an oxyfluoride glass ceramic: Optical study using Eu3+ probe ion. J. Lumin. 2014, 146, 539–543. [Google Scholar] [CrossRef]
- Fujihara, S.; Kitta, S.; Kimura, T. Porous Phosphor thin films of oxyfluoride SiO2-BaMgF4: Eu2+ glass-ceramics prepared by sol-gel method. Chem. Lett. 2003, 32, 928–929. [Google Scholar] [CrossRef]
- Kitta, S.; Fujihara, S.; Kimura, T. Porous SiO2-BaMgF4:Eu(II) glass-ceramic thin films and their strong blue photoluminescence. J. Sol-Gel Sci. Technol. 2004, 32, 263–266. [Google Scholar] [CrossRef]
- Blasse, G.; van den Heuvel, G.P.M.; Van Dijk, T. Energy transfer from Gd3+ to Tb3+ and Eu3+. Chem. Phys. Lett. 1979, 62, 600–602. [Google Scholar] [CrossRef]
- Grzyb, T.; Runowski, M.; Lis, S. Facile synthesis, structural and spectroscopic properties of GdF3:Ce3+, Ln3+ (Ln3+ = Sm3+, Eu3+, Tb3+, Dy3+) nanocrystals with bright multicolor luminescence. J. Lumin. 2014, 154, 479–486. [Google Scholar] [CrossRef]
- Pokhrel, M.; Mimun, L.C.; Yust, B.; Kumar, G.A.; Dhanale, A.; Tang, L.; Sardara, D.K. Stokes emission in GdF3:Nd3+ nanoparticles for bioimaging probes. Nanoscale 2014, 6, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, S.; Tada, M.; Kimura, T. Sol-gel processing of LaF3 thin films. J. Ceram. Soc. JPN 1998, 106, 124–126. [Google Scholar] [CrossRef]
- Fujihara, S.; Tada, M.; Kimura, T. Formation of LaF3 microcrystals in sol-gel silica. J. Non-Cryst. Solids 1999, 244, 267–274. [Google Scholar] [CrossRef]
- Tada, M.; Fujihara, S.; Kimura, T. Sol-gel processing and characterization of alkaline earth and rare-earth fluoride thin films. J. Mater. Res. 1999, 14, 1610–1616. [Google Scholar] [CrossRef]
- Fujihara, S.; Kato, T.; Kimura, T. Influence of solution composition on the formation of SiO2/LaF3 composites in the sol-gel process. J. Mater. Sci. 2000, 35, 2763–2767. [Google Scholar] [CrossRef]
- Fujihara, S.; Tada, M.; Kimura, T. Controlling factors for the conversion of trifluoroacetate sols into thin metal fluoride coatings. J. Sol-Gel Sci. Technol. 2000, 19, 311–314. [Google Scholar] [CrossRef]
- Fujihara, S.; Kato, T.; Kimura, T. Sol-gel synthesis of silica-based oxyfluoride glass-ceramic thin films: incorporation of Eu3+ activators into crystallites. J. Am. Ceram. Soc. 2001, 84, 2716–2718. [Google Scholar] [CrossRef]
- Ribeiro, S.J.L.; Araújo, C.C.; Bueno, L.A.; Gonçalves, R.R.; Messaddeq, Y. Sol-gel Eu3+/Tm3+ doped transparent glass-ceramic waveguides. J. Non-Cryst. Solids 2004, 348, 180–184. [Google Scholar] [CrossRef]
- Biswas, A.; Maciel, G.S.; Friend, C.S.; Prasad, P.N. Upconversion properties of a transparent Er3+-Yb3+co-doped LaF3-SiO2 glass-ceramics prepared by sol-gel method. J. Non-Cryst. Solids 2003, 316, 393–397. [Google Scholar] [CrossRef]
- Yanes, A.C.; del-Castillo, J.; Méndez-Ramos, J.; Rodríguez, V.D.; Torres, M.E.; Arbiol, J. Luminescence and structural characterization of transparent nanostructured Eu3+-doped LaF3-SiO2 glass-ceramics prepared by sol-gel method. Opt. Mater. 2007, 9, 999–1003. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Yanes, A.C.; del Castillo, J.; Méndez-Ramos, J.; Rodríguez, V.D. Optical properties of Ho3+-Yb3+ co-doped nanostructured SiO2-LaF3 glass-ceramics prepared by sol-gel method. Phys. Status Solidi A 2007, 204, 1762–1768. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Velázquez, J.J.; Yanes, A.C.; del Castillo, J.; Rodríguez, V.D. Up-conversion in nanostructured Yb3+-Tm3+ co-doped sol-gel derived SiO2-LaF3 transparent glass-ceramics. Phys. Status Solidi A 2008, 205, 330–334. [Google Scholar] [CrossRef]
- Yanes, A.C.; Velázquez, J.J.; del Castillo, J.; Méndez-Ramos, J.; Rodríguez, V.D. Colour tuneability and white light generation in Yb3+-Ho3+-Tm3+ co-doped SiO2-LaF3 nano-glass-ceramics prepared by sol-gel method. J. Sol-Gel Sci. Technol. 2009, 51, 4–9. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Rodríguez, V.D.; Yanes, A.C.; del Castillo, J.; Méndez-Ramos, J. Increase in the Tb3+ green emission in SiO2-LaF3 nano-glass-ceramics by codoping with Dy3+ ions. J. Appl. Phys. 2010, 108, 113530–113536. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Rodríguez, V.D.; Yanes, A.C.; del Castillo, J.; Méndez-Ramos, J. Photon down-shifting by energy transfer from Sm3+ to Eu3+ ions in sol-gel SiO2-LaF3 nano-glass-ceramics for photovoltaics. Appl. Phys. B 2012, 108, 577–583. [Google Scholar] [CrossRef]
- Dejneka, M.J. The luminescence and structure of novel transparent oxyfuoride glass-ceramics. J. Non-Cryst. Solids 1998, 239, 149–155. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Cheng, Y.; Bao, F.; Zhou, L. Crystallization and structural evolution of YF3-SiO2 xerogel. Mater. Sci. Eng. B 2006, 127, 218–223. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Santana-Alonso, A.; Yanes, A.C.; del Castillo, J.; Rodríguez, V.D. Rare-earth doped YF3 nanocrystals embedded in sol-gel silica glass matrix for white light generation. J. Lumin. 2010, 130, 2508–2511. [Google Scholar] [CrossRef]
- Santana-Alonso, A.; Méndez-Ramos, J.; Yanes, A.C.; del Castillo, J.; Rodríguez, V.D. White light up-conversion in transparent sol-gel derived glass-ceramics containing Yb3+-Er3+-Tm3+ triply-doped YF3 nanocrystals. Mater. Chem. Phys. 2010, 124, 699–703. [Google Scholar] [CrossRef]
- Yanes, A.C.; Santana-Alonso, A.; Méndez-Ramos, J.; del Castillo, J.; Rodríguez, V.D. Novel sol-gel nano-glass-ceramics comprising Ln3+-Doped YF3 nanocrystals: structure and high efficient UV up-conversion. Adv. Funct. Mater. 2011, 21, 3136–3142. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Yu, Y.; Huang, P. Structure and optical spectroscopy of Eu-doped glass ceramics containing GdF3 nanocrystals. J. Phys. Chem. C 2008, 112, 18943–18947. [Google Scholar] [CrossRef]
- Shan, Z.; Chen, D.; Yu, Y.; Huang, P.; Lin, H.; Wang, Y. Luminescence in rare earth-doped transparent glass ceramics containing GdF3 nanocrystals for lighting applications. J. Mater. Sci. 2010, 45, 2775–2779. [Google Scholar] [CrossRef]
- Yin, W.; Zhao, L.; Zhou, L.; Gu, Z.; Liu, X.; Tian, G.; Jin, S.; Yan, L.; Ren, W.; Xing, G.; Zhao, Y. Enhanced red emission from GdF3:Yb3+, Er3+ upconversion nanocrystals by Li+ doping and their application for bioimaging. Chem. Eur. J. 2012, 18, 9239–9245. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, S.; Koji, S.; Kimura, T. Structure and optical properties of (Gd,Eu)F3-nanocrystallized sol-gel silica films. J. Mater. Chem. 2004, 14, 1331–1335. [Google Scholar] [CrossRef]
- Szpikowska-Sroka, B.; Zur, L.; Czoik, R.; Goryczka, T.; Żądło, M.; Pisarski, W.A. Ultraviolet-to-visible downconversion luminescence in solgel oxyfluoride glass ceramics containing Eu3+:GdF3 nanocrystals. Opt. Lett. 2014, 39, 3181–3184. [Google Scholar] [CrossRef] [PubMed]
- Szpikowska-Sroka, B.; Pawlik, N.; Goryczka, T.; Pisarski, W.A. Influence of silicate sol-gel host matrices and catalyst agents on the luminescent properties of Eu3+/Gd3+ under different excitation wavelengths. RSC Adv. 2015, 5, 98773–98782. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Sołtys, M.; Pisarski, W.A. Optical properties of silica sol-gel materials singly- and doubly-doped with Eu3+and Gd3+ ions. J. Rare Earth 2016, 34, 786–795. [Google Scholar] [CrossRef]
- Kano, T.; Yamamoto, H.; Otomo, Y. NaLnF4: Yb3+, Er3+ (Ln: Y, Gd, La): Efficient green-emitting infrared-excited phosphors. J. Electrochem. Soc. 1972, 119, 1561–1564. [Google Scholar] [CrossRef]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Liu, F.; Ma, E.; Chen, D.; Yu, Y.; Wang, Y. Tunable red-green upconversion luminescence in novel transparent glass ceramics containing Er: NaYF4 nanocrystals. J. Phys. Chem. B 2006, 110, 20843–20846. [Google Scholar] [CrossRef] [PubMed]
- De Pablos-Martín, A.; Méndez-Ramos, J.; del Castillo, J.; Durán, A.; Rodríguez, V.D.; Pascual, M.J. Crystallization and up-conversion luminescence properties of Er3+/Yb3+-doped NaYF4-based nano-glass-ceramics. J. Eur. Ceram. Soc. 2015, 35, 1831–1840. [Google Scholar] [CrossRef]
- Yanes, A.C.; Santana-Alonso, A.; Méndez-Ramos, J.; del Castillo, J.; Rodríguez, V.D. Yb3+-Er3+ co-doped sol-gel transparent nano-glass-ceramics containing NaYF4 nanocrystals for tuneable up-conversion phosphors. J. Alloy. Compd. 2009, 480, 706–710. [Google Scholar] [CrossRef]
- Santana-Alonso, A.; Yanes, A.C.; Méndez-Ramos, J.; del Castillo, J.; Rodríguez, V.D. Sol-gel transparent nano-glass-ceramics containing Eu3+-doped NaYF4 nanocrystals. J. Non-Cryst. Solids 2010, 356, 933–936. [Google Scholar] [CrossRef]
- Santana-Alonso, A.; Méndez-Ramos, J.; Yanes, A.C.; del Castillo, J.; Rodríguez, V.D. Up-conversion in sol-gel derived nano-glass-ceramics comprising NaYF4 nano-crystals doped with Yb3+, Ho3+ and Tm3+. Opt. Mater. 2010, 32, 903–908. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Yanes, A.C.; Santana-Alonso, A.; del Castillo, J.; Rodríguez, V.D. Colour tuneability in sol-gel nano-glass-ceramics comprising Yb3+-Er3+-Tm3+ Co-Doped NaYF4 nanocrystals. J. Nanosci. Nanotechno. 2010, 10, 1273–1277. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Yanes, A.C.; Santana-Alonso, A.; del Castillo, J. Highly efficient up-conversion and bright white light in RE co-doped KYF4 nanocrystals in sol-gel silica matrix. Chem. Phys. Lett. 2013, 555, 196–201. [Google Scholar] [CrossRef]
- Yanes, A.C.; Santana-Alonso, A.; Méndez-Ramos, J.; del Castillo, J. Structure and intense UV up-conversion emissions in RE3+-doped sol-gel glass-ceramics containing KYF4 nanocrystals. Appl. Phys. B 2013, 113, 589–596. [Google Scholar] [CrossRef]
- Del Castillo, J.; Yanes, A.C.; Santana-Alonso, A.; Méndez-Ramos, J. Efficient dual-wavelength excitation of Tb3+ emission in rare-earth doped KYF4 cubic nanocrystals dispersed in silica sol-gel matrix. Opt. Mater. 2014, 37, 511–515. [Google Scholar] [CrossRef]
- Yanes, A.C.; del Castillo, J. Enhanced emission via energy transfer in RE co-doped SiO2-KYF4 nano-glass-ceramics for white LEDs. J. Alloy. Compd. 2016, 658, 170–178. [Google Scholar] [CrossRef]
- Deng, D.; Xu, S.; Zhao, S.; Li, C.; Wang, H.; Ju, H. Enhancement of upconversion luminescence in Tm3+/Er3+/Yb3+-codoped glass ceramic containing LiYF4 nanocrystals. J. Lumin. 2009, 129, 1266–1270. [Google Scholar] [CrossRef]
- Kawamura, G.; Yoshimura, R.; Ota, K.; Oh, S.Y.; Hakiri, N.; Muto, H.; Hayakawa, T.; Matsuda, A. A unique approach to characterization of sol-gel-derived rare-earth-doped oxyfluoride glass-ceramics. J. Am. Ceram. Soc. 2013, 96, 476–480. [Google Scholar] [CrossRef]
- Secu, C.E.; Negrea, R.F.; Secu, M. Eu3+ probe ion for rare-earth dopant site structure in sol-gel derived LiYF4 oxyfluoride glass-ceramic. Opt. Mater. 2013, 35, 2456–2460. [Google Scholar] [CrossRef]
- Kawamura, G.; Yoshimura, R.; Ota, K.; Oh, S.Y.; Muto, H.; Hayakawa, T.; Matsuda, A. Extraction of Nd3+-doped LiYF4 phosphor from sol-gel-derived oxyfluoride glass ceramics by hydrofluoric acid treatment. Opt. Mater. 2013, 45, 1879–1881. [Google Scholar] [CrossRef]
- Secu, M.; Secu, C.E. Up-conversion luminescence of Er3+/Yb3+ co-doped LiYF4 nanocrystals in sol-gel derived oxyfluoride glass-ceramics. J. Non-Cryst. Solids 2015, 426, 78–82. [Google Scholar] [CrossRef]
- Del Castillo, J.; Yanes, A.C.; Abe, S.; Smet, P.F. Site selective spectroscopy in BaYF5:RE3+ (RE = Eu, Sm) nano-glass-ceramics. J. Alloy. Compd. 2015, 635, 136–141. [Google Scholar] [CrossRef]
- Del Castillo, J.; Yanes, A.C. Bright luminescence of Gd3+ sensitized RE3+-doped SiO2-BaGdF5 glass-ceramics for UV-LEDs colour conversion. J. Alloy. Compd. 2017, 695, 3736–3743. [Google Scholar] [CrossRef]
- Chiodini, N.; Paleari, A.; DiMartino, D.; Spinolo, G. SnO2 nanocrystals in SiO2 : A wide-band-gap quantum-dot system. Appl. Phys. Lett. 2022, 81, 1702–1704. [Google Scholar] [CrossRef]
- Du, J.; Zhao, R.; Xie, Y.; Li, J. Size-controlled synthesis of SnO2 quantum dots and their gas-sensing performance. Appl Surf. Sci. 2015, 346, 256–262. [Google Scholar] [CrossRef]
- Nogami, M.; Enomoto, T.; Hayakawa, T. Enhanced fluorescence of Eu3+ induced by energy transfer from nanosized SnO2 crystals in glass. J. Lumin. 2002, 97, 147–152. [Google Scholar] [CrossRef]
- Chiodini, N.; Paleari, A.; Spinolo, G.; Crespi, P. Photorefractivity in SiO2:SnO2 glass-ceramics by visible light. J. Non-Cryst. Solids 2003, 322, 266–271. [Google Scholar] [CrossRef]
- Yanes, A.C.; del Castillo, J.; Torres, M.; Peraza, J.; Rodríguez, V.D.; Méndez-Ramos, J. Nanocrystal-size selective spectroscopy in SnO2: Eu3+ semiconductor quantum dots. Appl. Phys. Lett. 2004, 85, 2343–2345. [Google Scholar] [CrossRef]
- Del Castillo, J.; Rodríguez, V.D.; Yanes, A.C.; Méndez-Ramos, J.; Torres, M.E. Luminescent properties of transparent nanostructured Eu3+ doped SnO2–SiO2 glass-ceramics prepared by the sol-gel method. Nanotechnology 2005, 16, S300–S303. [Google Scholar] [CrossRef]
- Del Castillo, J.; Yanes, A.C.; Velázquez, J.J.; Méndez-Ramos, J.; Rodríguez, V.D. Luminescent properties of Eu3+-Tb3+-doped SiO2-SnO2-based nano-glass-ceramics prepared by sol-gel method. J. Alloy. Compd. 2009, 473, 571–575. [Google Scholar] [CrossRef]
- Morais, E.A.; Ribeiro, S.J.L.; Scalvi, L.V.A.; Santilli, C.V.; Ruggiero, L.O.; Pulcinelli, S.H.; Messadeq, Y. Optical characteristics of Er3+-Yb3+ doped SnO2 xerogels. J. Alloy. Compd. 2002, 344, 217–220. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Si Bui, T.; Turrell, S.; Capoen, B.; Roussel, P.; Bouazaoui, M.; Ferrari, M.; Cristini, O.; Kinowski, C. Controlled SnO2 nanocrystal growth in SiO2-SnO2 glass-ceramic monoliths. J. Raman Spectrosc. 2012, 43, 869–875. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Turrell, S.; Capoen, B.; Le Van, H.; Ferrari, M.; Ristic, D.; Boussekey, L.; Kinowski, C. Environment segregation of Er3+ emission in bulk sol-gel-derived SiO2-SnO2 glass ceramics. J. Mater. Sci. 2014, 49, 8226–8233. [Google Scholar] [CrossRef]
- Gonçalves, R.R.; Messaddeq, Y.; Chiasera, A.; Jestin, Y.; Ferrari, M.; Ribeiro, S.J.L. Erbium-activated silica-zirconia planar waveguides prepared by sol-gel route. Thin Solid Films 2008, 516, 3094–3097. [Google Scholar] [CrossRef]
- Gonçalves, R.R.; Guimarães, J.J.; Ferrari, J.L.; Maia, L.J.Q.; Ribeiro, S.J.L. Active planar waveguides based on sol-gel Er3+-doped SiO2-ZrO2 for photonic applications: Morphological, structural and optical properties. J. Non-Cryst. Solids 2008, 354, 4846–4851. [Google Scholar] [CrossRef]
- Suhaimi, N.F.M.; Rashid, S.N.M.; Junit, N.F.H.M.; Iznie Razakia, N.; Abd-Rahmana, M.K.; Ferrari, M. Effect of Zirconia in Er3+-doped SiO2-ZrO2 for planar waveguide laser. In Proceedings of the 2012 IEEE 3rd International Conference on Photonics (ICP), Penang, Malaysia, 1–3 October 2012. [Google Scholar]
- Dos Santos Cunha, C.; Ferrari, J.L.; de Oliveira, D.C.; Maia, L.J.Q.; Gomes, A.S.L.; Ribeiro, S.J.L.; Gonçalves, R.R. NIR luminescent Er3+/Yb3+ co-doped SiO2-ZrO2 nanostructured planar and channel waveguides: Optical and structural properties. Mater. Chem. Phys. 2012, 136, 120–129. [Google Scholar] [CrossRef]
- Ribeiro, S.J.L.; Messaddeq, Y.; Gonçalves, R.R.; Ferrari, M.; Montagna, M.; Aegerter, M.A. Low optical loss planar waveguides prepared in an organic-inorganic hybrid system. Appl. Phys. Lett. 2000, 77, 3502–3504. [Google Scholar] [CrossRef]
- Jestin, Y.; Armellini, C.; Chiappini, A.; Chiaser, A.; Ferrari, M.; Goyes, C.; Montagna, M.; Moser, E.; Nunzi Conti, G.; Pelli, S.; et al. Erbium activated HfO2 based glass-ceramics waveguides for photonics. J. Non-Cryst. Solids 2007, 353, 494–497. [Google Scholar] [CrossRef]
- Ferrari, J.L.; Lima, K.O.; Maia, L.J.Q.; Ribeiro, S.J.L.; Gonçalves, R.R. Structural and spectroscopic properties of luminescent Er3+-Doped SiO2-Ta2O5 nanocomposites. J. Am. Ceram. Soc. 2011, 94, 1230–1237. [Google Scholar] [CrossRef]
- Aquino, F.T.; Ferrari, J.L.; Ribeiro, S.J.L.; Ferrier, A.; Goldner, P.; Gonçalves, R.R. Broadband NIR emission in novel sol-gel Er3+-doped SiO2-Nb2O5 glass ceramic planar waveguides for photonic applications. Opt. Mater. 2013, 35, 387–396. [Google Scholar] [CrossRef]
- Secu, M.; Secu, C.E.; Bartha, C. Crystallization and luminescence properties of a new Eu3+-doped LaOCl nano-glass-ceramic. J. Eur. Ceram. Soc. 2016, 36, 203–207. [Google Scholar] [CrossRef]
- Innocenzi, P.; Abdirashid, M.O.; Guglielmi, M. Structure and properties of sol-gel coatings from methyltriethoxysilane and tetraethoxysilane. J. Sol-Gel Sci. Technol. 1994, 3, 47–55. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lee, H.R.; Tsuru, T. Study on preparation and hydrophobicity of MTES derived silica sol and gel. Adv. Mater. Res. 2012, 535–537, 2563–2566. [Google Scholar] [CrossRef]
- Jia, Y.Q. Crystal Radii and effective ionic radii of the rare earth ions. J. Sol. State Chem. 1991, 95, 184–187. [Google Scholar] [CrossRef]
- Gorni, G.; Pascual, M.J.; Caballero, A.; Velázquez, J.J.; Mosa, J.; Castro, Y.; Durán, A. Crystallization mechanism in sol-gel oxyfluoride glass-ceramics. J. Non-Cryst. Solids 2018. accepted. [Google Scholar]
- Gorni, G.; Balda, R.; Ferández, J.; Velázquez, J.J.; Pascual, L.; Mosa, J.; Durán, A.; Castro, Y. 80SiO2-20LaF3 oxyfluoride glass ceramic coatings doped with Nd3+ for optical applications. Int. J. Appl. Glass Sci. 2017, in press. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Mosa, J.; Gorni, G.; Balda, R.; Ferández, J.; Pascual, L.; Durán, A.; Castro, Y. Transparent SiO2-GdF3 sol-gel nano-glass ceramics for optical applications. J. Sol-Gel Sci. Technol. 2018. under-review. [Google Scholar]
- Chen, D.; Yu, Y.; Huang, P.; Wang, Y. Nanocrystallization of lanthanide trifluoride in an aluminosilicate glass matrix: Dimorphism and rare earth partition. CrystEngComm 2009, 11, 1686–1690. [Google Scholar] [CrossRef]
- Bartha, C.; Secu, C.E.; Matei, E.; Secu, M. Crystallization kinetics mechanism investigation of sol-gel-derived NaYF4:(Yb,Er) up-converting phosphors. CrystEngComm 2017, 19, 4992–5000. [Google Scholar] [CrossRef]
- Agarwal, B.K.; Verma, L.P. A rule for chemical shifts of X-ray absorption edges. J. Phys. C Solid State 1970, 3, 535–537. [Google Scholar] [CrossRef]
- Gorni, G.; Velázquez, J.J.; Mather, G.C.; Durán, A.; Chen, G.; Sundararaja, M.; Balda, R.; Ferández, J.; Pascual, M.J. Selective excitation in transparent oxyfluoride glass-ceramics doped with Nd3+. J. Eur. Ceram. Soc. 2017, 37, 1695–1706. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorni, G.; Velázquez, J.J.; Mosa, J.; Balda, R.; Fernández, J.; Durán, A.; Castro, Y. Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials. Materials 2018, 11, 212. https://doi.org/10.3390/ma11020212
Gorni G, Velázquez JJ, Mosa J, Balda R, Fernández J, Durán A, Castro Y. Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials. Materials. 2018; 11(2):212. https://doi.org/10.3390/ma11020212
Chicago/Turabian StyleGorni, Giulio, Jose J. Velázquez, Jadra Mosa, Rolindes Balda, Joaquin Fernández, Alicia Durán, and Yolanda Castro. 2018. "Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials" Materials 11, no. 2: 212. https://doi.org/10.3390/ma11020212
APA StyleGorni, G., Velázquez, J. J., Mosa, J., Balda, R., Fernández, J., Durán, A., & Castro, Y. (2018). Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials. Materials, 11(2), 212. https://doi.org/10.3390/ma11020212







