Improvement on the Repair Effect of Electrochemical Chloride Extraction Using a Modified Electrode Configuration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimen Preparation
2.2. Electrochemical Treatment
2.3. Measurements
2.3.1. Steel Potential Measurement
2.3.2. Cl−, OH−, Na+, and K+ Contents
2.3.3. Corrosion Potential and Corrosion Current Density of the Steel
3. Results
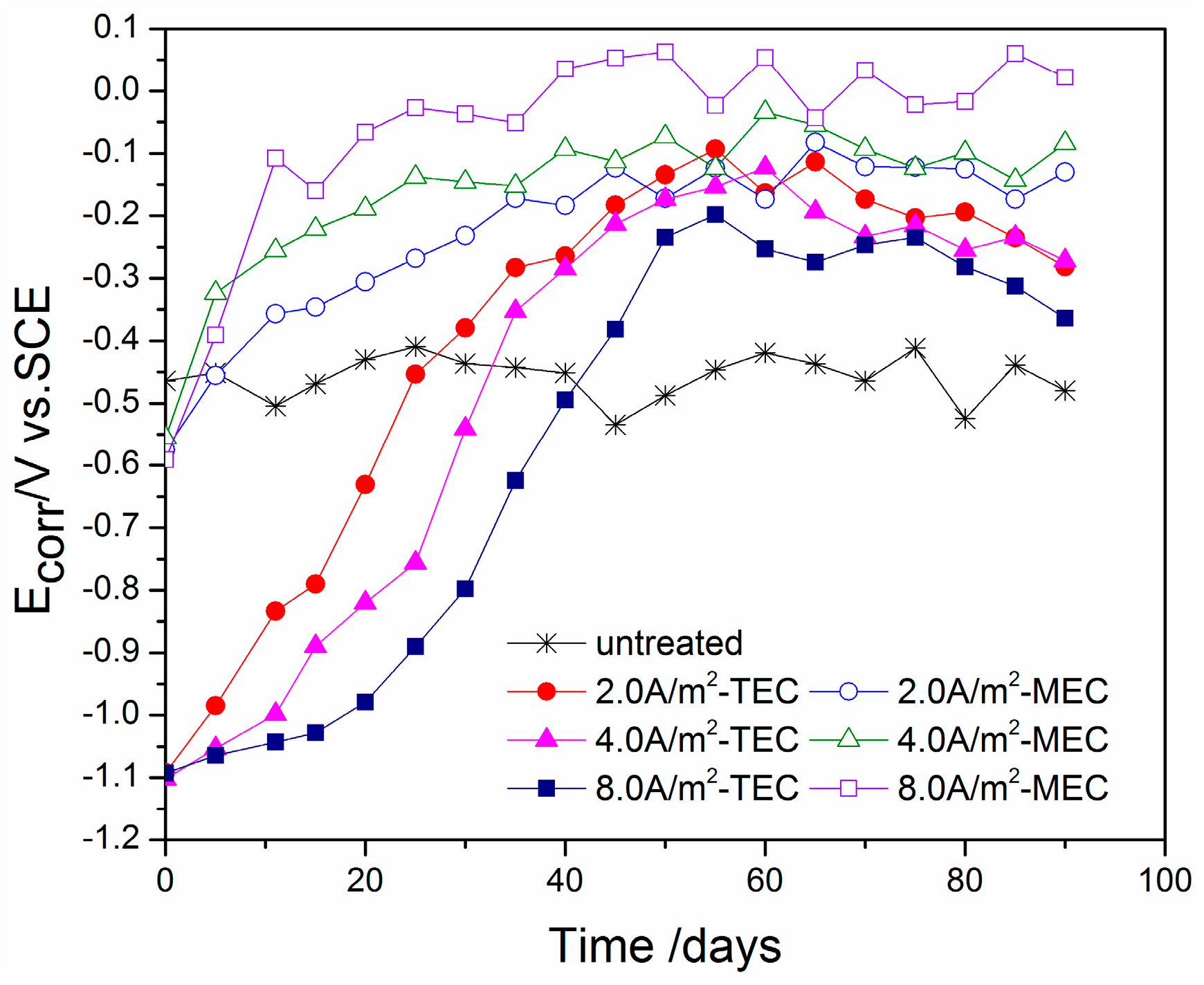
3.1. Change of Steel Potential with Time
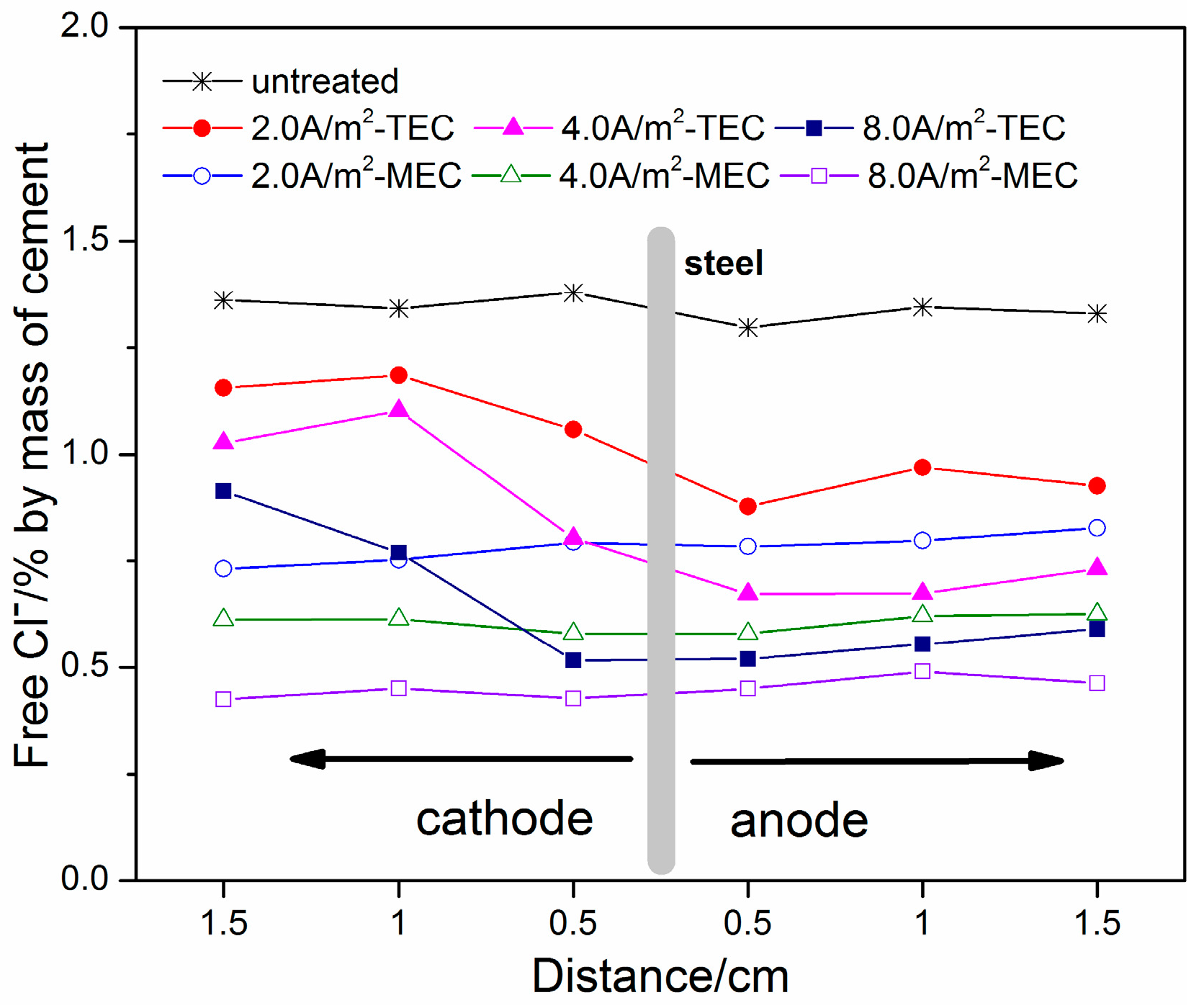
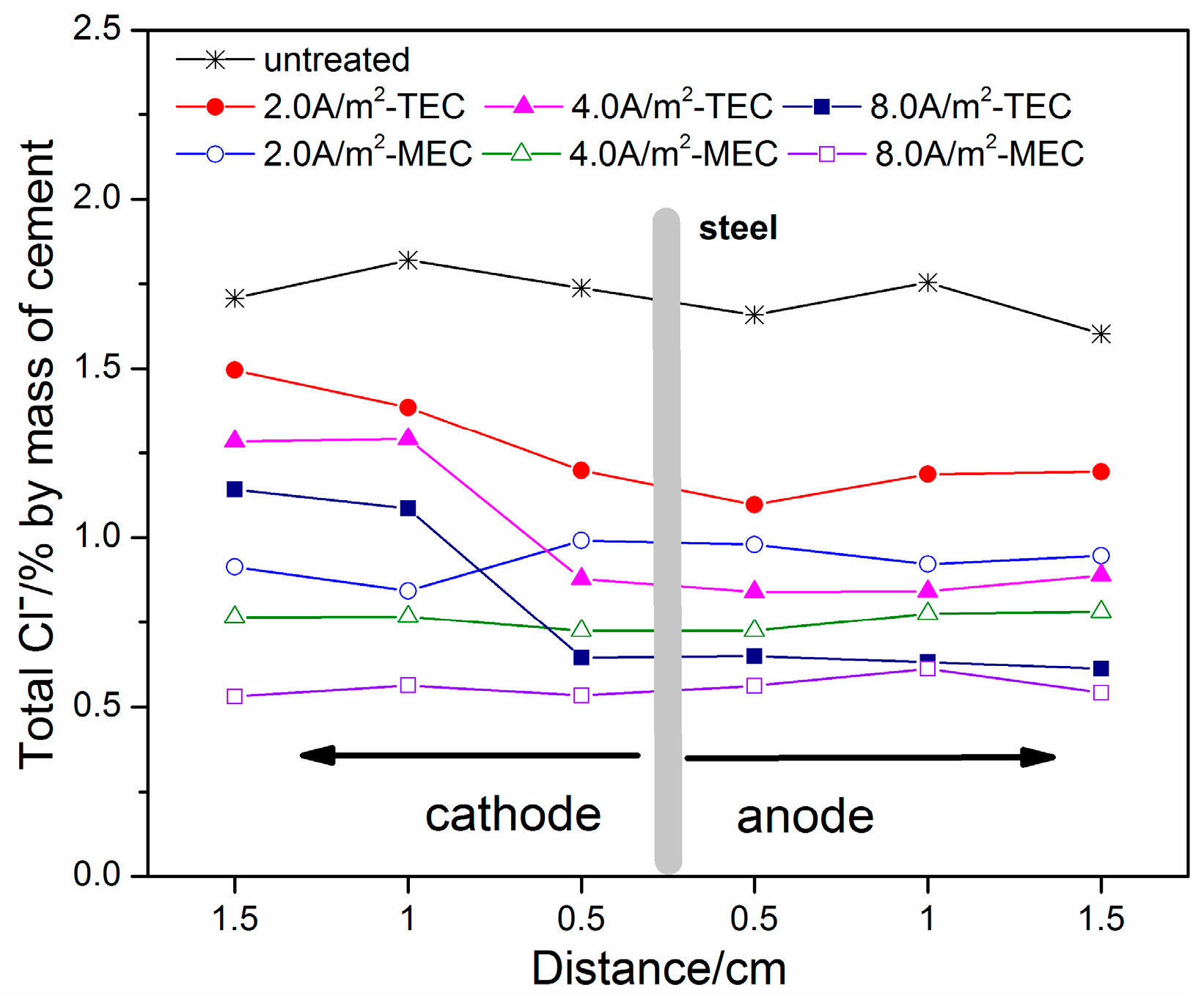
3.2. Extracted Chloride, Free Chloride, and Total Chloride Contents
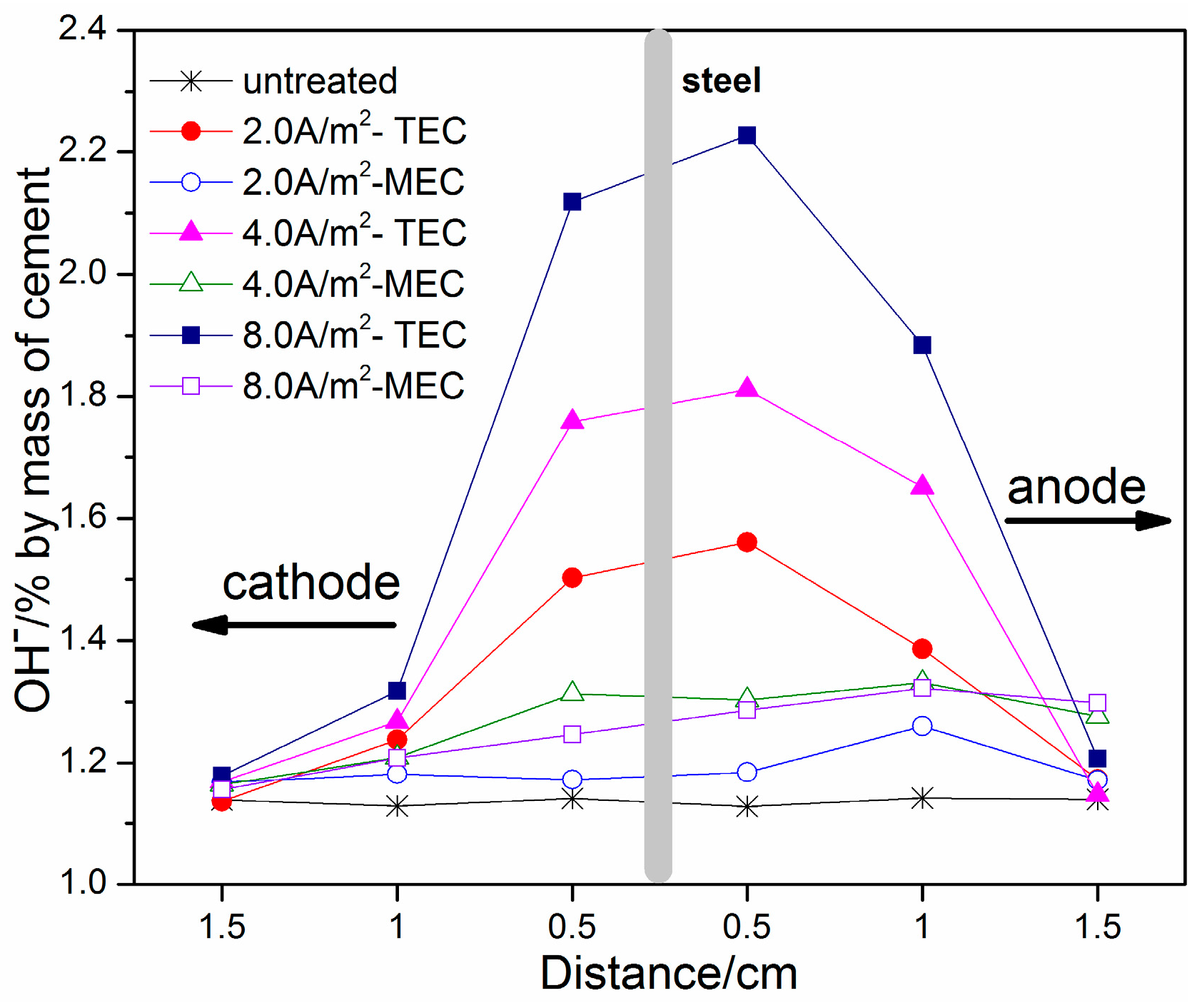
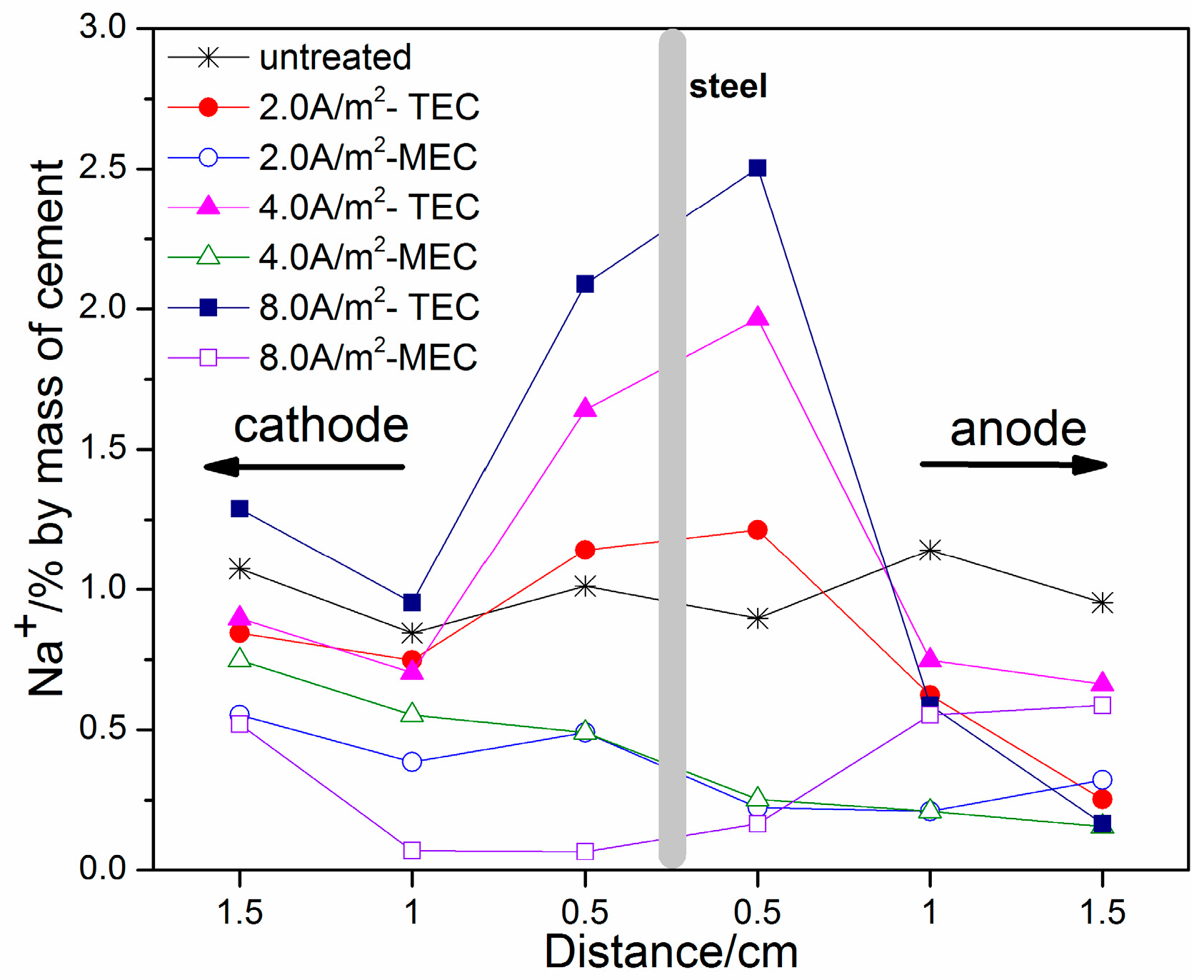
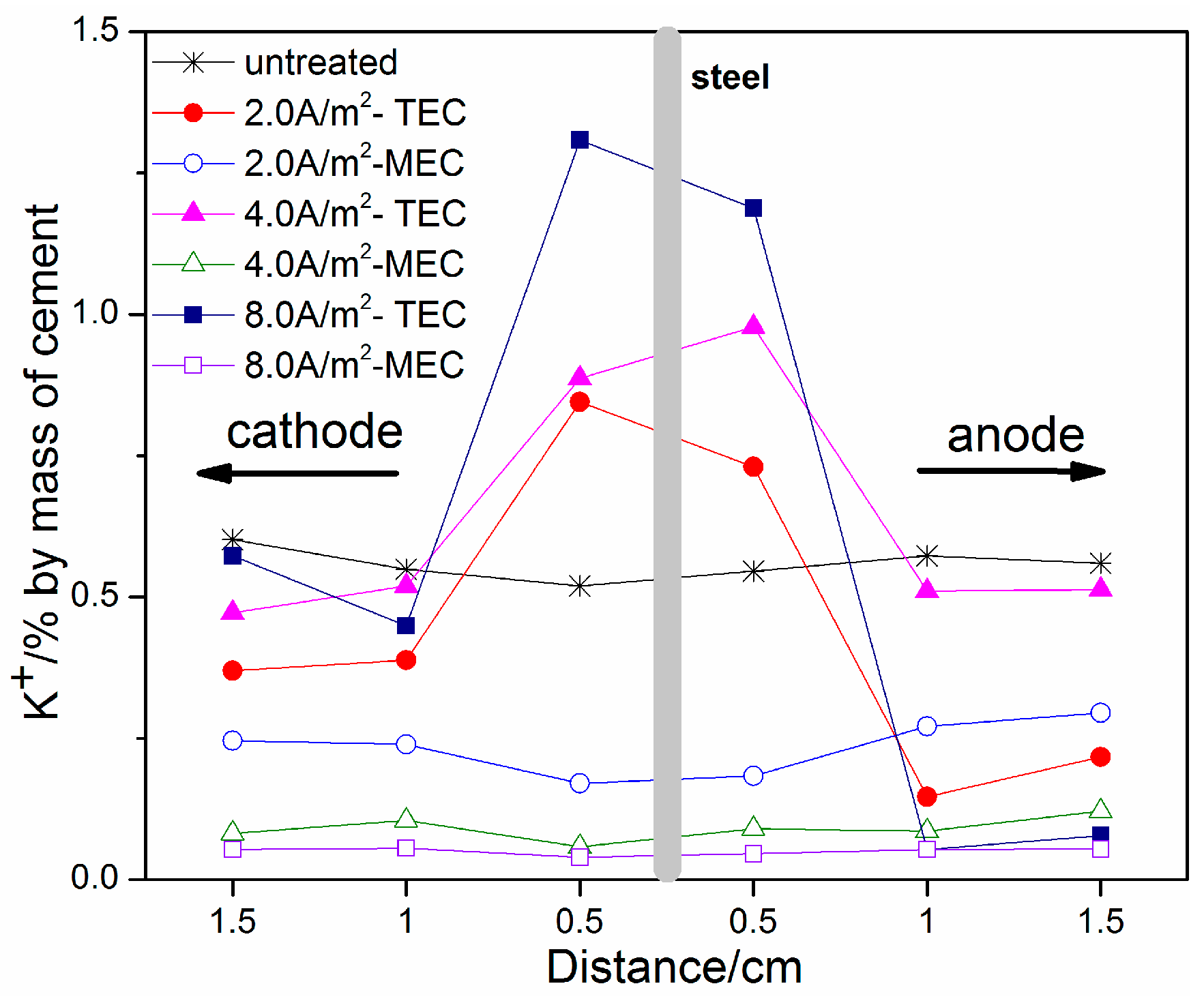
3.3. OH−, Na+, and K+ Contents
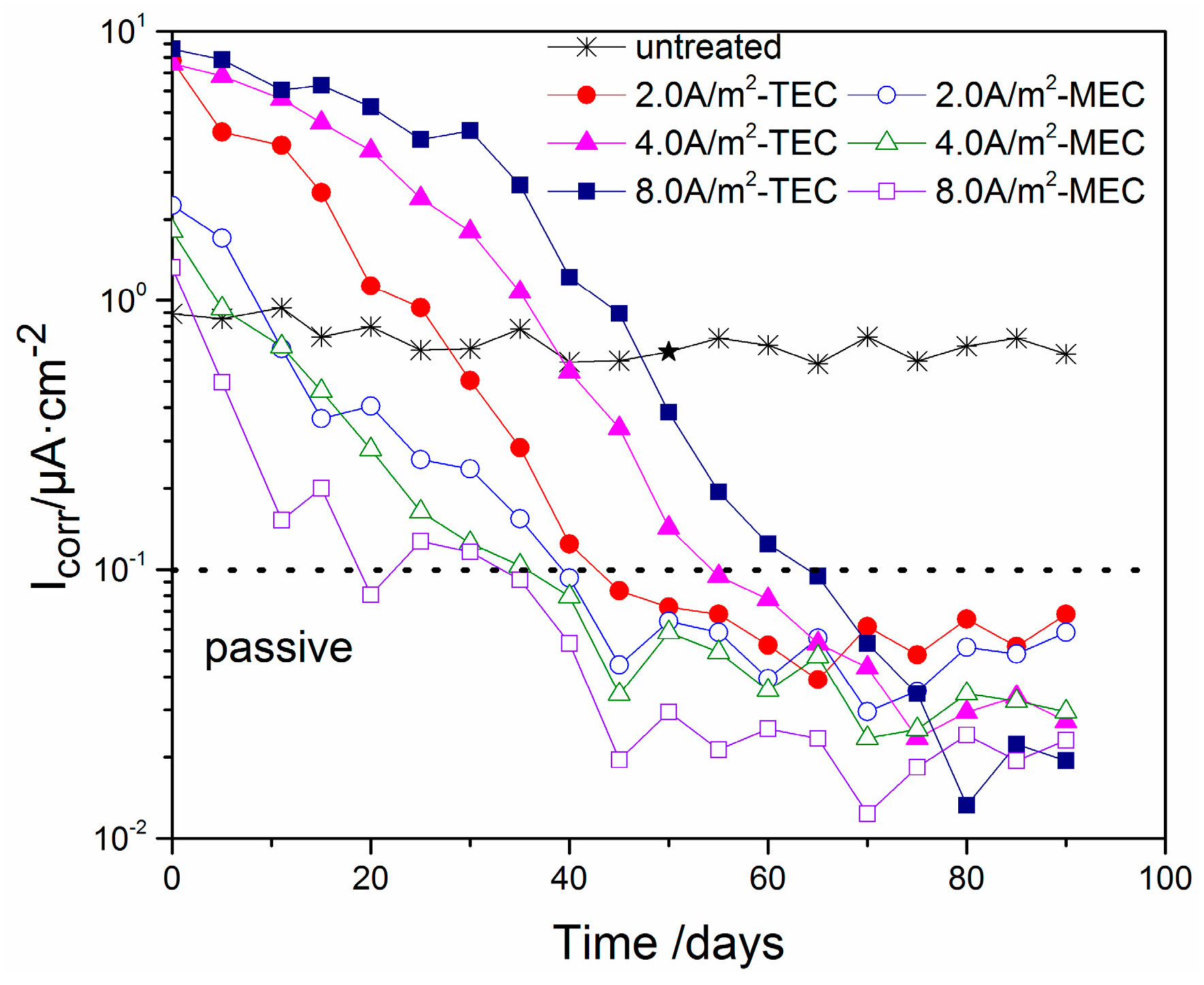
3.4. Corrosion Potential and Corrosion Current Density of the Steel
4. Discussion
5. Conclusions
- (1)
- The potential of the steel during the MEC treatment has a value of −800 mV versus the SCE, which is far lower than that during the TEC treatment. Besides, the applied current density does not change obviously the potential of the steel during the MEC treatment. This potential cannot induce hydrogen evolution so that hydrogen embrittlement of the steel can be avoided by using the MEC treatment.
- (2)
- For both the TEC treatment and MEC treatment, most of the chloride contents are extracted during the first 2 weeks of application. Compared to the TEC treatment, the chloride content in the anolyte and the free chloride content in the mortar are obviously decreased, but the total chloride content in the mortar for the MEC treatment is increased when the same current density is applied. Therefore, the MEC treatment has a better chloride removal ratio than the TEC treatment.
- (3)
- An obvious increase of OH−, Na+, and K+ contents near the steel surface can be obtained for the TEC treatment. In contrast, the OH−, Na+, and K+ contents near the steel surface are softly reduced after the MEC treatment. Therefore, the MEC treatment cannot induce the accumulation of OH−, Na+, and K+ near the steel surface.
- (4)
- The corrosion potentials for the specimens after all of the TEC treatments have an extremely lower initial value than the corrosion potential of the specimen without the treatment. Contrary to this, all of the potentials of the steel have a higher value when the equilibrium state of the steel is attained. However, the corrosion potentials for the specimens after all of the MEC treatments are slightly lower than the corrosion potential for the specimen without the treatment. The initial values of the corrosion current density after the MEC treatments are lower than those after the TEC treatments. In addition, the re-passivation of the steel is more quickly attained after the MEC treatment.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, L.; Wang, W.; Jiang, Y. Influence of CaCl2 and NaCl from different sources on chloride threshold value for the corrosion of steel reinforcement in concrete. Constr. Build. Mater. 2011, 25, 663–669. [Google Scholar] [CrossRef]
- Justnes, H.; Kim, M.O.; Ng, S.; Qian, X. Methodology of calculating required chloride diffusion coefficient for intended service life as function of concrete cover in reinforced marine structures. Cem. Concr. Compos. 2016, 73, 316–323. [Google Scholar] [CrossRef]
- Elsener, B.; Angst, U. Mechanism of electrochemical chloride removal. Corros. Sci. 2007, 49, 4504–4522. [Google Scholar] [CrossRef]
- Carmona, J.; Climent, M.; Antón, C.; Vera, G.D.; Garcés, P. Shape Effect of Electrochemical Chloride Extraction in Structural Reinforced Concrete Elements Using a New Cement-Based Anodic System. Materials 2015, 8, 2901–2917. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, G.; Escadeillas, G.; Arliguie, G. Electrochemical chloride extraction (ECE) from steel-reinforced concrete specimens contaminated by ‘‘artificial’’ sea-water. Corros. Sci. 2006, 48, 110–125. [Google Scholar] [CrossRef]
- Arya, C.; Sa’Id-Shawqi, Q.; Vassie, P.R.W. Factors influencing electrochemical removal of chloride from concrete. Cem. Concr. Res. 1996, 26, 851–860. [Google Scholar] [CrossRef]
- Siegwart, M.; Lyness, J.F.; Mcfarland, B.J.; Doyle, G. The effect of electrochemical chloride extraction on pre-stressed concrete. Constr. Build. Mater. 2005, 19, 585–594. [Google Scholar] [CrossRef]
- Marcotte, T.D.; Hansson, C.M.; Hope, B.B. The effect of the electrochemical chloride extraction treatment on steel-reinforced mortar Part I: Electrochemical measurements. Cem. Concr. Res. 1999, 29, 1555–1560. [Google Scholar] [CrossRef]
- Orellan, J.C.; Escadeillas, G.; Arliguie, G. Electrochemical chloride extraction: Efficiency and side effects. Cem. Concr. Res. 2004, 34, 227–234. [Google Scholar] [CrossRef]
- Abdelaziz, G.E.; Abdelalim, A.M.K.; Fawzy, Y.A. Evaluation of the short and long-term efficiencies of electro-chemical chloride extraction. Cem. Concr. Res. 2009, 39, 727–732. [Google Scholar] [CrossRef]
- Chang, J.J. Bond degradation due to the desalination process. Constr. Build. Mater. 2003, 17, 281–287. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Q.; Deng, C.; Wei, J.; Wen, Z. Change of electrochemical property of reinforced concrete after electrochemical chloride extraction. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 764–769. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wei, L.; Wang, Z.; Liang, C.K.; Fang, Y.; Xing, F. Application of carbon-fiber-reinforced polymer anode in electrochemical chloride extraction of steel-reinforced concrete. Constr. Build. Mater. 2016, 120, 275–283. [Google Scholar] [CrossRef]
- Martínez, I.; Andrade, C. Polarization resistance measurements of bars embedded in concrete with different chloride concentrations: EIS and DC comparison. Mater. Corros. 2015, 62, 932–942. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.Y.; Jiang, C.C.; Liu, B.; Li, N.B.; Zhu, R.F.; Lu, Y.P. Influence of process parameters on microstructure and corrosion properties of hopeite coating on stainless steel. Corros. Sci. 2015, 94, 428–437. [Google Scholar] [CrossRef]
- Boubitsas, D.; Tang, L. The influence of reinforcement steel surface condition on initiation of chloride induced corrosion. Mater. Struct. 2015, 48, 2641–2658. [Google Scholar] [CrossRef]
- Gartner, N.; Kosec, T.; Legat, A. The efficiency of a corrosion inhibitor on steel in a simulated concrete environment. Mater. Chem. Phys. 2016, 184, 31–40. [Google Scholar] [CrossRef]
- Liu, Q.F.; Xia, J.; Easterbrook, D.; Yang, J.; Li, L.Y. Three-phase modelling of electrochemical chloride removal from corroded steel-reinforced concrete. Constr. Build. Mater. 2014, 70, 410–427. [Google Scholar] [CrossRef]
- Yeih, W.; Chang, J.J.; Chang, C.C.; Chen, K.L.; Chi, M.C. Electrochemical chloride removal for reinforced concrete with steel rebar cage using auxiliary electrodes. Cem. Concr. Compos. 2016, 74, 136–146. [Google Scholar] [CrossRef]
- Siegwart, M.; Siegwart, M. Influence of Electrochemical Chloride Extraction on the Performance of Prestressed Concrete under Dynamic Loading Conditions. J. Mater. Civ. Eng. 2006, 18, 800–812. [Google Scholar] [CrossRef]
- Bikul’Chus, G. Chloride Removal from Reinforced Concrete and Relevant Loss of Strength. Prot. Met. Phys. Chem. 2005, 41, 484–486. [Google Scholar] [CrossRef]
- Otero, E.; González, J.A.; Cobo, A.; González, M.N. Electrochemical chloride removal from reinforced concrete structures and its ability to repassivate prerusted steel surfaces. Mater. Corros. 2001, 52, 581–589. [Google Scholar] [CrossRef]
- Gowers, K.R.; Millard, S.G.; Gill, J.S.; Gill, R.P. Programmable Linear Polarisation Meter for Determination of Corrosion Rate of Reinforcement in Concrete Structures. Br. Corros. J. 2013, 29, 25–32. [Google Scholar] [CrossRef]
- Page, M.M.; Ngala, V.T.; Page, C.L. Corrosion inhibitors in concrete repair systems. Mag. Concr. Res. 2000, 52, 25–37. [Google Scholar] [CrossRef]
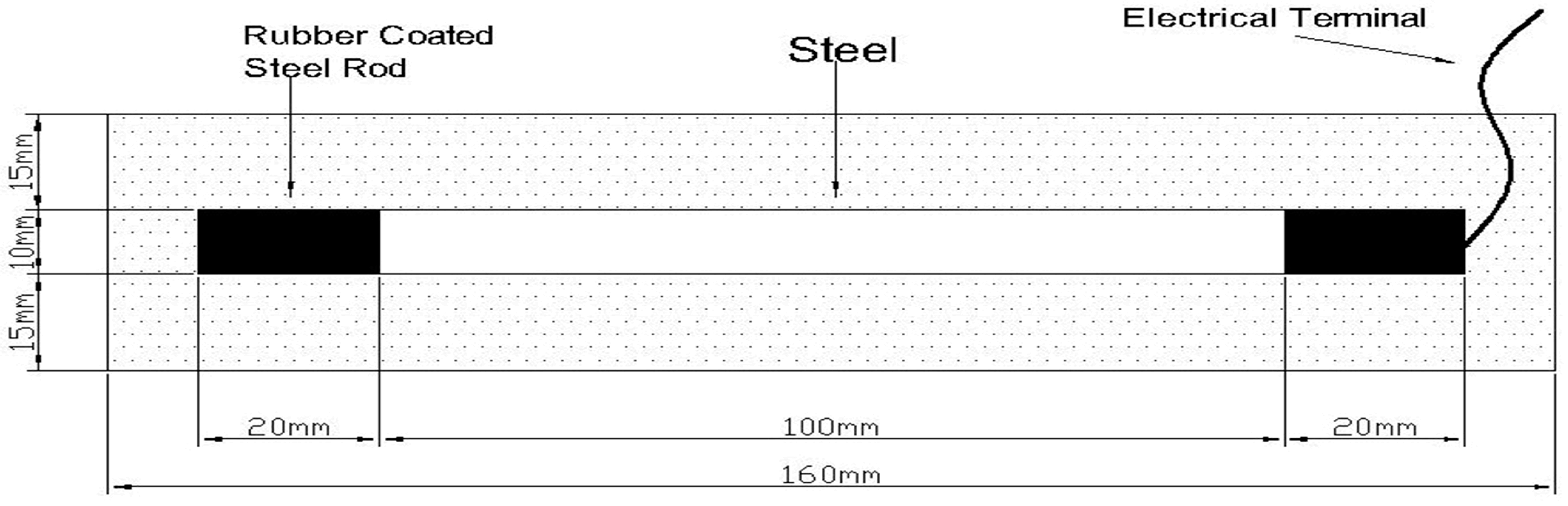



| Oxide | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | Total Cl | SO3 | Ignition Loss |
|---|---|---|---|---|---|---|---|---|---|---|
| Composition (wt.%) | 57.27 | 24.99 | 9.32 | 3.11 | 0.86 | 0.83 | 0.28 | 0.05 | 1.13 | 2.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Xu, J.; Jiang, L.; Song, Y.; Cao, Y.; Tan, Q. Improvement on the Repair Effect of Electrochemical Chloride Extraction Using a Modified Electrode Configuration. Materials 2018, 11, 225. https://doi.org/10.3390/ma11020225
Feng W, Xu J, Jiang L, Song Y, Cao Y, Tan Q. Improvement on the Repair Effect of Electrochemical Chloride Extraction Using a Modified Electrode Configuration. Materials. 2018; 11(2):225. https://doi.org/10.3390/ma11020225
Chicago/Turabian StyleFeng, Wei, Jinxia Xu, Linhua Jiang, Yingbin Song, Yalong Cao, and Qiping Tan. 2018. "Improvement on the Repair Effect of Electrochemical Chloride Extraction Using a Modified Electrode Configuration" Materials 11, no. 2: 225. https://doi.org/10.3390/ma11020225
APA StyleFeng, W., Xu, J., Jiang, L., Song, Y., Cao, Y., & Tan, Q. (2018). Improvement on the Repair Effect of Electrochemical Chloride Extraction Using a Modified Electrode Configuration. Materials, 11(2), 225. https://doi.org/10.3390/ma11020225




