Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments
Abstract
:1. Introduction
2. Experimental Procedures
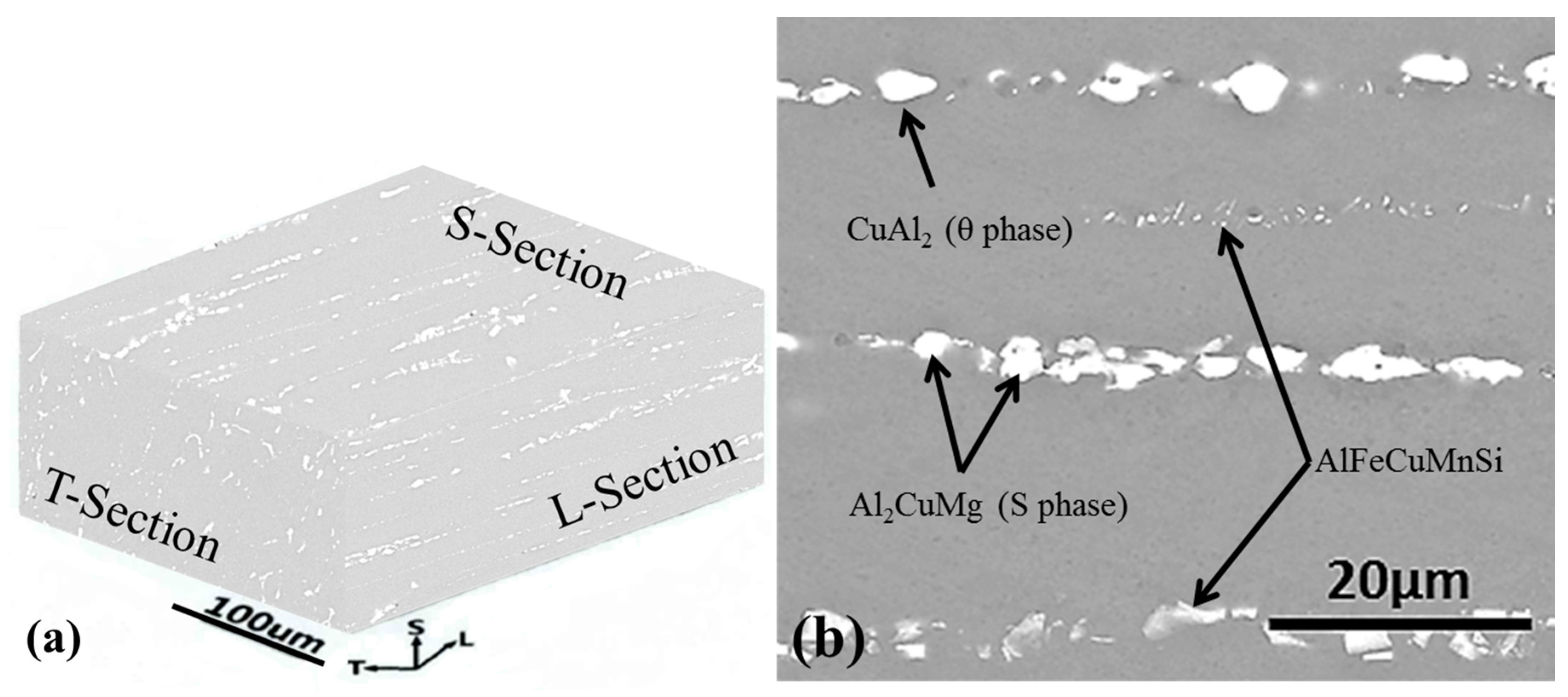
2.1. Materials Preparation
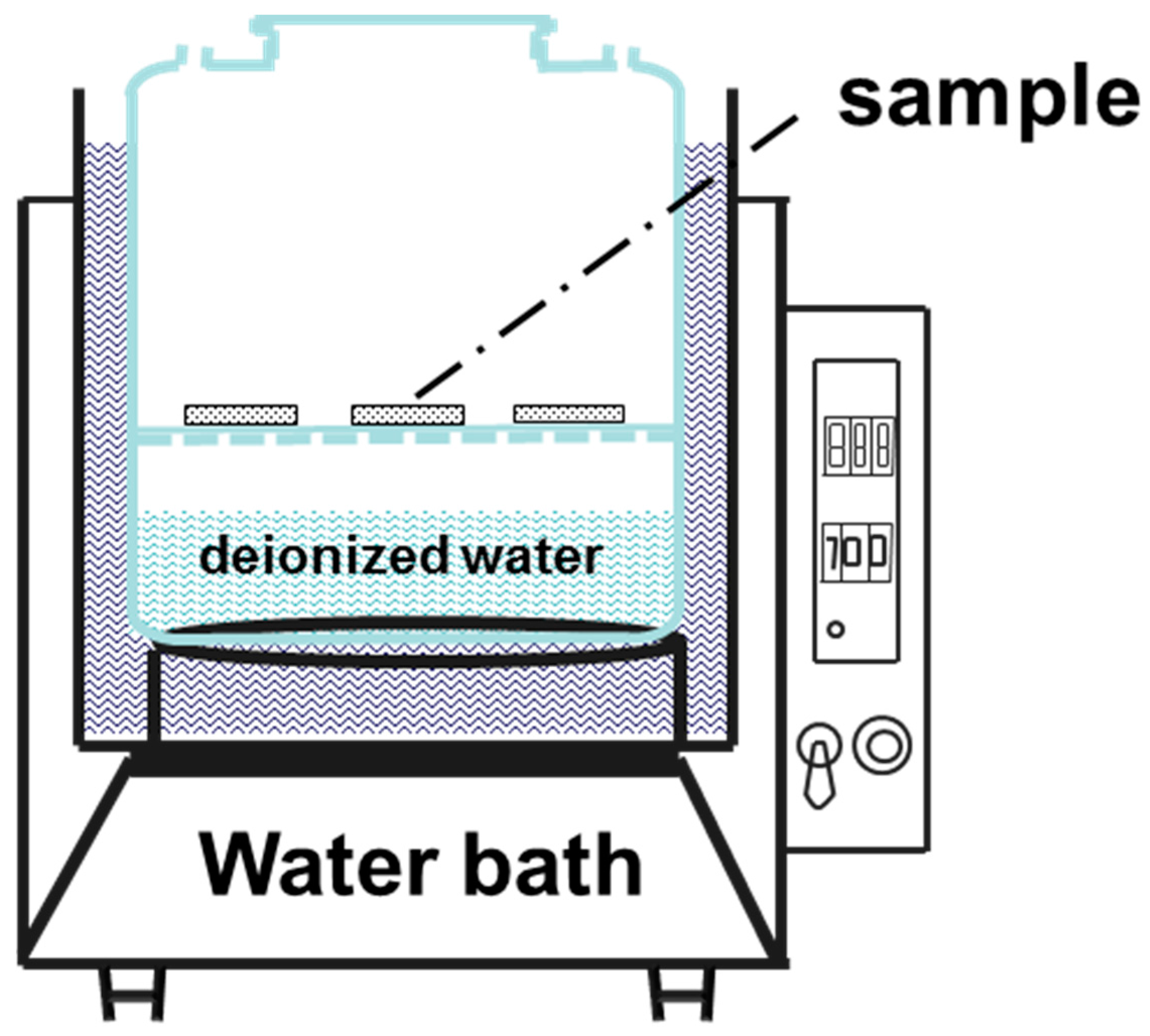
2.2. Corrosion Testing
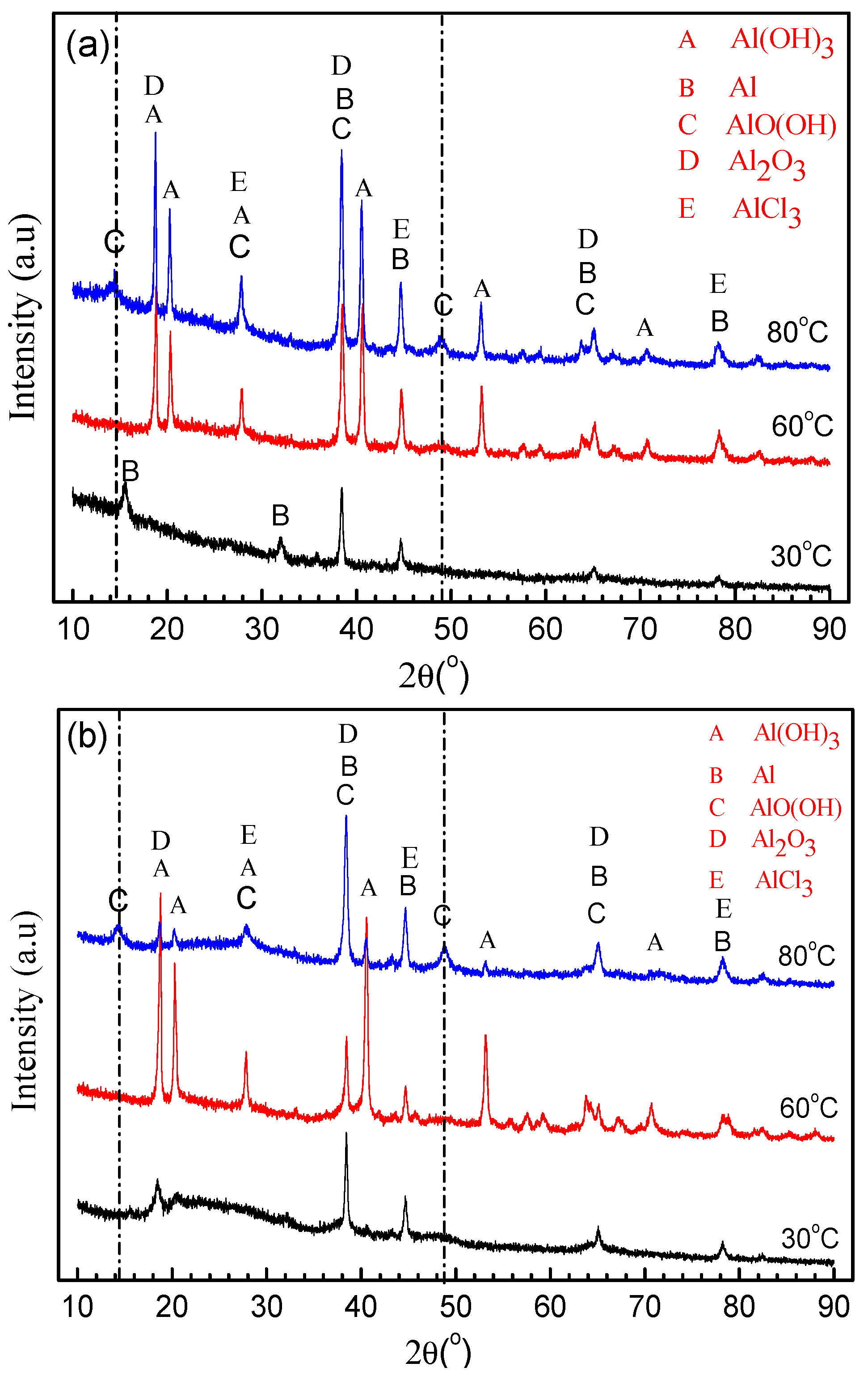
3. Results
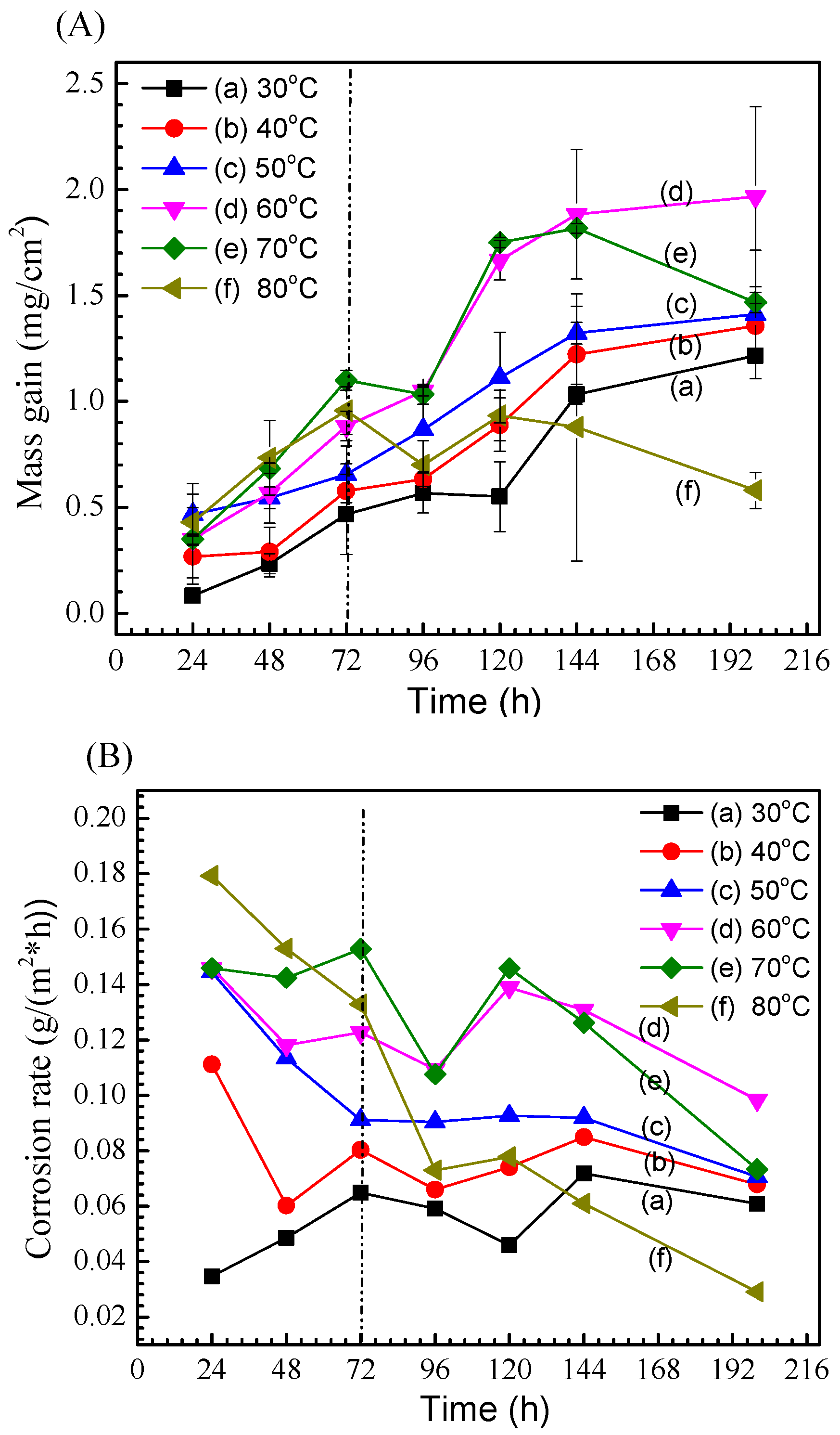
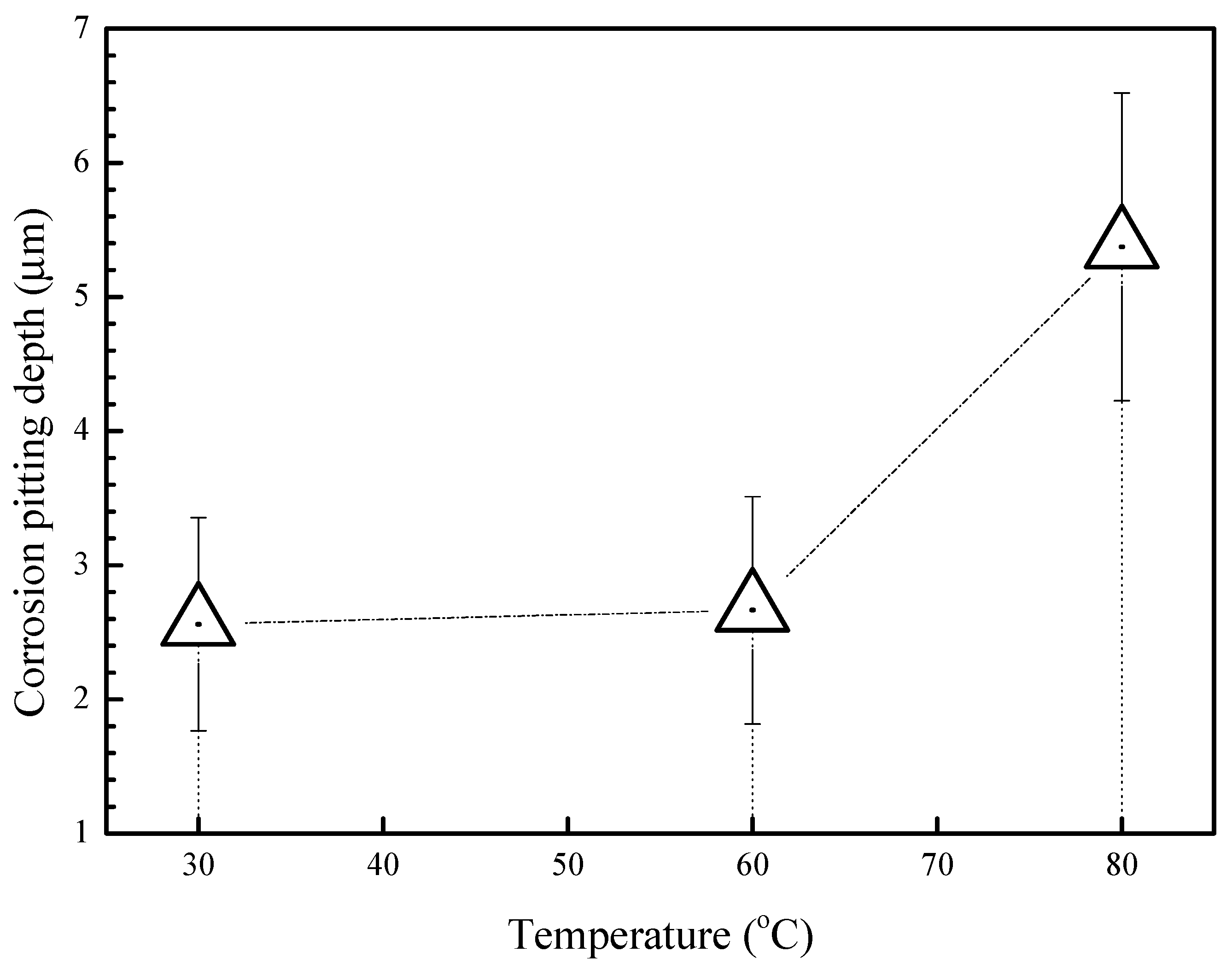
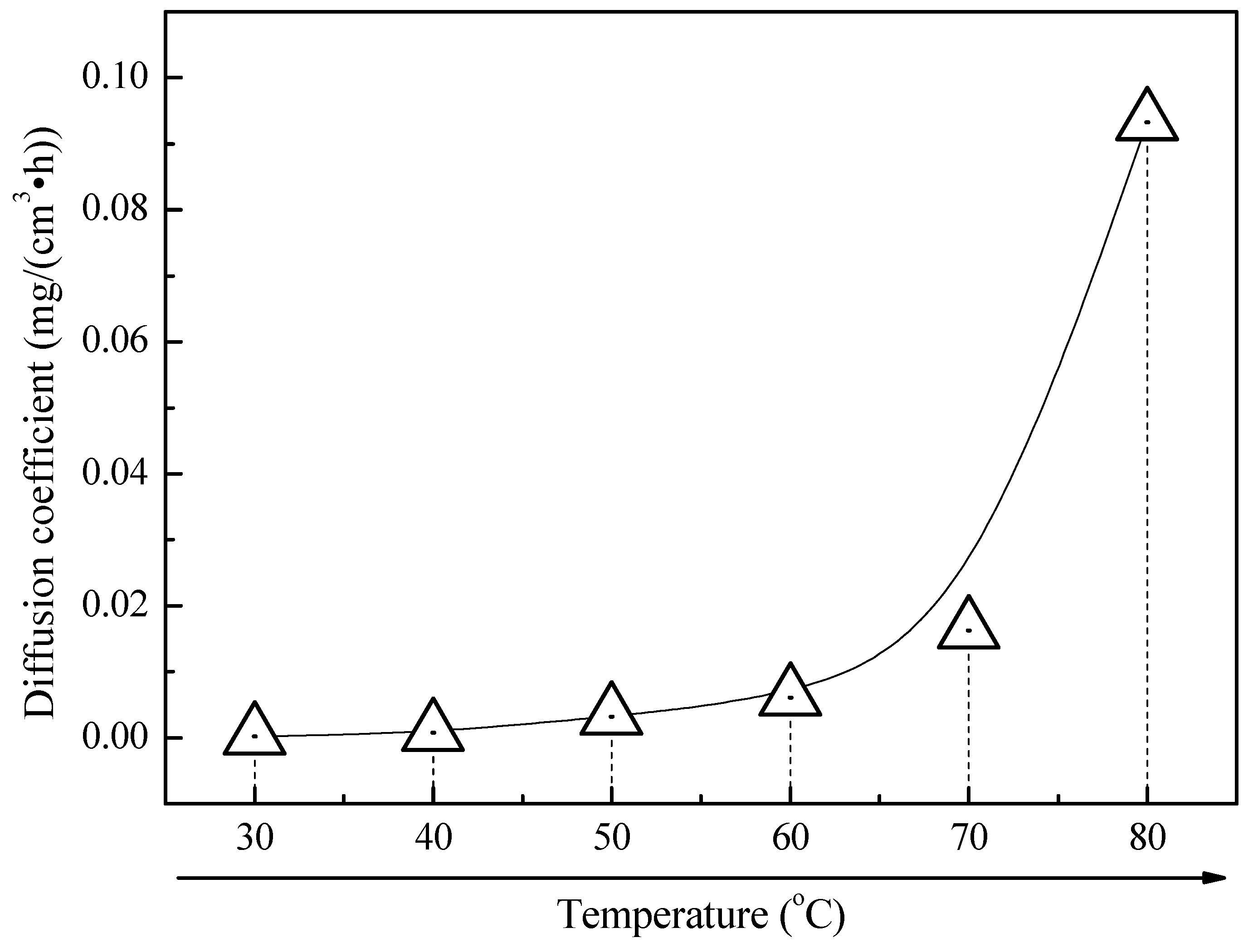
3.1. Corrosion Kinetics
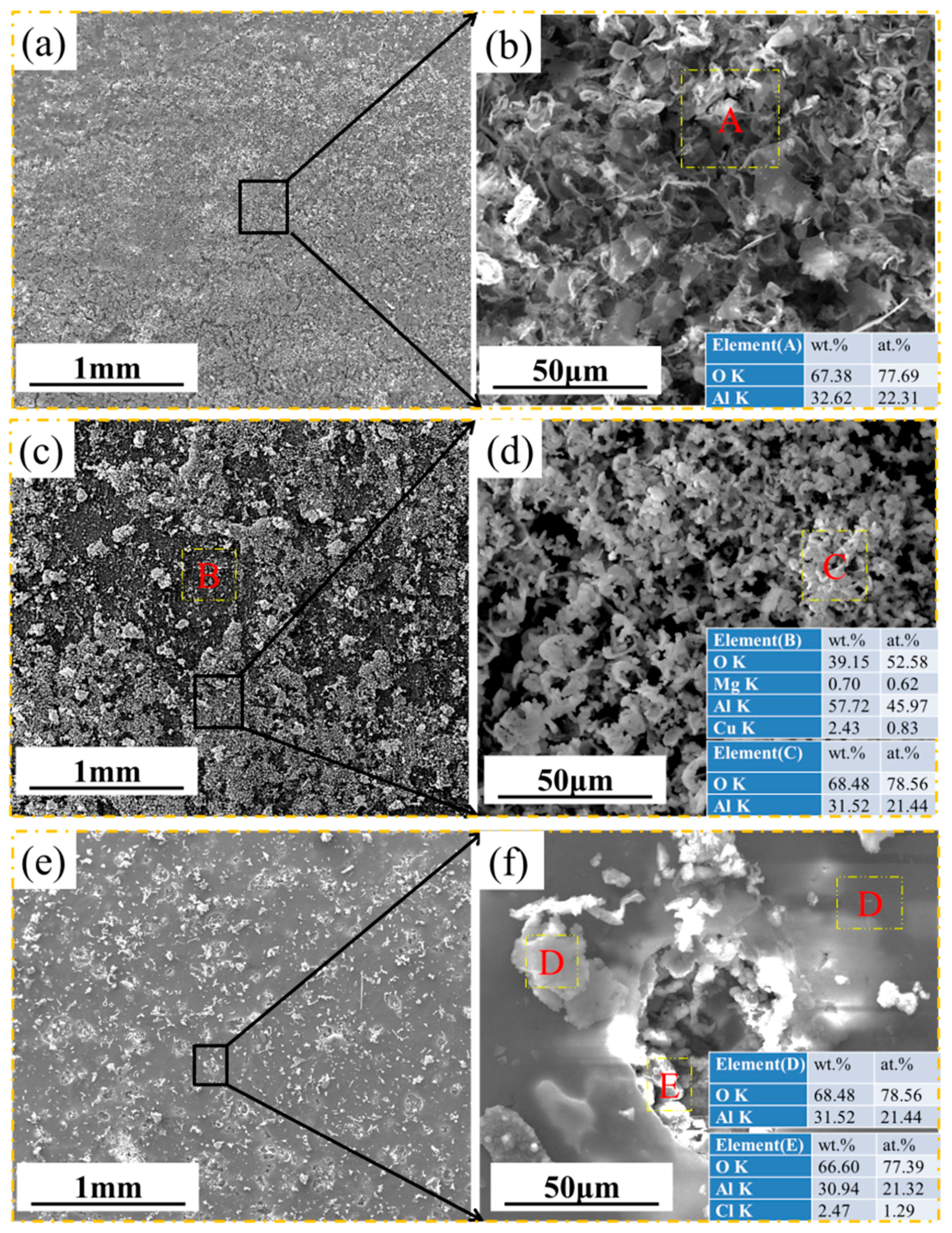
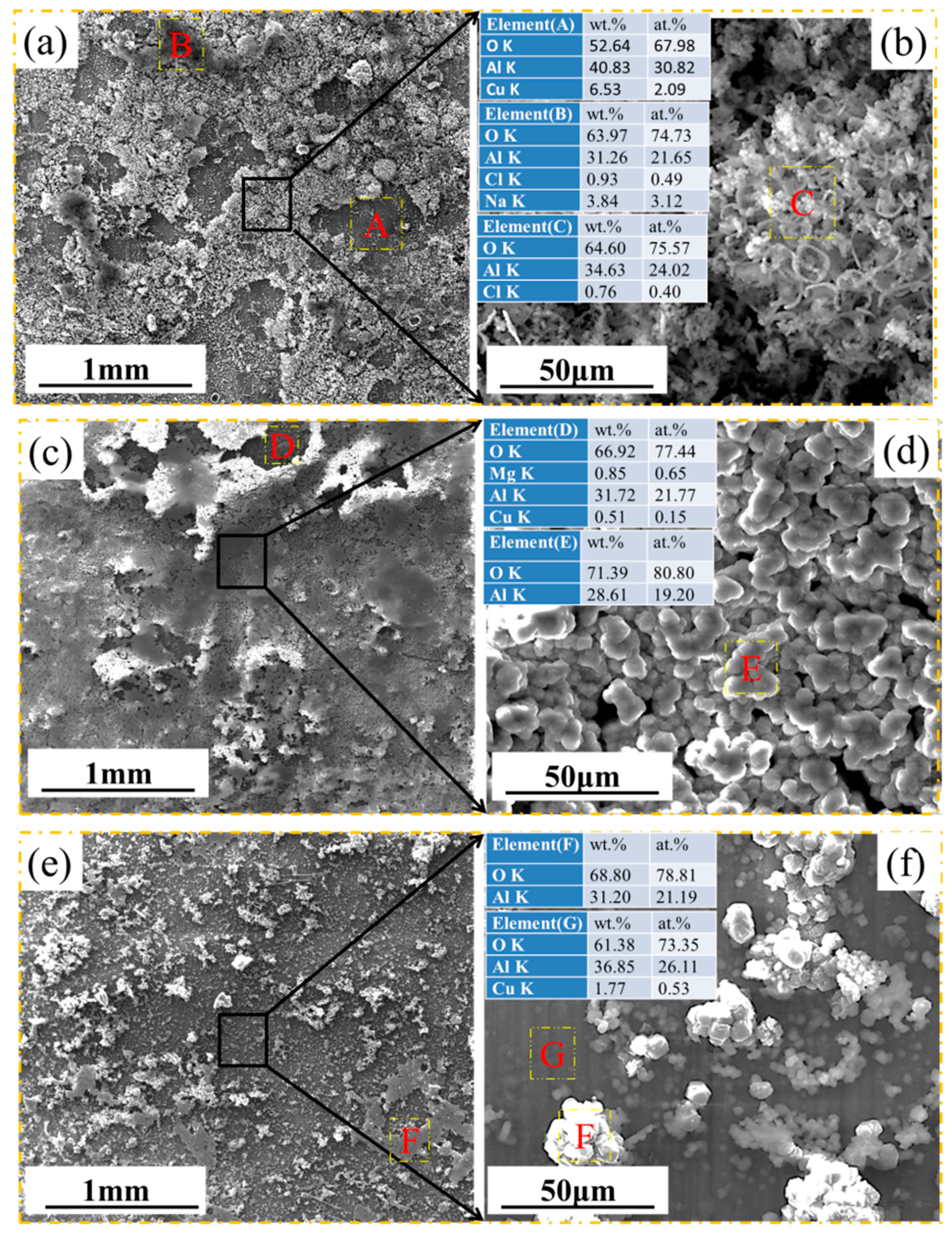
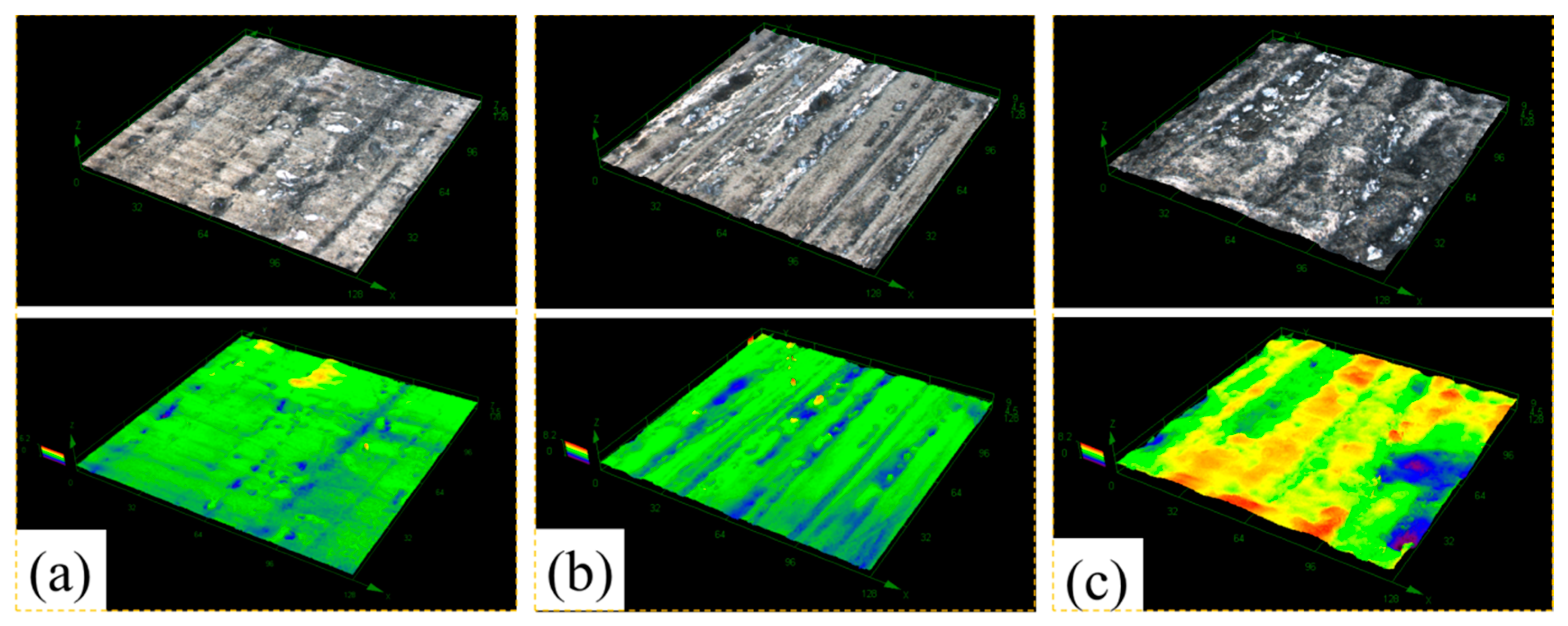
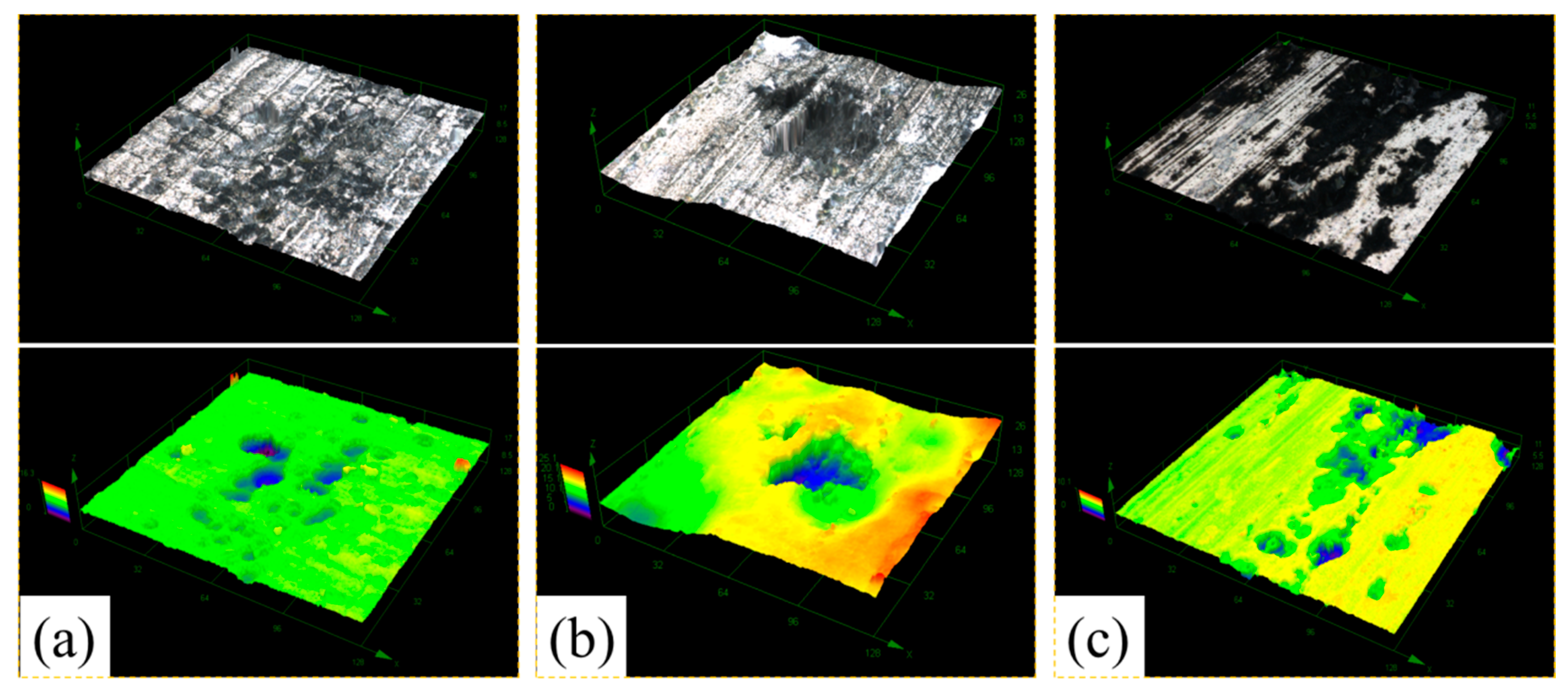
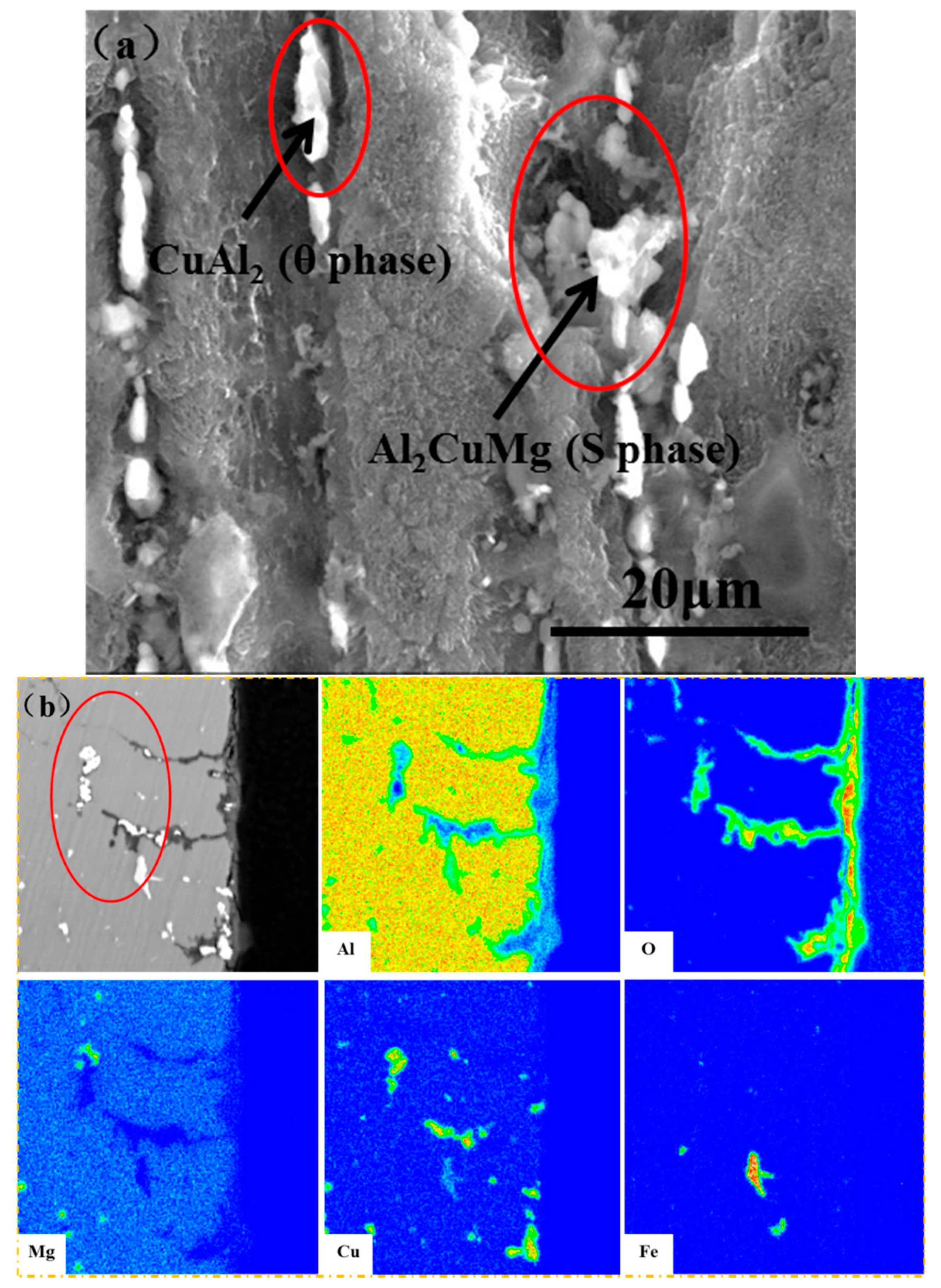
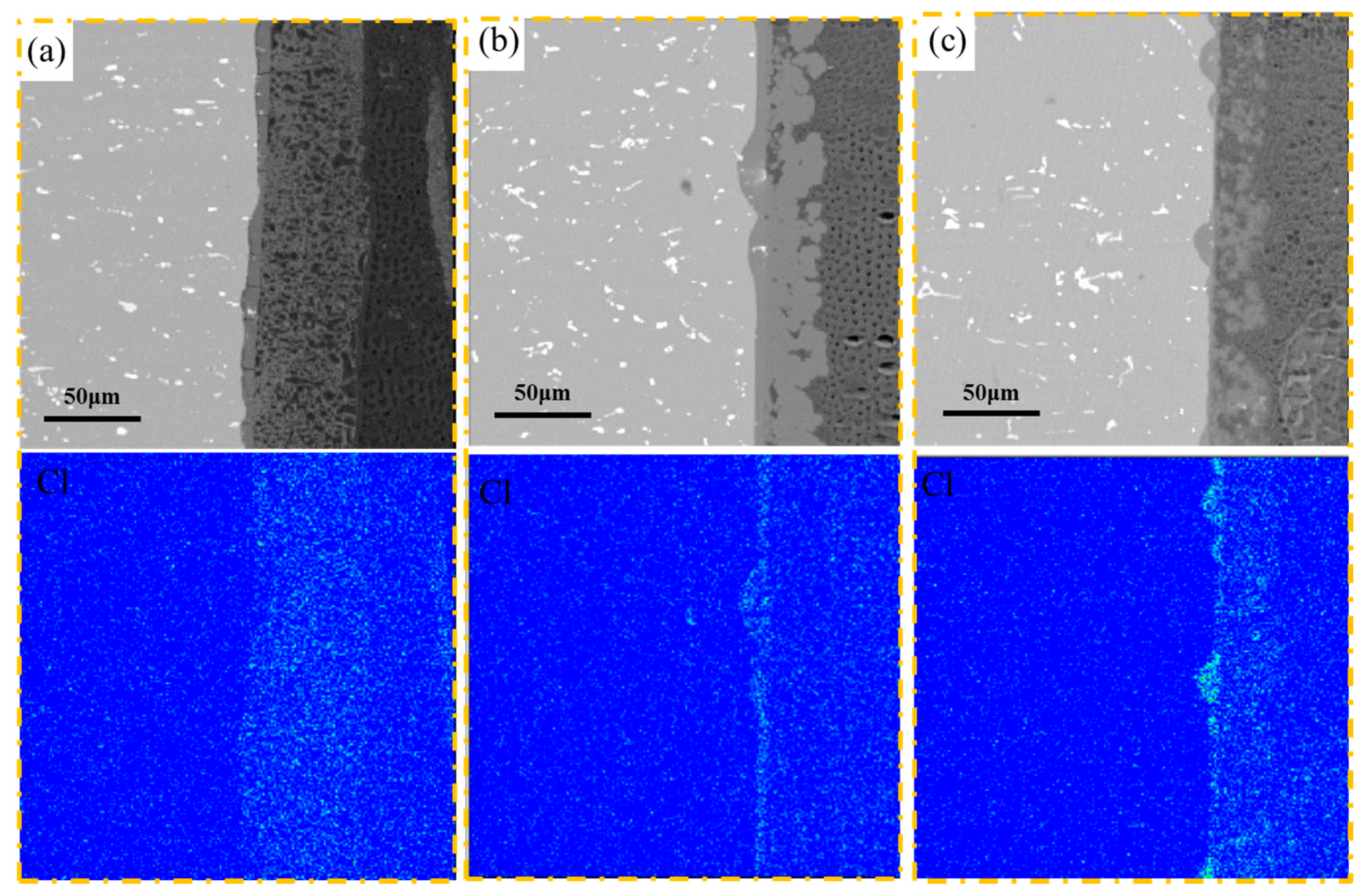
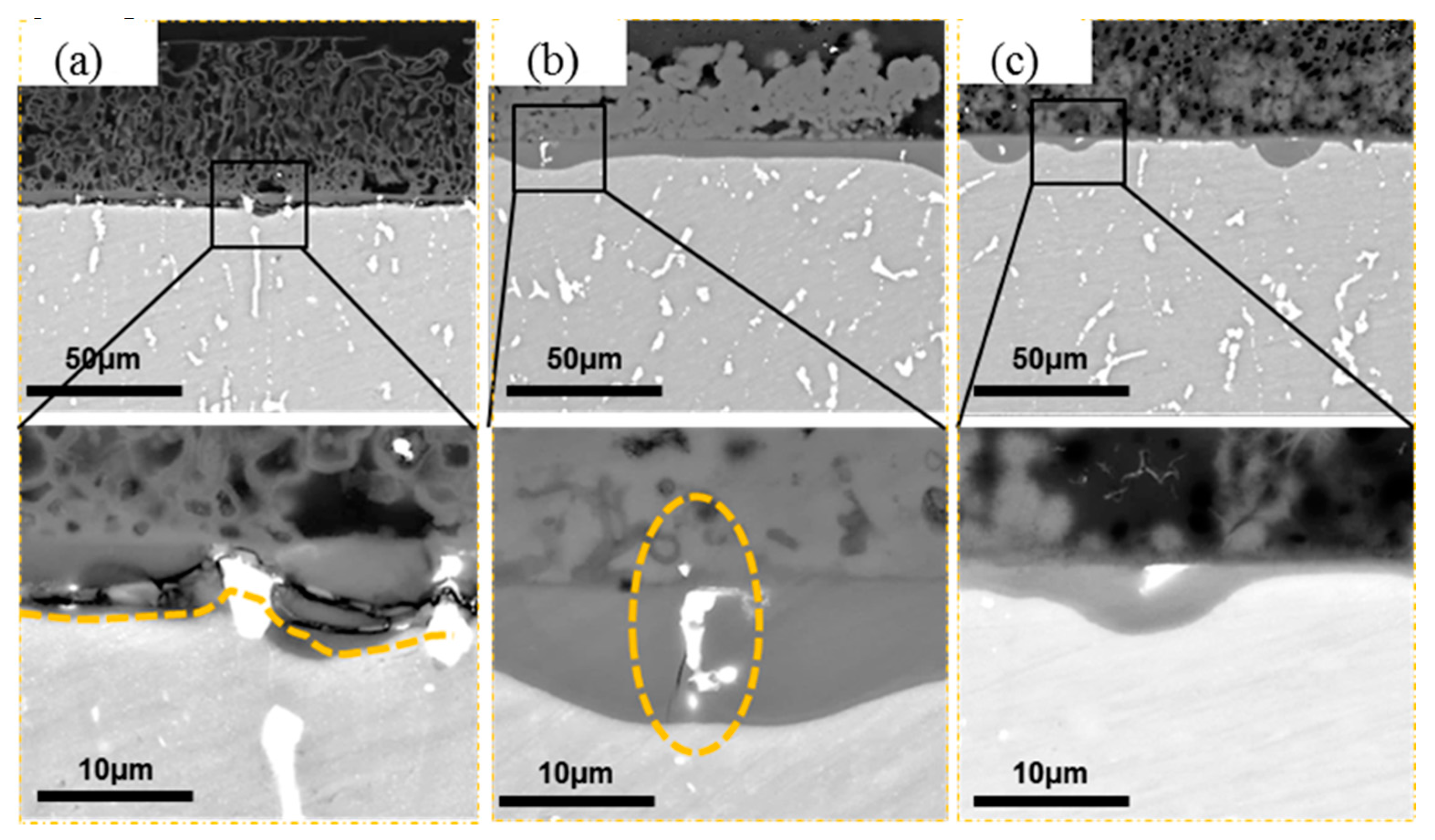
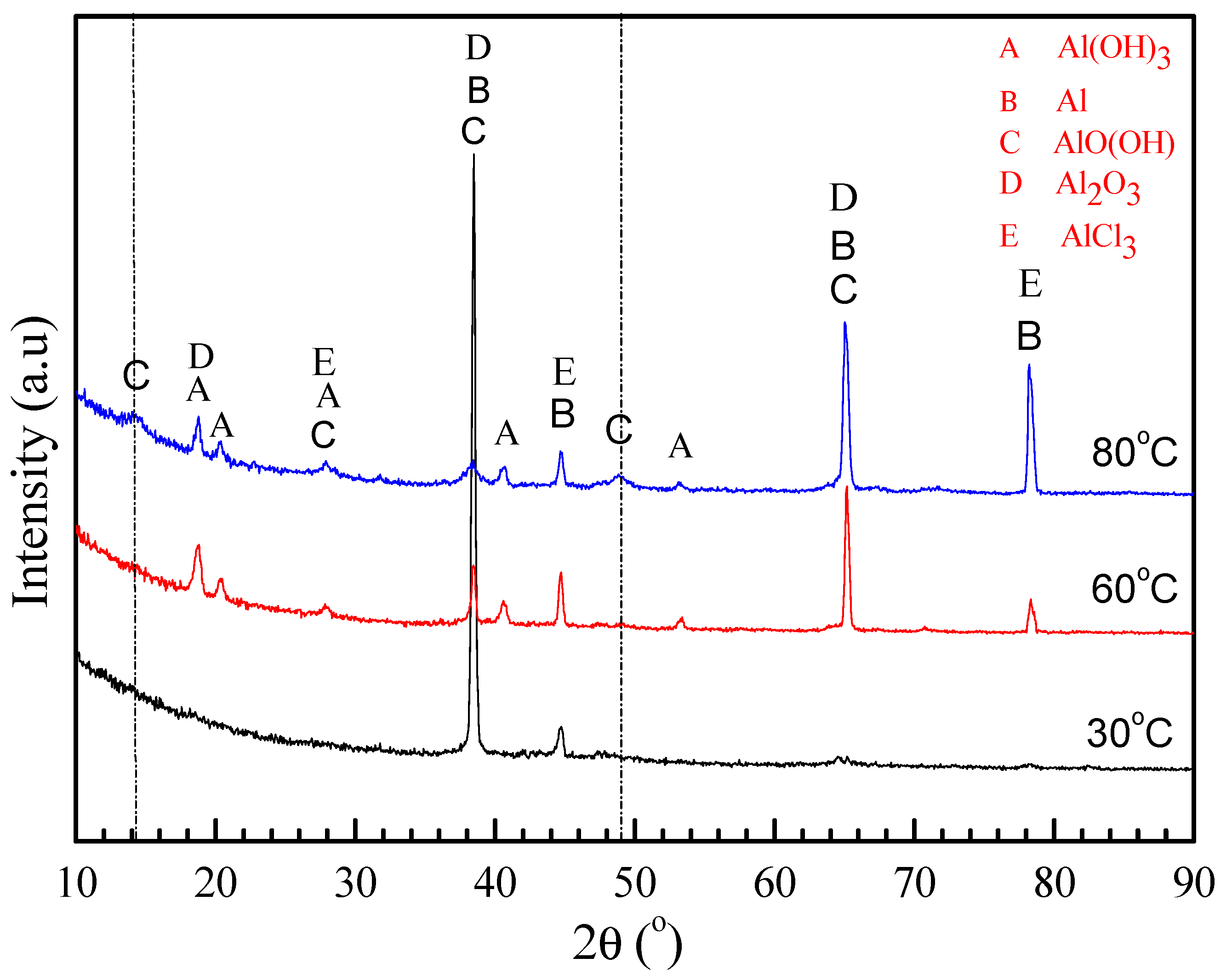
3.2. Morphology and Composition
4. Discussion
4.1. Pitting Corrosion of 2A02 Al Alloy in the Accelerated Modified Marine Atmospheric Tests
4.2. Effect of Temperature on the Corrosion Behavior of 2A02 Al Alloy in Accelerated Modified Marine Atmospheric Tests
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Z.Y.; Ma, T.; Han, W.; Yu, G.C. Corrosion behavior on aluminum alloy LY12 in simulated atmospheric corrosion process. Trans. Nonferrous Metal. Soc. 2007, 17, 326–334. [Google Scholar] [CrossRef]
- Cai, J.P.; Liu, M.; Luo, Z.H.; Tang, Z.H.; Li, B.; Zhang, X.Y.; Lu, F.; Tao, C.H. Study on accelerated tests for aluminum alloy atmospheric corrosion. J. Chin. Soc. Corros. Prot. 2005, 25, 262–266. [Google Scholar]
- Wang, B.B.; Wang, Z.Y.; Han, W.; Wang, C.; Ke, W. Effects of magnesium chloride-based multicomponent salts on atmospheric corrosion of aluminum alloy 2024. Trans. Nonferrous Metal. Soc. 2013, 23, 1199–1208. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Zhang, Z.; Su, J.X.; Cao, F.H.; Zhang, J.Q. Electrochemical noise study on 2024-T3 aluminum alloy corrosion in simulated acid rain under cyclic wet-dry condition. Electrochim. Acta 2006, 51, 4977–4986. [Google Scholar] [CrossRef]
- Astarita, A.; Bitondo, C.; Squillace, A.; Armentani, E.; Bellucci, F. Stress corrosion cracking behaviour of conventional and innovative aluminium alloys for aeronautic applications. Surf. Interface Anal. 2013, 45, 1610–1618. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Sun, S.Q.; Wen, J.G.; Li, D.F. Effects of the soluble salts in desert dust on atmospheric corrosion of aluminium. J. Chin. Soc. Corros. Prot. 2010, 30, 72–77. [Google Scholar]
- Liu, M.; Cai, J.P.; Zhang, X.Y.; Lu, F. Study on simulative and accelerated tests of 2A12 aluminum alloy marine corrosion. J. Mater. Eng. 2010, z1, 348–351, 376. [Google Scholar]
- Cheng, Y.L.; Zhang, Z.; Cao, F.H.; Li, J.F.; Zhang, J.Q.; Wang, J.M.; Cao, C.N. A study of the corrosion of aluminum alloy 2024-T3 under thin electrolyte layers. Corros. Sci. 2004, 46, 1649–1667. [Google Scholar] [CrossRef]
- Blucher, D.B.; Svensson, J.E.; Johansson, L.G. The influence of CO2, AlCl3·6H2O, MgCl2·6H2O, Na2SO4 and NaCl on the atmospheric corrosion of aluminium. Corros. Sci. 2006, 48, 1848–1866. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Cao, F.H.; Zhang, Z.; Zhang, J.Q.; Wang, J.M.; Cao, C.N. Electrochemical noises of LC4, LY12 and pure Al in NaCl solution. Acta Metall. Sin. 2002, 38, 74–78. [Google Scholar]
- Moutarlier, V.; Gigandet, M.P.; Normand, B.; Pagetti, J. EIS characterisation of anodic films formed on 2024 aluminium alloy, in sulphuric acid containing molybdate or permanganate species. Corros. Sci. 2005, 47, 937–951. [Google Scholar] [CrossRef]
- Dan, Z.; Muto, I.; Hara, N. Effects of environmental factors on atmospheric corrosion of aluminium and its alloy under constant dew point conditions. Corros. Sci. 2012, 57, 22–29. [Google Scholar] [CrossRef]
- Wang, B.B.; Wang, Z.Y.; Wang, C.; Han, W.; Ke, W. Corrosion behaviour of Al alloy LC4 in simulated salt lake atmospheric environment. In Proceedings of the 7th Cross-Strait Conference on Materials, Kunming, China, 25–31 July 2010; Volume 79. [Google Scholar]
- Natesan, M.; Venkatachari, G.; Palaniswamy, N. Kinetics of atmospheric corrosion of mild steel, zinc, galvanized iron and aluminium at 10 exposure stations in India. Corros. Sci. 2006, 48, 3584–3608. [Google Scholar] [CrossRef]
- Rafi, H.K.; Ram, G.D.J.; Phanikumar, G.; Rao, K.P. Microstructure and tensile properties of friction welded aluminum alloy AA7075-T6. Mater. Des. 2010, 31, 2375–2380. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, G.Z.; Cheng, Y.F. Electronic structure and pitting behavior of 3003 aluminum alloy passivated under various conditions. Electrochim. Acta 2009, 54, 4155–4163. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent Developments in Advanced Aircraft Aluminium Alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Ferrer, K.S.; Kelly, R.G. Comparison of methods for removal of corrosion product from AA2024-T3. Corrosion 2001, 57, 110–117. [Google Scholar] [CrossRef]
- Graedel, T.E.; Leygraf, C. Corrosion mechanisms for nickel exposed to the atmosphere. J. Electrochem. Soc. 2000, 147, 1010–1014. [Google Scholar] [CrossRef]
- Vera, R.; Delgado, D.; Rosales, B.M. Effect of atmospheric pollutants on the corrosion of high power electrical conductors: Part 1. aluminium and AA6201 alloy. Corros. Sci. 2006, 48, 2882–2900. [Google Scholar] [CrossRef]
- Wranglen, G. An Introduction to Corrosion and Protection of Metals; Chapman and Hall: London, UK, 1985. [Google Scholar]
- Liu, Y.; Wang, Z.; Ke, W. Study on influence of native oxide and corrosion products on atmospheric corrosion of pure Al. Corros. Sci. 2014, 80, 169–176. [Google Scholar] [CrossRef]
- Nakazato, R.Z.; Codaro, E.N.; Horovistiz, A.; Hein, R. A metallurgical study of aluminium alloys used as aircraft components. Prakt. Metallogr. 2001, 38, 74–87. [Google Scholar]
- Oguzie, E.E. Corrosion inhibition of aluminum in acidic and alkaline media by sansevieria trifasciata extract. Corros. Sci. 2007, 49, 1527–1539. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, Q.; Li, D.; Wen, J. Long-term atmospheric corrosion behaviour of aluminium alloys 2024 and 7075 in urban, coastal and industrial environments. Corros. Sci. 2009, 51, 719–727. [Google Scholar] [CrossRef]
- Knight, S.; Salagaras, M.; Trueman, A. The study of intergranular corrosion in aircraft aluminium alloys using X-ray tomography. Corros. Sci. 2011, 53, 727–734. [Google Scholar] [CrossRef]
- Sun, S.Q.; Zheng, Q.F.; Li, D.F.; Hu, S.Q.; Wen, J.G. Exfoliation corrosion of extruded 2024-T4 in the coastal environments in china. Corros. Sci. 2011, 53, 2527–2538. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Gudic, S.; Smoljko, I.; Kliskic, M. Electrochemical behavior of aluminum alloys containing indium and tin in NaCl solution. Mater. Chem. Phys. 2010, 121, 561–566. [Google Scholar] [CrossRef]
- Lin, F.; Cai, J.; Qi, Z. Corrosion behavior of LY12 aluminum alloy with cyclic immersion corrosion test. J. Chin. Soc. Corros. Prot. 2009, 29, 215–219. [Google Scholar]
- Abiola, O.K. Adsorption of 3-(4-amino-2-methyl-5-pyrimidyl methyl)-4-methyl thiazolium chloride on mild steel. Corros. Sci. 2006, 48, 3078–3090. [Google Scholar] [CrossRef]
- Prosek, T.; Lversen, A.; Taxén, C.; Thierry, D. Low-temperature stress corrosion cracking of stainless steels on the atmosphere in the presence of chloride deposits. Corrosion 2009, 65, 105–117. [Google Scholar] [CrossRef]
- Hastuty, S.; Nishikata, A.; Tsuru, T. Pitting corrosion of type 430 stainless steel under chloride solution droplet. Corros. Sci. 2010, 52, 2035–2043. [Google Scholar] [CrossRef]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Han, D.; Li, D. Influence of temperature on initial corrosion of LY12 in marine atmosphere. Mater. Prot. 2007, 40, 7–19. [Google Scholar]
- Xu, N.X.; Zhao, L.Y.; Ding, C.H.; Zhang, C.D.; Li, R.S.; Zhong, Q.D. Laboratory observation of dew formation at an early stage of atmospheric corrosion of metals. Corros. Sci. 2002, 44, 163–170. [Google Scholar] [CrossRef]
- Sharifi-Asla, S.; Mao, F.; Lu, P.; Kursten, B.; Macdonald, D.D. Exploration of the effect of chloride ion concentration and temperature on pitting corrosion of carbon steel in saturated Ca(OH)2 solution. Corros. Sci. 2015, 98, 708–715. [Google Scholar] [CrossRef]
- Anwar, M.S.; Prifiharni, S.; Mabruri, E. The effect of tempering temperature on pitting corrosion resistance of 420 stainless steels. AIP Conference Proc. 2016, 1725, 020005. [Google Scholar] [CrossRef]
- Liu, J.; Alfantazi, A.; Asselin, E. Effects of Temperature and sulfate on the pitting corrosion of titanium in high-temperature chloride solutions. J. Electrochem. Soc. 2015, 162, 189–196. [Google Scholar] [CrossRef]
- Blücher, D.B.; Svensson, J.E.; Johansson, L.G. The NaCl-induced atmospheric corrosion of aluminum: The influence of carbon dioxide and temperature. J. Electrochem. Soc. 2003, 150, B93–B98. [Google Scholar] [CrossRef]
- Esmaily, M.; Shahabi-Navid, M.; Svensson, J.-E.; Halvarsson, M.; Nyborg, L.; Cao, Y.; Johansson, L.-G. Influence of temperature on the atmospheric corrosion of the Mg–Al alloy AM50. Corros. Sci. 2015, 90, 420–433. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Sun, S.Q.; Wen, J.G.; Li, D.F. Atmospheric corrosion behaviours of aluminum and aluminum alloys in desert atmosphere of southern xinjiang province, china. Chin. J. Nonferrous Met. 2009, 19, 353–359. [Google Scholar]
- Sun, S.Q.; Zheng, Q.F.; Li, D.F.; Chen, J.; Wen, J.G. Long-term atmospheric corrosion behavior of LY12 aluminum alloy. J. Chin. Soc. Corros. Prot. 2009, 29, 442–446. [Google Scholar]
- Wang, B.B.; Wang, Z.Y.; Han, W.; Ke, W. Atmospheric corrosion of aluminium alloy 2024-T3 exposed to salt lake environment in western china. Corros. Sci. 2012, 59, 63–70. [Google Scholar] [CrossRef]
- Fuente, D.D.L.; Otero-Huerta, E.; Morcillo, M. Studies of long-term weathering of aluminium in the atmosphere. Corros. Sci. 2007, 49, 3134–3148. [Google Scholar] [CrossRef]
- He, Y.; Cui, R.; An, T. Long-Term atmospheric corrosion of aluminum alloy 2024-T4 in a coastal environment. J. Mater. Eng. Perform. 2015, 24, 2764–2773. [Google Scholar]
- Shu, Y.; Wang, F.; Wu, W. Synergestic effect of NaCl and water vapor on the corrosion of 1Cr11Ni2W2MoV steel at 500–700 °C. Oxid. Met. 1999, 51, 97–110. [Google Scholar] [CrossRef]
- Shu, Y.; Wang, F.; Wu, W. Corrosion behavior of Ti60 alloy coated with a solid NaCl deposit in O2 plus water vapor at 500–700 °C. Oxid. Met. 1999, 52, 463–473. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Van Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl)propyl] tetrasulfide in neutral sodium chloride solution. Part 1: corrosion of AA 2024-T3. Corros. Sci. 2003, 45, 2163–2175. [Google Scholar] [CrossRef]
- Shao, M.H.; Fu, Y.; Hu, R.G.; Lin, C. A study on pitting corrosion of aluminum alloy 2024-T3 by scanning microreference electrode technique. Mater. Sci. Eng. A 2003, 344, 323–327. [Google Scholar] [CrossRef]
- Mol, J.M.C. A filiform corrosion and potentiodynamic polarisation study of some aluminium alloys. J. Mater. Sci. 2000, 35, 1629–1639. [Google Scholar] [CrossRef]
- Lunt, T.T.; Scully, J.R.; Brusamarello, V.; Mikhailov, A.S.; Hudson, J.L. Spatial Interactions among Localized Corrosion Sites: Experiments and Modeling. J. Electrochem. Soc. 2002, 149, B163–B173. [Google Scholar] [CrossRef]
- Lucente, A.M.; Scully, J.R. Pitting and Alkaline Dissolution of anAmorphous–Nanocrystalline Alloy with Solute-Lean Nanocrystals. Corros. Sci. 2007, 49, 2351–2361. [Google Scholar] [CrossRef]
- Strehblow, H.H. Mechanisms of Pitting Corrosion in Corrosion Mechanisms in Theory and Practice; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Li, T.; Li, X.G.; Dong, C.F.; Cheng, Y.F. Characterization of atmospheric corrosion of 2A12 aluminum alloy in tropical marine environment. J. Mater. Eng. Perform. 2010, 19, 591–598. [Google Scholar] [CrossRef]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Liang, Y.J.; Che, Y.C. Thermodynamic Data of Inorganic Compounds; Northeastern University Press: Shenyang, China, 1993. [Google Scholar]
- Zhou, Q.; He, C.; Cai, Q.K.; Shao, Z. Research progress in sol-gel technology applied in the surface treatment for aluminum alloy. Mater. Rev. 2007, 21, 83–86. [Google Scholar]
- Zhu, Z. Anodized and Surface Treatment Technology on Al Alloy; Chemical Industry Press: Beijing, China, 2004; pp. 100–110. [Google Scholar]
- Xie, W.; Liu, Y.; Zhang, F.; Li, Y.; Guo, Y. Preparation of bochmite sol by sol-gel method. Membr. Sci. Technol. 2005, 25, 71–73. [Google Scholar]
- Seinfeld, J.H.; Pandis, S.N. Amospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]


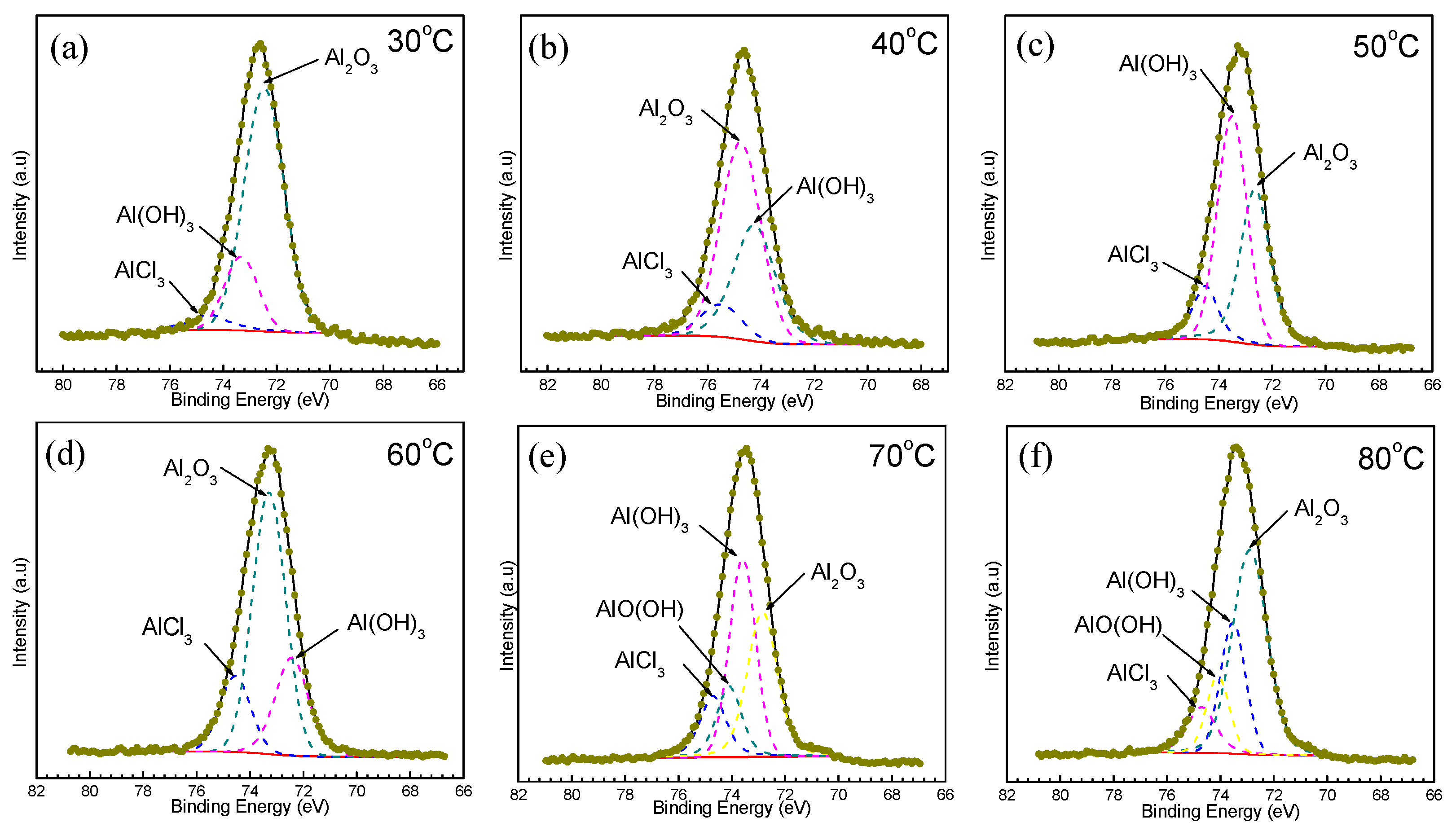
| Contents | 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C |
|---|---|---|---|---|---|---|
| 0.06 | 0.09 | 0.12 | 0.16 | 0.14 | 0.11 | |
| 0.75 | 0.56 | 0.39 | 0.22 | 0.35 | 0.53 | |
| 0 | 0 | 0 | 0 | 0.15 | 0.13 | |
| 0.19 | 0.35 | 0.49 | 0.62 | 0.36 | 0.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Liu, L.; Fan, L.; Yu, Z.; Li, Y.; Oguzie, E.E.; Wang, F. Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments. Materials 2018, 11, 235. https://doi.org/10.3390/ma11020235
Cao M, Liu L, Fan L, Yu Z, Li Y, Oguzie EE, Wang F. Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments. Materials. 2018; 11(2):235. https://doi.org/10.3390/ma11020235
Chicago/Turabian StyleCao, Min, Li Liu, Lei Fan, Zhongfen Yu, Ying Li, Emeka E. Oguzie, and Fuhui Wang. 2018. "Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments" Materials 11, no. 2: 235. https://doi.org/10.3390/ma11020235
APA StyleCao, M., Liu, L., Fan, L., Yu, Z., Li, Y., Oguzie, E. E., & Wang, F. (2018). Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments. Materials, 11(2), 235. https://doi.org/10.3390/ma11020235




