Wafer-Scale Hierarchical Nanopillar Arrays Based on Au Masks and Reactive Ion Etching for Effective 3D SERS Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
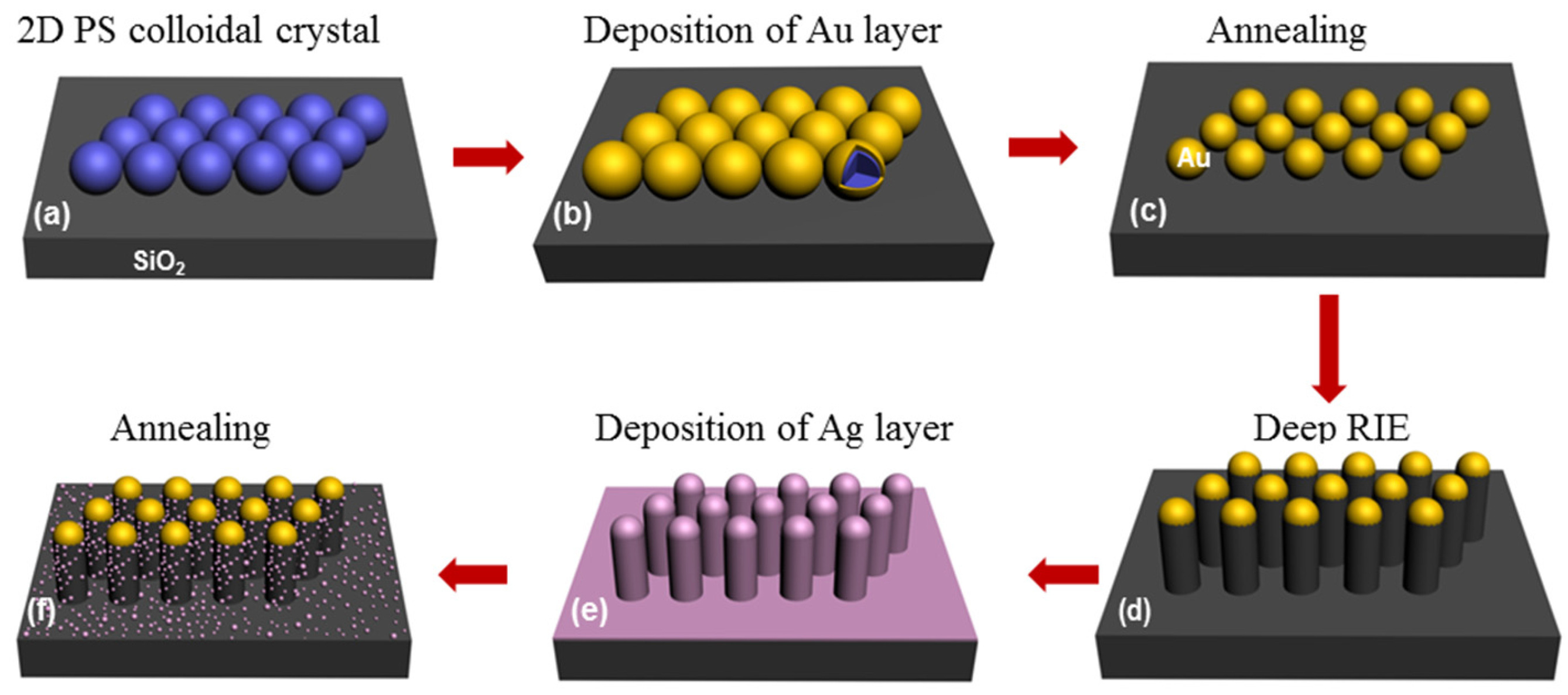
2.2. Fabrication of the SiO2 Nanopillar Arrays Decorated with Ag NPs
2.3. Characterization
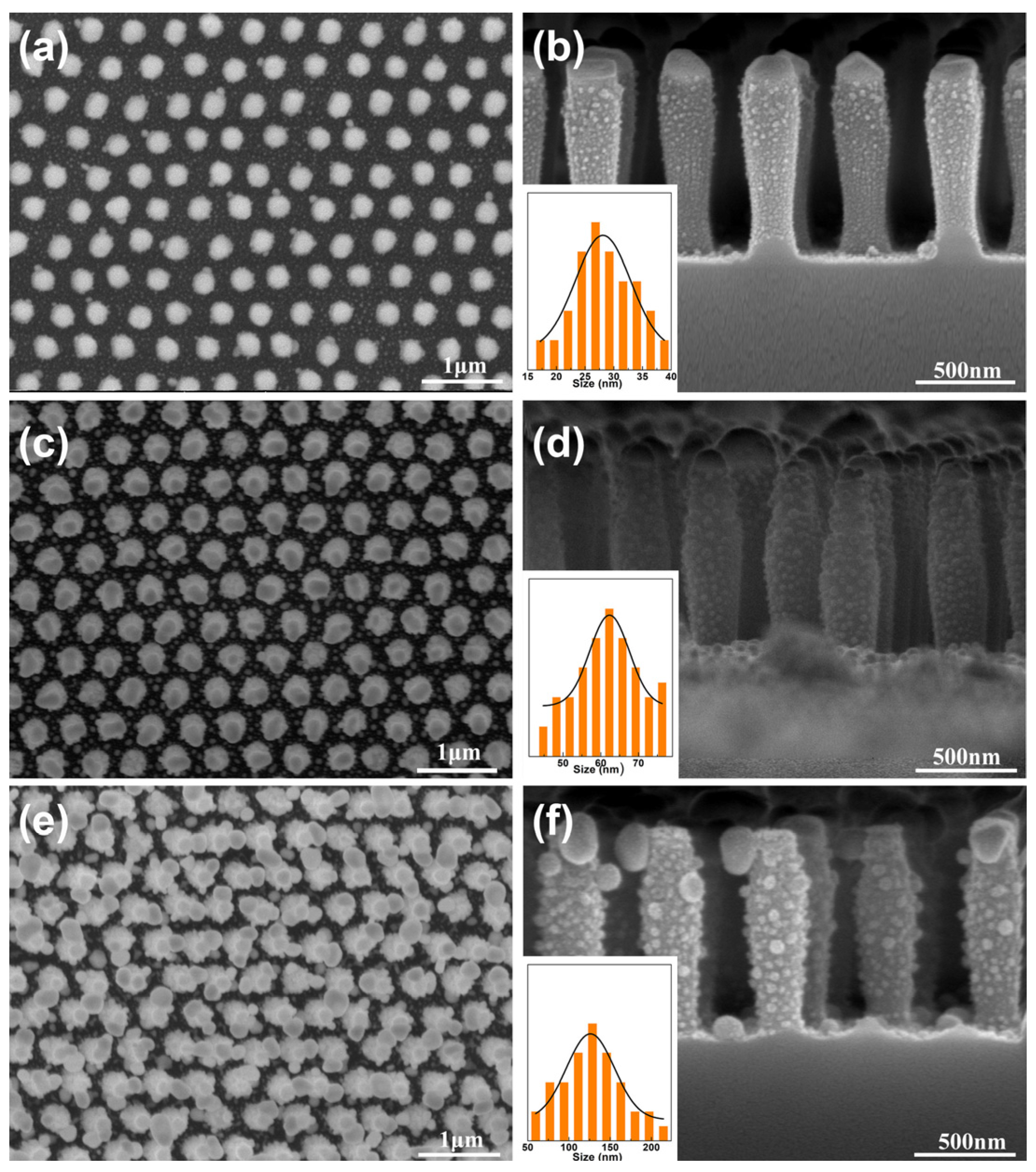
3. Results and Discussion
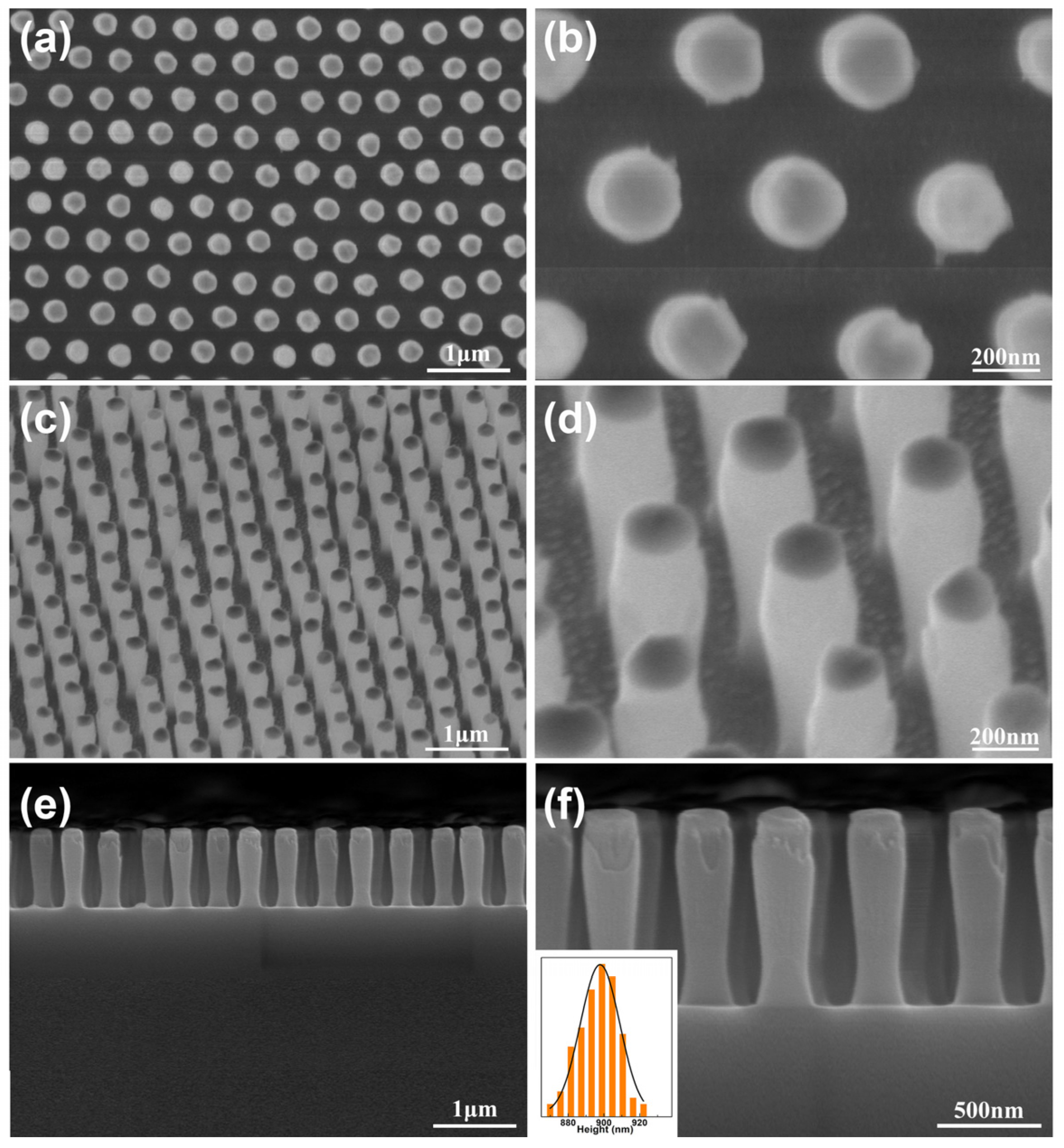
3.1. The SiO2 Nanopillar Arrays
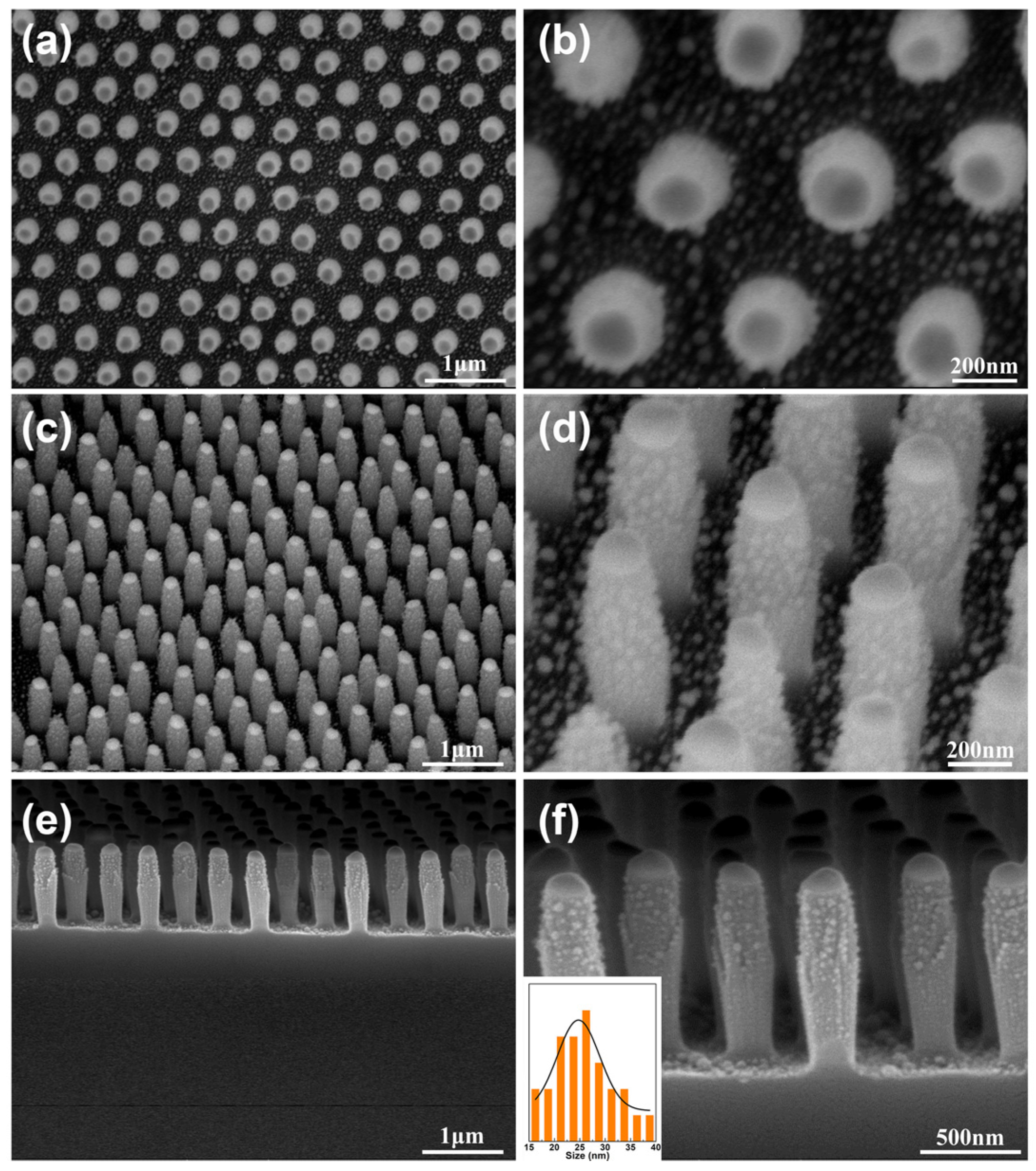
3.2. The Hierarchical SiO2 Nanopillar Decorated with Ag NPs Arrays
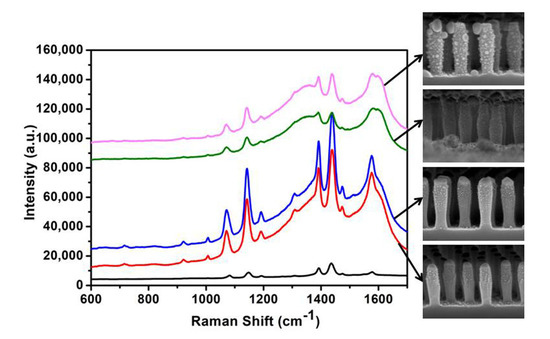
3.3. SERS Enhancement of Hierarchical SiO2 Nanopillar Arrays Decorated with Ag NPs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kleinman, S.L.; Sharma, B.; Blaber, M.G.; Henry, A.I.; Valley, N.; Freeman, R.G.; Natan, M.J.; Schatz, G.C.; Van Duyne, R.P. Structure enhancement factor relationships in single gold nanoantennas by surface-enhanced Raman excitation spectroscopy. J. Am. Chem. Soc. 2013, 135, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Zheng, M.J.; Xiang, Q.; Hu, H.L.; Duan, H.G. Optimization of the particle density to maximize the SERS enhancement factor of periodic plasmonic nanostructure array. Opt. Express 2016, 24, 20613–20620. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.B.; Li, P.; Liu, H.L.; Tang, X.H.; Liu, J.H. A dynamic surface enhanced Raman spectroscopy method for ultra-sensitive detection: From the wet state to the dry state. Chem. Soc. Rev. 2015, 44, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Yang, Z.L.; Meng, L.Y.; Sun, Y.D.; Wang, J.; Yang, L.B.; Liu, J.H.; Tian, Z.Q. Three-Dimensional and Time-Ordered Surface-Enhanced Raman Scattering Hotspot Matrix. J. Am. Chem. Soc. 2014, 136, 5332–5341. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, E.A.; Sonntag, M.D.; Jiang, N.; Klingsporn, J.M.; Hersam, M.C.; Van Duyne, R.P. Tip-enhanced Raman imaging: An Emergent Tool for Probing Biology at the Nanoscale. ACS Nano 2013, 7, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, M.D.; Klingsporn, J.M.; Zrimsek, A.B.; Sharma, B.; Ruvuna, L.K.; Van Duyne, R.P. Molecular plasmonics for nanoscale spectroscopy. Chem. Soc. Rev. 2014, 43, 1230–1247. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.L.; Meng, G.W.; Huang, Q.; Yang, Y.J.; Zhu, C.H.; Tang, C.L. Improved SERS Performance from Au Nanopillar Arrays by Abridging the Pillar Tip Spacing by Ag Sputtering. Adv. Mater. 2010, 22, 4136–4139. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Rana, U.; Malik, S. Facile Decoration of Polyaniline Fiber with Ag Nanoparticles for Recyclable SERS Substrate. ACS Appl. Mater. Interfaces 2015, 7, 10457–10465. [Google Scholar] [CrossRef] [PubMed]
- Si, S.R.; Liang, W.K.; Sun, Y.H.; Huang, J.; Ma, W.L.; Liang, Z.Q.; Bao, Q.L.; Jiang, L. Facile Fabrication of High-Density Sub-1-nm Gaps from Au Nanoparticle Monolayers as Reproducible SERS Substrates. Adv. Funct. Mater. 2016, 26, 8137–8145. [Google Scholar] [CrossRef]
- Xin, W.; Yang, J.M.; Li, C.; Goorsky, M.S.; Carlson, L.; De Rosa, I.M. Novel Strategy for One-Pot Synthesis of Gold Nanoplates on Carbon Nanotube Sheet As an Effective Flexible SERS Substrate. ACS Appl. Mater. Interfaces 2017, 9, 6246–6254. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Men, D.D.; Sun, Y.Q.; Liu, D.L.; Li, X.Y.; Li, L.B.; Li, C.C.; Cai, W.P.; Li, Y. Surface enhanced Raman scattering properties of dynamically tunable Nanogaps between Au nanoparticles self-assembled on hydrogel microspheres controlled by pH. J. Colloid Interface Sci. 2017, 505, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Q.; Liu, G.Q.; Zhang, H.W.; Li, Y.; Cai, W.P. Temperature regulation growth of Au nanocrystals: From concave Trisoctahedron to dendritic structures and their ultrasensitive SERS-based detection of Lindane. J. Mater. Chem. C 2017, 5, 10399–10405. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Large, N.; Nordlander, P.; Wang, H. Porous Au Nanoparticles with Tunable Plasmon Resonances and Intense Field Enhancements for Single-Particle SERS. J. Phys. Chem. Lett. 2014, 5, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, F.; Hang, L.F.; Sun, Y.Q.; Liu, D.L.; Li, H.L.; Liu, G.Q.; Lyu, X.J.; Li, C.C.; Cai, W.P.; et al. Controlled synthesis of sponge-like porous Au-Ag alloy Nanocubes for surface-enhanced Raman scattering properties. J. Mater. Chem. C 2017, 5, 11039–11045. [Google Scholar] [CrossRef]
- Yan, Y.; Radu, A.I.; Rao, W.; Wang, H.M.; Chen, G.; Weber, K.; Wang, D.; Cialla-May, D.; Popp, J.; Schaaf, P. Mesoscopically Bi-continuous Ag-Au Hybrid Nanosponges with Tunable Plasmon Resonances as Bottom-up Substrates for Surface-Enhanced Raman Spectroscopy. Chem. Mater. 2016, 28, 7673–7682. [Google Scholar] [CrossRef]
- Fan, M.; Lai, F.J.; Chou, H.L.; Lu, W.T.; Hwang, B.J.; Brolo, A.G. Surface-enhanced Raman scattering (SERS) from Au:Ag bimetallic nanoparticles: The effect of the molecular probe. Chem. Sci. 2013, 4, 509–515. [Google Scholar] [CrossRef]
- Liu, K.; Bai, Y.C.; Zhang, L.; Yang, Z.B.; Fan, Q.K.; Zheng, H.Q.; Yin, Y.D.; Gao, C.B. Porous Au-Ag Nanospheres with High-Density and Highly Accessible Hotspots for SERS Analysis. Nano Lett. 2016, 16, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Liu, D.L.; Hang, L.F.; Li, X.Y.; Liu, G.Q.; Cai, W.P.; Li, Y. Effective SERS-active substrates composed of hierarchical micro/nanostructured arrays based on reactive ion etching and colloidal masks. Nanotechnology 2016, 27, 395304. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Cai, W.P.; Kong, L.C.; Duan, G.T.; Li, Y.; Wang, J.J.; Cheng, Z.X. Trace detection of cyanide based on SERS effect of Ag Nanoplate-built hollow microsphere arrays. J. Hazard. Mater. 2013, 248–249, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Liu, M.; Zhou, F.; Liu, D.L.; Liu, G.Q.; Duan, G.T.; Cai, W.P.; Li, Y. Physical Deposition Improved SERS Stability of Morphology Controlled PeriodicMicro/Nanostructured Arrays Based on Colloidal Templates. Small 2015, 11, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Zhou, F.; Liu, M.; Liu, D.L.; Men, D.D.; Cai, W.P.; Duan, G.T.; Li, Y. Spherical Nanoparticle Arrays with Tunable Nanogaps and Their Hydrophobicity Enhanced Rapid SERS Detection by Localized Concentration of Droplet Evaporation. Adv. Mater. Interfaces 2015, 2, 1500031. [Google Scholar] [CrossRef]
- Zhu, C.H.; Meng, G.W.; Zheng, P.; Huang, Q.; Li, Z.B.; Hu, X.Y.; Wang, X.J.; Huang, Z.L.; Li, F.D.; Wu, N.Q. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Men, D.D.; Zhou, F.; Hang, L.F.; Li, X.Y.; Duan, G.T.; Cai, W.P.; Li, Y. A functional hydrogel film attached with a 2D Au nanosphere array and its ultrahigh optical diffraction intensity as a visualized sensor. J. Mater. Chem. C 2016, 4, 2117–2122. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Hang, L.F.; Men, D.D.; Li, H.L.; Liu, D.L.; Li, X.Y.; Wen, L.L.; Li, Y. Periodic nanostructured Au arrays on an Si electrodeor high-performance electrochemical detection of hydrogen peroxide without an enzyme. J. Mater. Chem. C 2016, 4, 9864–9871. [Google Scholar] [CrossRef]
- Wang, H.H.; Liu, C.Y.; Wu, S.B.; Liu, N.W.; Peng, C.Y.; Chan, T.H.; Hsu, C.F.; Wang, J.K.; Wang, Y.L. Highly Raman-Enhancing Substrates Based on Silver Nanoparticle Arrays with Tunable Sub-10 nm Gaps. Adv. Mater. 2006, 18, 491–495. [Google Scholar] [CrossRef]
- Yokota, Y.; Ueno, K.; Misawa, H. Essential Nanogap effects on surface-enhanced Raman scattering signals from closely spaced gold nanoparticles. Chem. Commun. 2011, 47, 3505–3507. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Men, D.; Wu, Y.; Wang, C.; Xiang, J.; Yang, G.; Wan, C.; Zhang, H. Wafer-Scale Hierarchical Nanopillar Arrays Based on Au Masks and Reactive Ion Etching for Effective 3D SERS Substrate. Materials 2018, 11, 239. https://doi.org/10.3390/ma11020239
Men D, Wu Y, Wang C, Xiang J, Yang G, Wan C, Zhang H. Wafer-Scale Hierarchical Nanopillar Arrays Based on Au Masks and Reactive Ion Etching for Effective 3D SERS Substrate. Materials. 2018; 11(2):239. https://doi.org/10.3390/ma11020239
Chicago/Turabian StyleMen, Dandan, Yingyi Wu, Chu Wang, Junhuai Xiang, Ganlan Yang, Changjun Wan, and Honghua Zhang. 2018. "Wafer-Scale Hierarchical Nanopillar Arrays Based on Au Masks and Reactive Ion Etching for Effective 3D SERS Substrate" Materials 11, no. 2: 239. https://doi.org/10.3390/ma11020239