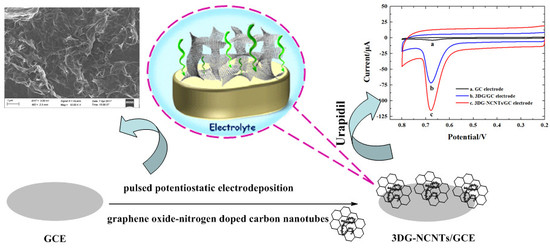
3D Graphene-Nitrogen Doped Carbon Nanotubes Network Modified Electrode as Sensing Materials for the Determination of Urapidil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO-NCNTs Dispersion
2.3. Preparation of 3DG-NCNTs Network Modified GC Electrode
2.4. Instruments and Characterizations
3. Results and Discussion
3.1. Characterization of GO-NCNTs Dispersion
3.2. Electrochemical Deposition and Characterization of 3DG-NCNTs Network
3.3. Voltammetric Behavior of Urapidil at 3DG-NCNTs Network Modified GC Electrode
3.4. Chronoamperometry and Chronocoulometry Investigations
3.5. Analytical Application and Method Validation
3.5.1. Optimization of Accumulation Conditions
3.5.2. Analytical Performances
3.5.3. Interference Studies
3.6. Determination of Urapidil in a Tablet Sample
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alijotas-Reig, J.; Bove-Farre, I.; de Cabo-Frances, F.; Angles-Coll, R. Effectiveness and safety of prehospital urapidil for hypertensive emergencies. Am. J. Emerg. Med. 2001, 19, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Hirschl, M.M.; Binder, M.; Bur, A.; Herkner, H.; Müllner, M.; Woisetschläger, C.; Laggner, A.N. Safety and efficacy of urapidil and sodium nitroprusside in the treatment of hypertensive emergencies. Intensive Care Med. 1997, 23, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Song, Z.; Wang, C. Rapid determination of subnanogram urapidil using flow injection enhancement chemiluminescence. J. Anal. Chem. 2006, 61, 295–299. [Google Scholar] [CrossRef]
- Lang, H.Y.; Li, Y.R.; Zhang, W.P.; Zhang, X.J.; Shen, Y.H. Determination of urapidil by flow injection chemiluminescence. Chem. J. Chin. Univ. 2003, 24, 618–620. [Google Scholar]
- Cai, D.; Zhang, Q. HPLC analysis of urapidil in pharmaceutical dosage form. Asian J. Chem. 2012, 24, 315–318. [Google Scholar]
- Ambavaram, V.B.R.; Nandigam, V.; Vemula, M.; Kalluru, G.R.; Gajulapalle, M. Liquid chromatography-tandem mass spectrometry method for simultaneous quantification of urapidil and aripiprazole in human plasma and its application to human pharmacokinetic study. Biomed. Chromatogr. 2013, 27, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lin, G.; Wang, X.; Li, J.; Wang, X.; Hu, L. Determination of urapidil hydrochloride in rabbit plasma by LC-MS-MS and its application to a pharmacokinetic study. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 307–316. [Google Scholar] [CrossRef]
- Sun, S.; Wei, Y.; Long, C.; Deng, B. Capillary electrophoresis with end-column electrochemiluminescence for ultrasensitive determination of urapidil hydrochloride in rat plasma and its application to pharmacokinetics study. J. Chromatogr. B 2015, 1006, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Lang, H.Y.; Wei, Y.F.; Zhang, W.P. Determination of urapidil by flow-injection indriect atomic absorption spectrometry. Chin. J. Anal. Lab. 2003, 22, 16–18. [Google Scholar]
- Zheng, L.; Song, J. Voltammetric behavior of urapidil and its determination at multi-wall carbon nanotube paste electrode. Talanta 2007, 73, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Yang, L.; Wang, W.; Ye, B. Sensitive determination of urapidil at an electrochemically pretreated glassy carbon electrode by linear sweep voltammetry. Anal. Methods 2014, 6, 6548–6554. [Google Scholar] [CrossRef]
- Biswas, C.; Lee, Y.H. Graphene versus carbon nanotubes in electronic devices. Adv. Funct. Mater. 2011, 21, 3806–3826. [Google Scholar] [CrossRef]
- Hou, J.B.; Shao, Y.Y.; Ellis, M.W.; Moore, R.B.; Yi, B.L. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y.H. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wei, T.; Shao, B.; Ma, F.Q.; Fan, Z.J.; Zhang, M.L.; Zheng, C.; Shang, Y.C.; Qian, W.Z.; Wei, F. Electrochemical properties of grapheme nanosheet/carbon black composites as electrodes for supercapacitors. Carbon 2010, 48, 1731–1737. [Google Scholar] [CrossRef]
- Choi, B.G.; Yang, M.H.; Hong, W.H.; Choi, J.W.; Huh, Y.S. 3D macroporous graphene frameworks for supercapacitors with high energy and power densities. ACS Nano 2012, 6, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, C.; Wang, X.L.; Shi, G.Q. On the gelation of graphene oxide. J. Phys. Chem. C 2011, 115, 5545–5551. [Google Scholar] [CrossRef]
- Chen, Z.P.; Ren, W.C.; Gao, L.B.; Liu, B.L.; Pei, S.F.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Chen, L.B.; Chen, Y.Q.; Bai, H.; Li, L. Three-dimensional porous graphene-based composite materials: Electrochemical synthesis and application. J. Mater. Chem. 2012, 22, 20968–20976. [Google Scholar] [CrossRef]
- Saidin, M.I.; Isa, I.M.; Ahmad, M.; Hashim, N.; Ab Ghani, S. Analysis of trace nickel by square wave stripping voltammetry using chloropalladium(II) complex-modified MWCNTs paste electrode. Sens. Actuator B Chem. 2017, 240, 848–856. [Google Scholar] [CrossRef]
- Yao, L.; Teng, J.; Zhu, M.; Zheng, L.; Zhong, Y.; Liu, G.; Xue, F.; Chen, W. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens. Bioelectron. 2016, 85, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Huang, C.; Jia, N. MWCNTs-PEI composites-based electrochemical sensor for sensitive detection of bisphenol A. Sens. Actuator B Chem. 2016, 235, 408–413. [Google Scholar] [CrossRef]
- Vikkisk, M.; Kruusenberg, I.; Joost, U.; Shulga, E.; Tammeveski, K. Electrocatalysis of oxygen reduction on nitrogen-containing multi-walled carbon nanotube modified glassy carbon electrodes. Electrochim. Acta 2013, 87, 709–716. [Google Scholar] [CrossRef]
- Tsierkezos, N.G.; Othman, S.H.; Ritter, U. Nitrogen-doped multi-walled carbon nanotubes for paracetamol sensing. Ionics 2013, 19, 1897–1905. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Wen, B.; Song, X.; Li, T. Preparation and electrochemical properties of nitrogen-doped multi-walled carbon nanotubes. Mater. Lett. 2011, 65, 49–52. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Lu, K.; Ye, B. A sensitive voltammetric sensor for taxifolin based on graphene nanosheets with certain orientation modified glassy carbon electrode. Sens. Actuator B Chem. 2015, 208, 188–194. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Lu, K.; Gao, L.; Ye, B. A simple, rapid and green method based on pulsed potentiostatic electrodeposition of reduced graphene oxide on glass carbon electrode for sensitive voltammetric detection of sophoridine. Electrochim. Acta 2014, 141, 82–88. [Google Scholar] [CrossRef]
- Mani, V.; Chen, S.M.; Lou, B.S. Three dimensional graphene oxide-carbon nanotubes and graphene-carbon nanotubes hybrids. Int. J. Electrochem. Sci. 2013, 8, 11641–11660. [Google Scholar]
- Asadian, E.; Shahrokhian, S.; Zad, A.I.; Ghorbani-Bidkorbeh, F. Glassy carbon electrode modified with 3D graphene-carbon nanotube network for sensitive electrochemical determination of methotrexate. Sens. Actuator B Chem. 2017, 239, 617–627. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Shi, L.; Zhu, X.; Lan, M.; Zhang, Q.; Fan, Z.H. Electrochemical sensing of nicotine using screen-printed carbon electrodes modified with nitrogen-doped graphene sheets. J. Electroanal. Chem. 2017, 784, 77–84. [Google Scholar] [CrossRef]
- Chen, P.H.; Fryling, M.A.; McCreery, R.L. Electron Transfer Kinetics at Modified Carbon Electrode Surfaces: The Role of Specific Surface Sites. Anal. Chem. 1995, 67, 3115–3122. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; pp. 148–150. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Anson, F.C. Application of Potentiostatic Current Integration to the Study of the Adsorption of Cobalt(III)-(Ethylenedinitrilo)tetraacetate on Mercury Electrodes. Anal. Chem. 1964, 36, 932–934. [Google Scholar] [CrossRef]
- Palanisamy, S.; Karuppiah, C.; Chen, S.M.; Emmanuel, R.; Muthukrishnan, P.; Prakash, P. Direct electrochemistry of myoglobin at silver nanoparticles/myoglobin biocomposite: Application for hydrogen peroxide sensing. Sens. Actuator B Chem. 2014, 202, 177–184. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 4th ed.; Pearson Education Limited: London, UK, 2000; pp. 120–125. [Google Scholar]









| Electrode | Methods | Linear Range (mol·L−1) | Detection Limit (mol·L−1) | Reference |
|---|---|---|---|---|
| Multi-wall carbon nanotube paste electrode | DPV | 5.0 × 10−8~2.0 × 10−6 | 3.8 × 10−8 | [10] |
| Electrochemically pretreated GC electrode | LSV | 2.0 × 10−8~5.0 × 10−6 | 2.0 × 10−8 | [11] |
| 3DG-NCNTs modified GC electrode | DPV | 1.0 × 10−8~2.0 × 10−6 | 5.0 × 10−9 | This work |
| DPASV (n = 3) a | UV-Vis | ||||||
|---|---|---|---|---|---|---|---|
| Samples | Original Found (μmol·L−1) | Standard Added (μmol·L−1) | Total Found (μmol·L−1) | RSD (%) | Recovery (%) | Original Found (μmol·L−1) | RSD (%) |
| Tablet b | 0.49(30.4 mg·tablet−1) | 0.30 | 0.78 | 3.4 | 96.7 | 0.50 | 1.9 |
| 0.50 | 1.01 | 2.9 | 104.0 | ||||
| 1.00 | 1.45 | 4.1 | 96.0 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhou, A.; Yang, H.; Wang, F.; Lu, K. 3D Graphene-Nitrogen Doped Carbon Nanotubes Network Modified Electrode as Sensing Materials for the Determination of Urapidil. Materials 2018, 11, 322. https://doi.org/10.3390/ma11020322
Wu Y, Zhou A, Yang H, Wang F, Lu K. 3D Graphene-Nitrogen Doped Carbon Nanotubes Network Modified Electrode as Sensing Materials for the Determination of Urapidil. Materials. 2018; 11(2):322. https://doi.org/10.3390/ma11020322
Chicago/Turabian StyleWu, Yanju, Anxing Zhou, Huimin Yang, Fei Wang, and Kui Lu. 2018. "3D Graphene-Nitrogen Doped Carbon Nanotubes Network Modified Electrode as Sensing Materials for the Determination of Urapidil" Materials 11, no. 2: 322. https://doi.org/10.3390/ma11020322
APA StyleWu, Y., Zhou, A., Yang, H., Wang, F., & Lu, K. (2018). 3D Graphene-Nitrogen Doped Carbon Nanotubes Network Modified Electrode as Sensing Materials for the Determination of Urapidil. Materials, 11(2), 322. https://doi.org/10.3390/ma11020322