Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline
Abstract
:1. Introduction
2. Experimentation
2.1. Sample Preparation
2.2. Synthesis of Coating Material
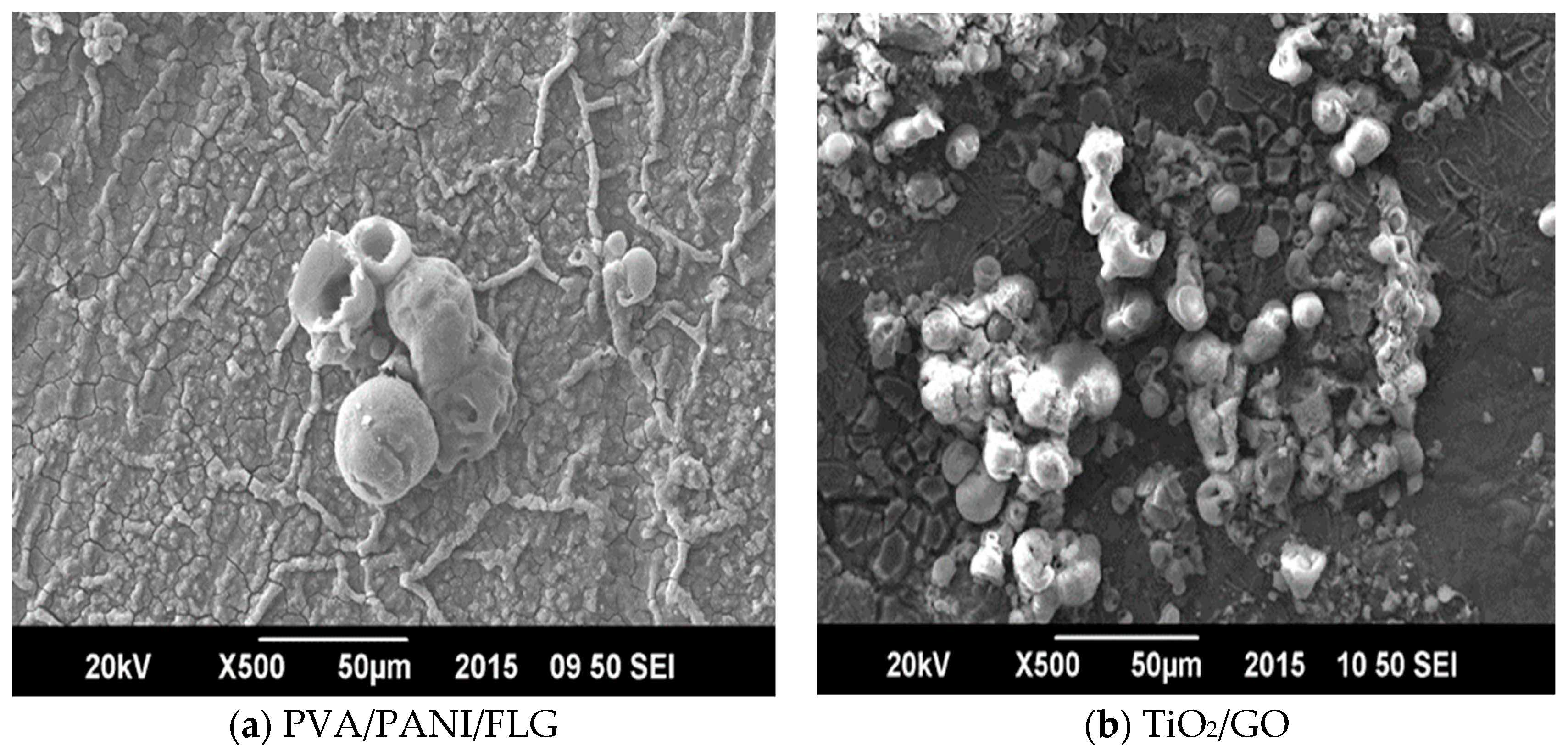
2.2.1. PVA/PANI/FLG
Preparation of Polyaniline
Preparation of PVA Solution
Preparation of Nanocomposite Solution
2.2.2. TiO2/GO Coating
Synthesis of TiO2 Nanoparticle
Synthesis of TiO2/Graphene Composite Solution
2.3. Dip Coating
2.4. Electrochemical Corrosion Studies
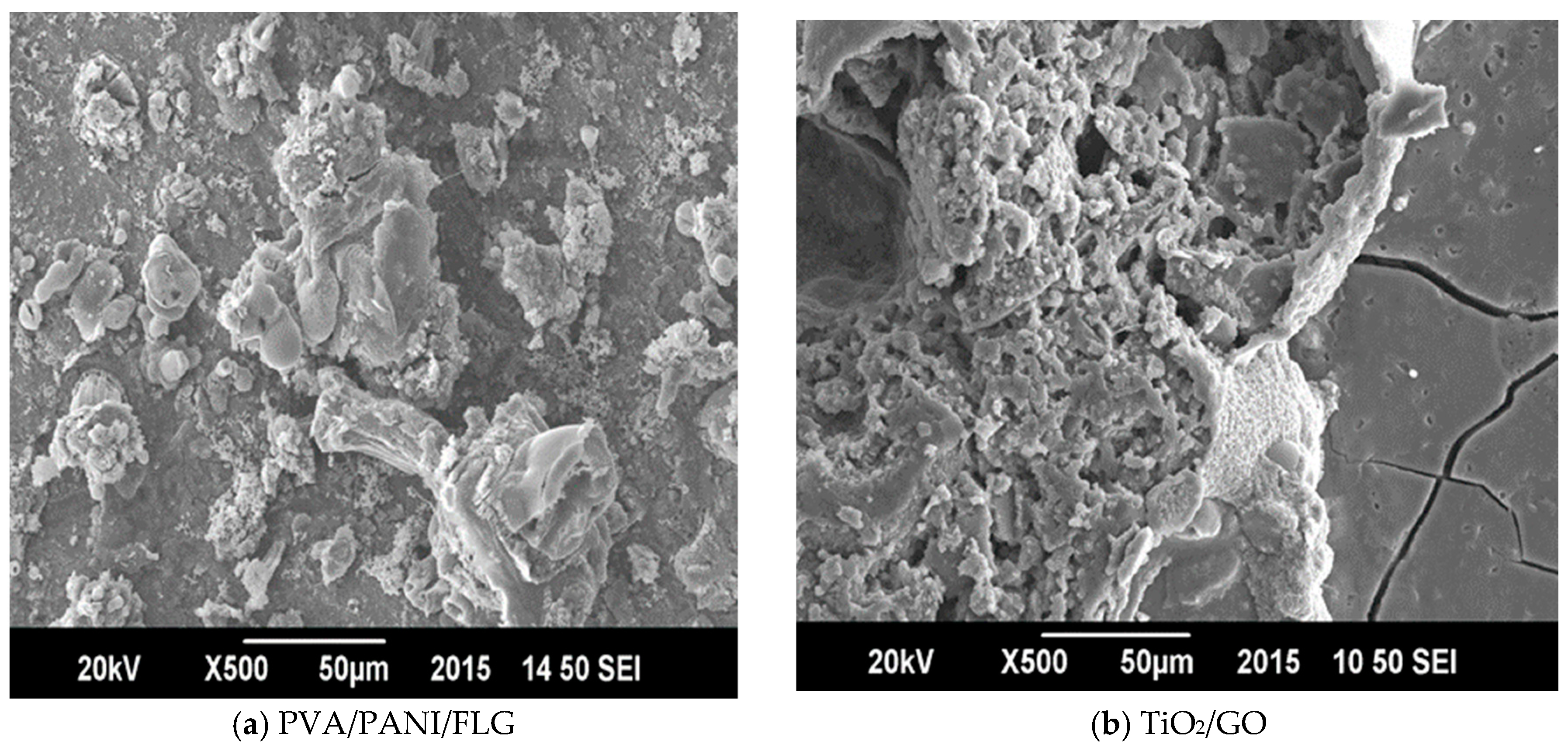
3. Results
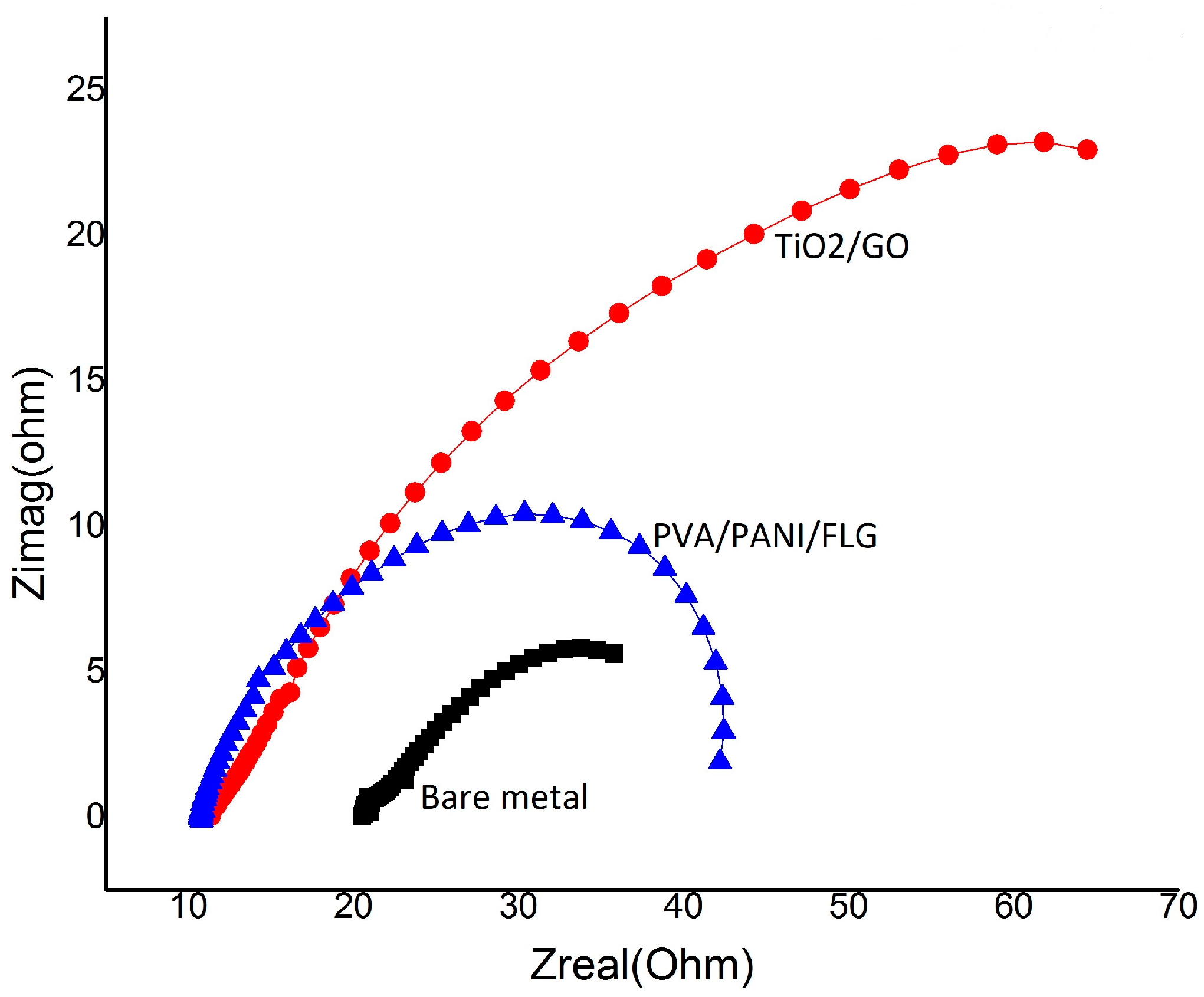
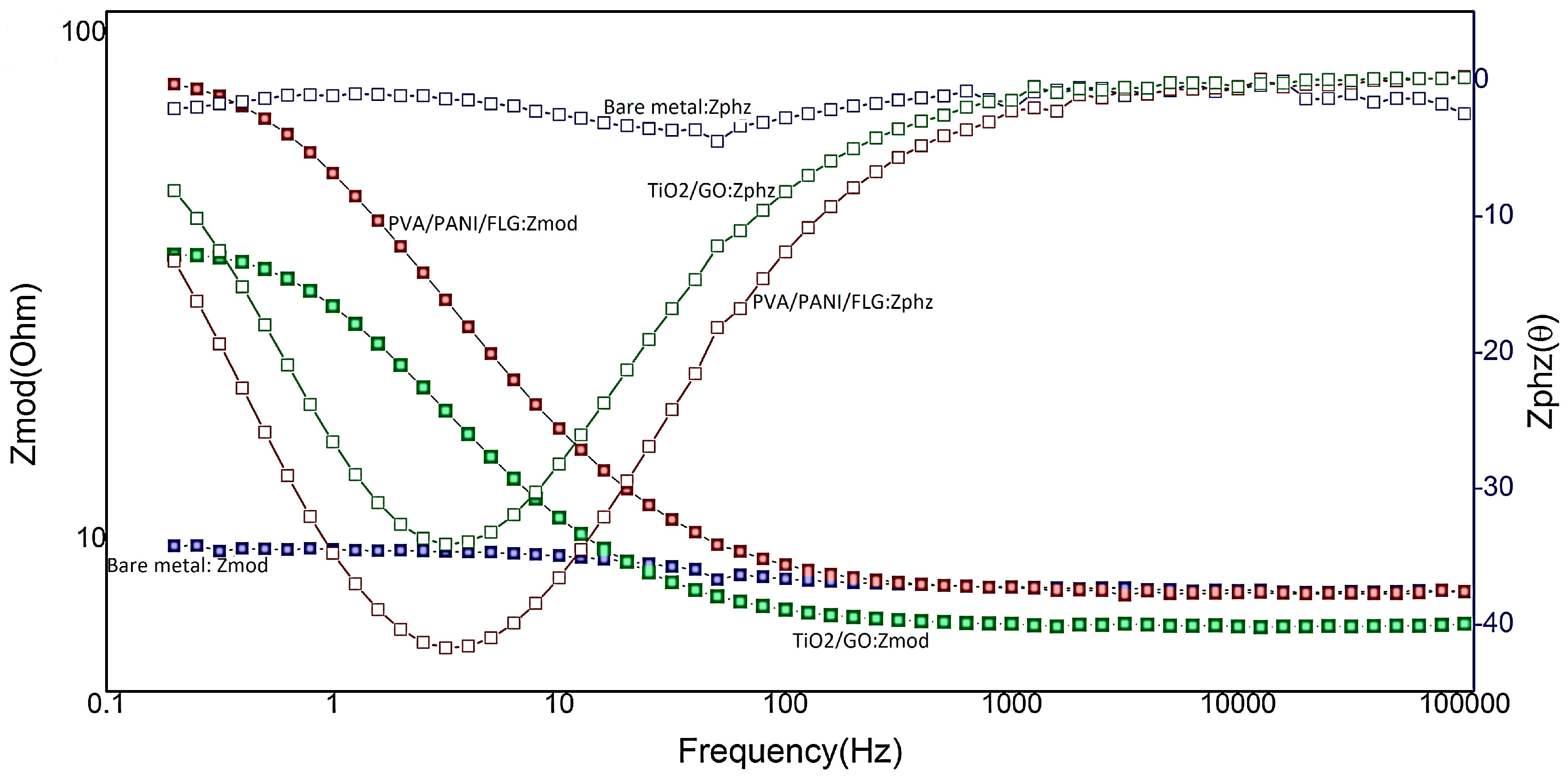
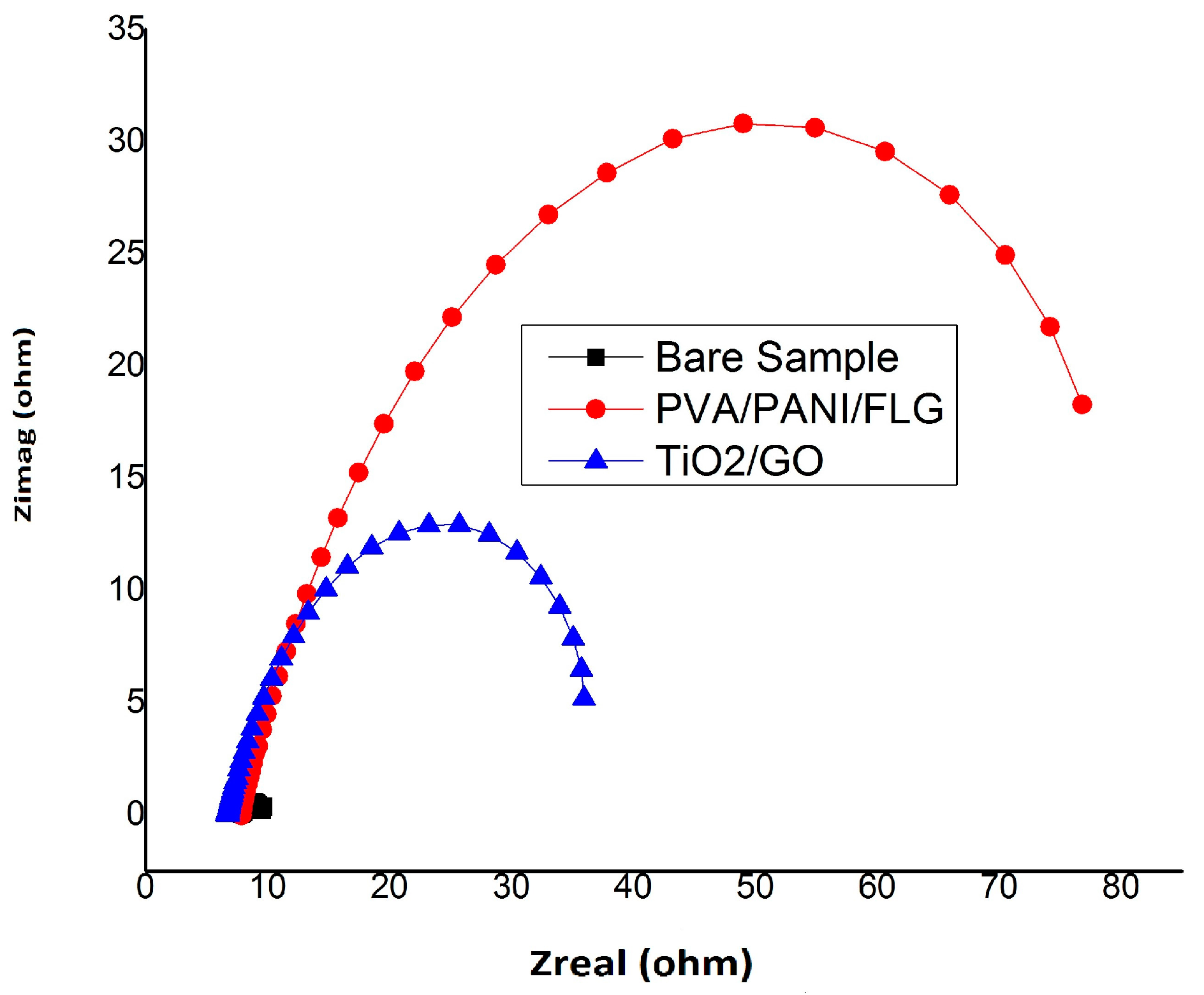
3.1. Equivalent Circuit Modelling
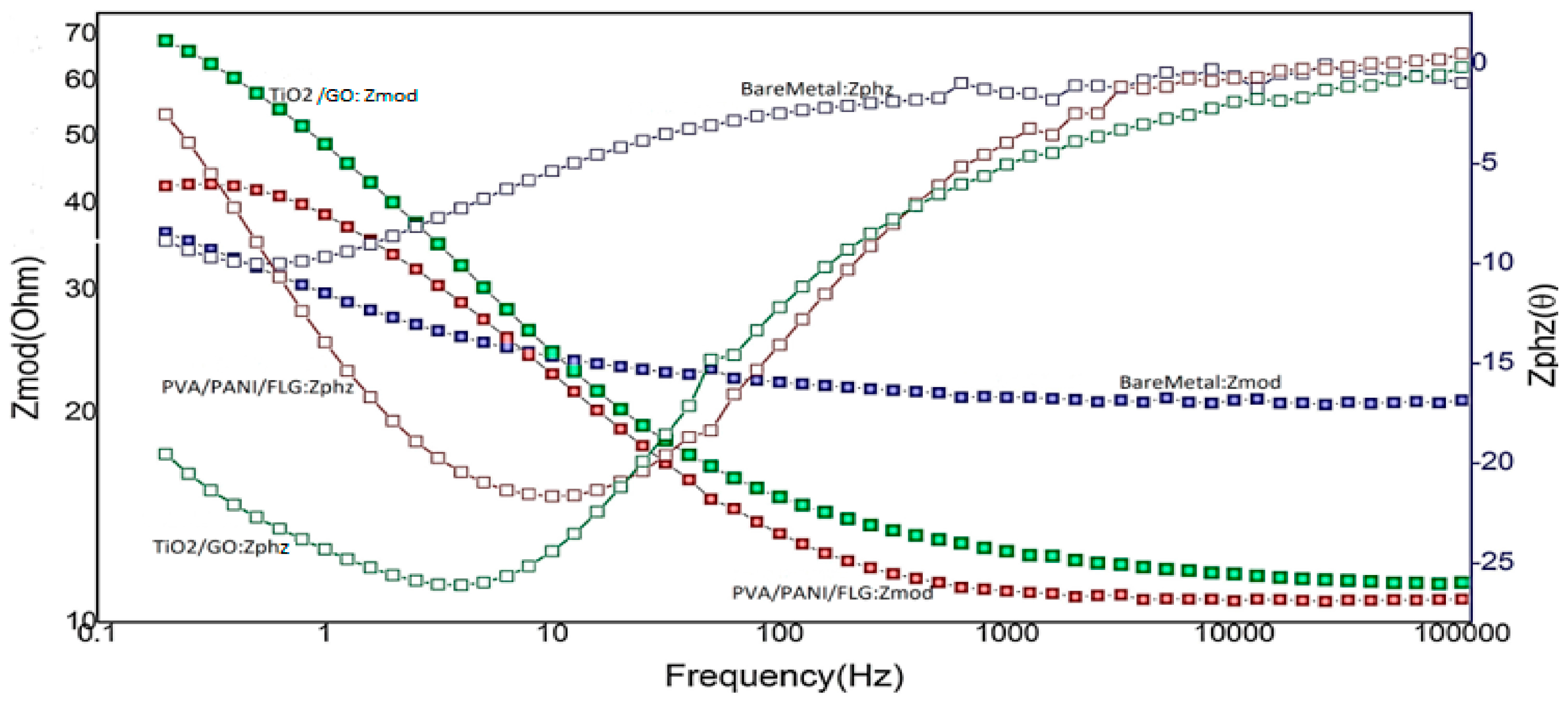
3.2. EIS in Seawater
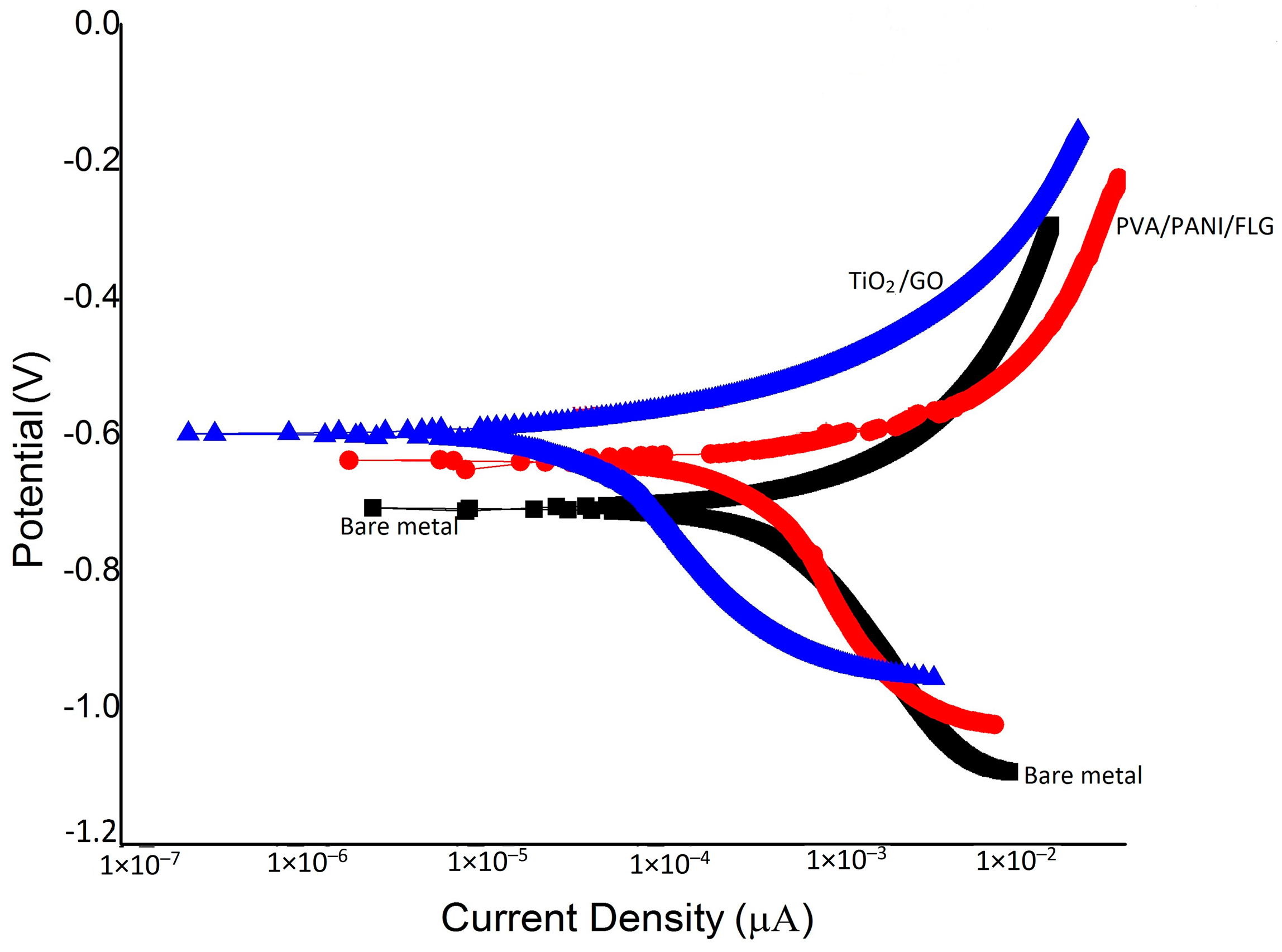
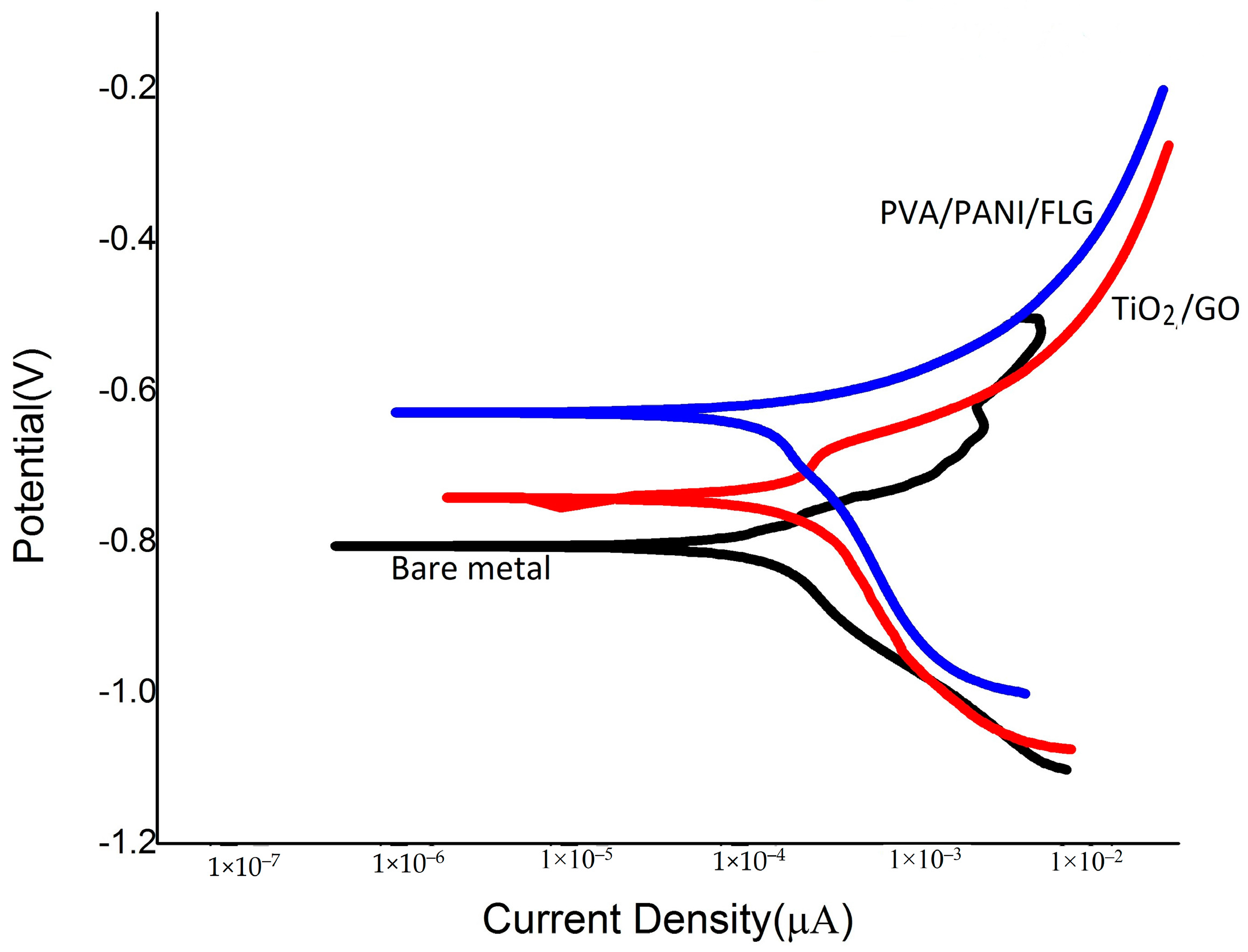
3.3. DC Corrosion Testing in Seawater
3.4. EIS in “Produced Water” from an Oil Exploration Plant
3.5. DC Corrosion Testing in “Produced Water”
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Melchers, R.E. Effect of Nutrient-Based Water Pollution on the Corrosion of Mild Steel in Marine Immersion Conditions. Corros. Sci. 2005, 61, 237–245. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Rawat, J. Inhibition of mild steel corrosion by some macrocyclic compounds in hot and concentrated hydrochloric acid. Mater. Chem. Phys. 2002, 73, 118–122. [Google Scholar] [CrossRef]
- Yun, H.; Li, J.; Chen, H.B.; Lin, C.J. A study on the N-, S- and Cl-modified nano-TiO2 coatings for corrosion protection of stainless steel. Electrochim. Acta 2007, 52, 6679–6685. [Google Scholar] [CrossRef]
- Shen, G.X.; Chen, Y.C.; Lin, C.J. Corrosion protection of 316 L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Films 2005, 489, 130–136. [Google Scholar] [CrossRef]
- Nazir, M.H.; Saeed, A.; Khan, Z. A comprehensive predictive corrosion model incorporating varying environmental gas pollutants applied to wider steel applications. Mater. Chem. Phys. 2017, 193, 19–34. [Google Scholar] [CrossRef]
- Wessling, B. Passivation of metals by coating with polyaniline: Corrosion potential shift and morphological changes. Adv. Mater. 1994, 6, 226–228. [Google Scholar] [CrossRef]
- Frau, A.F.; Pernites, R.B.; Advincula, R.C. A Conjugated Polymer Network Approach to Anticorrosion Coatings: Poly(vinylcarbazole) Electrodeposition. Ind. Eng. Chem. Res. 2010, 49, 9789–9797. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Stokes, K. A model for cathodic blister growth in coating degradation using mesomechanics approach: A model for cathodic blister growth in coating degradation. Mater. Corros. 2016, 67, 495–503. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2013, 5, 535–550. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering, 3rd ed.; McGraw Hill Education (pvt) Ltd.: New York, NY, USA, 2005. [Google Scholar]
- Srimathi, M.; Rajalakshmi, R.; Subhashini, S. Polyvinyl alcohol–sulphanilic acid water soluble composite as corrosion inhibitor for mild steel in hydrochloric acid medium. Arab. J. Chem. 2014, 7, 647–656. [Google Scholar] [CrossRef]
- Shen, G.X.; Chen, Y.C.; Lin, L.; Lin, C.J.; Scantlebury, D. Study on a hydrophobic nano-TiO2 coating and its properties for corrosion protection of metals. Electrochim. Acta. 2005, 50, 5083–5089. [Google Scholar] [CrossRef]
- Ming, H.-L.; Wang, S.-Y.; Zhang, Z.-M.; Wang, J.-Q.; Han, E.-H.; Ke, W. Oxidation and Electrochemical Behavior of Monolayer-Graphene-Coated Copper in Simulated Primary Water. Acta Metall. Sin. Engl. Lett. 2016, 29, 89–93. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Water on graphene: Hydrophobicity and dipole moment using density functional theory. Phys. Rev. B. 2009, 79, 235440. [Google Scholar] [CrossRef]
- Loveday, D.; Peterson, P. Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy; Part 1; Gamry Instruments: Warminster, PA, USA, 2004. [Google Scholar]
- Nazir, M.H.; Khan, Z.A.; Stokes, K. Optimisation of interface roughness and coating thickness to maximise coating-substrate adhesion-a failure prediction and reliability assessment modelling. J. Adhes. Sci. Technol. 2015, 29, 1415–1445. [Google Scholar] [CrossRef]
- Khan, M. Mechanical and Dielectric Properties of Three Component Hybrid Nanocomposite system of PVA/PANI/Graphene. Master’s Thesis, National University of Sciences & Technology, Islamabad, Pakistan, 2015. [Google Scholar]
- Saleem, H.; Habib, A. Study of band gap reduction of TiO2 thin films with variation in GO contents and use of TiO2/Graphene composite in hybrid solar cell. J. Alloys Compd. 2016, 679, 177–183. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Zoski, C.G. Handbook of Electrochemistry; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Fontana, M.G.; Greene, N.D. Corrosion Engineering; McGraw Hill Education (pvt) Ltd.: New York, NY, USA, 1987. [Google Scholar]
- Gamry Instuments. Available online: https://www.gamry.com/application-notes/corrosion-coatings/rapid-electrochemical-assessment-of-paint/ (accessed on 23 March 2016).

| Material | Manufacturer |
|---|---|
| Polyvinyl alcohol | ERKOL |
| Polyaniline | Prepared in lab |
| Few layer graphene | I. Janowska |
| PVA/PANI/FLG | TiO2/GO | ||
|---|---|---|---|
| Rpore | 4.219 ohms | Rpore | 7.626 ohms |
| Cc | 24.87 × 10−3 F | Cc | 11.50 × 10−3 F |
| Bare Metal | PVA/PANI/FLG | TiO2/GO |
|---|---|---|
| 19.56 | 9.477 | 1.315 |
| PVA/PANI/FLG | TiO2/GO | ||
|---|---|---|---|
| Rpore | 303.9 × 10−3 ohms | Rpore | 166.1 × 10−3 ohms |
| Cc | 17.59 × 10−3 F | Cc | 28.79 × 10−3 F |
| Bare Metal | PVA/PANI/FLG | TiO2/GO |
|---|---|---|
| 21.76 | 3.140 | 9.315 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, A.U.; Shahid, M.; Ahmed, M.K.; Khan, M.; Khalid, A.; Khan, Z.A. Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline. Materials 2018, 11, 332. https://doi.org/10.3390/ma11030332
Ammar AU, Shahid M, Ahmed MK, Khan M, Khalid A, Khan ZA. Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline. Materials. 2018; 11(3):332. https://doi.org/10.3390/ma11030332
Chicago/Turabian StyleAmmar, Ameen Uddin, Muhammad Shahid, Muhammad Khitab Ahmed, Munawar Khan, Amir Khalid, and Zulfiqar Ahmad Khan. 2018. "Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline" Materials 11, no. 3: 332. https://doi.org/10.3390/ma11030332
APA StyleAmmar, A. U., Shahid, M., Ahmed, M. K., Khan, M., Khalid, A., & Khan, Z. A. (2018). Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline. Materials, 11(3), 332. https://doi.org/10.3390/ma11030332






