High Efficient Reduction of Graphene Oxide via Nascent Hydrogen at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reduction of GO
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
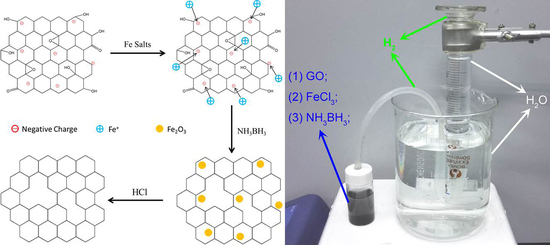
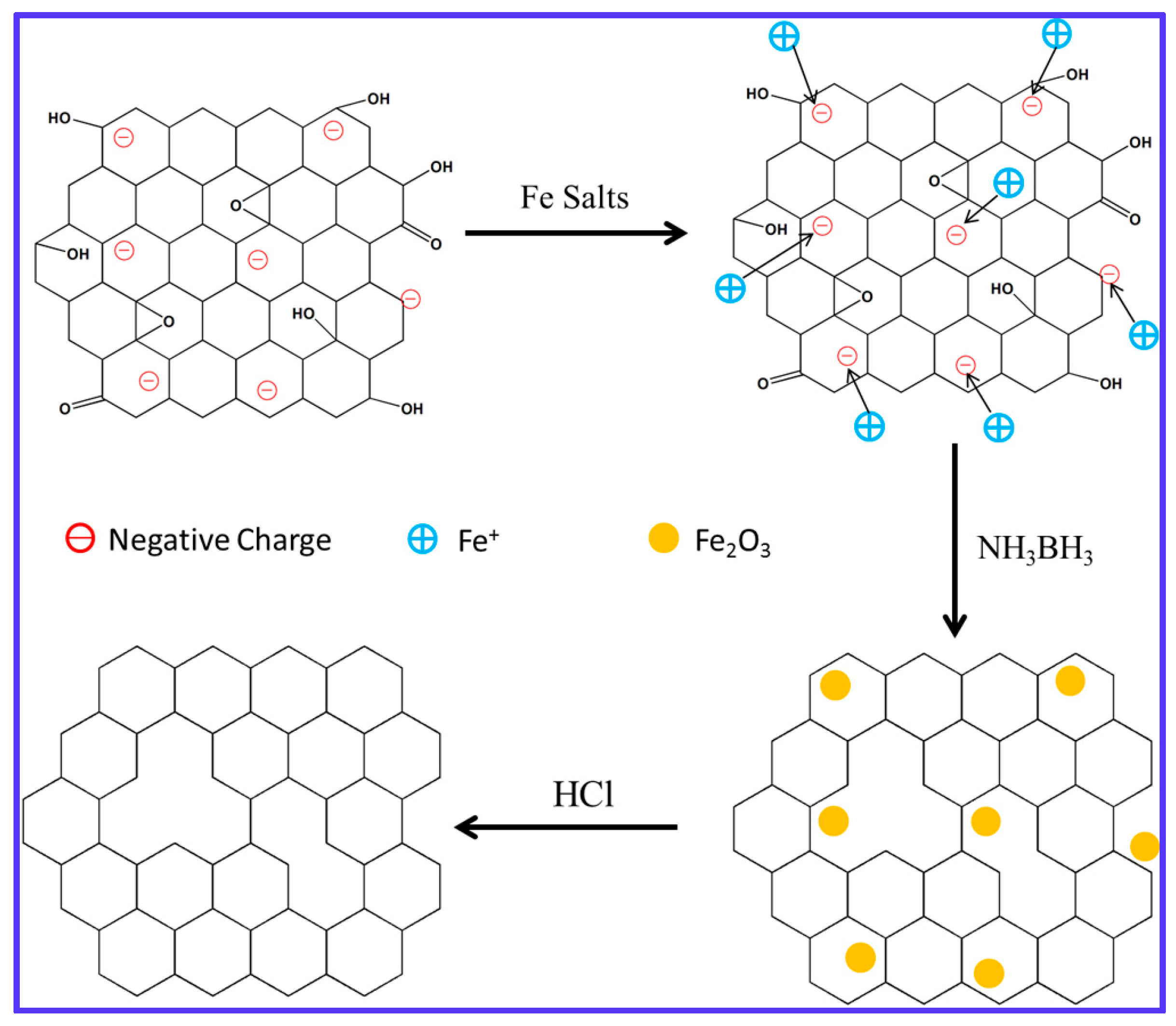
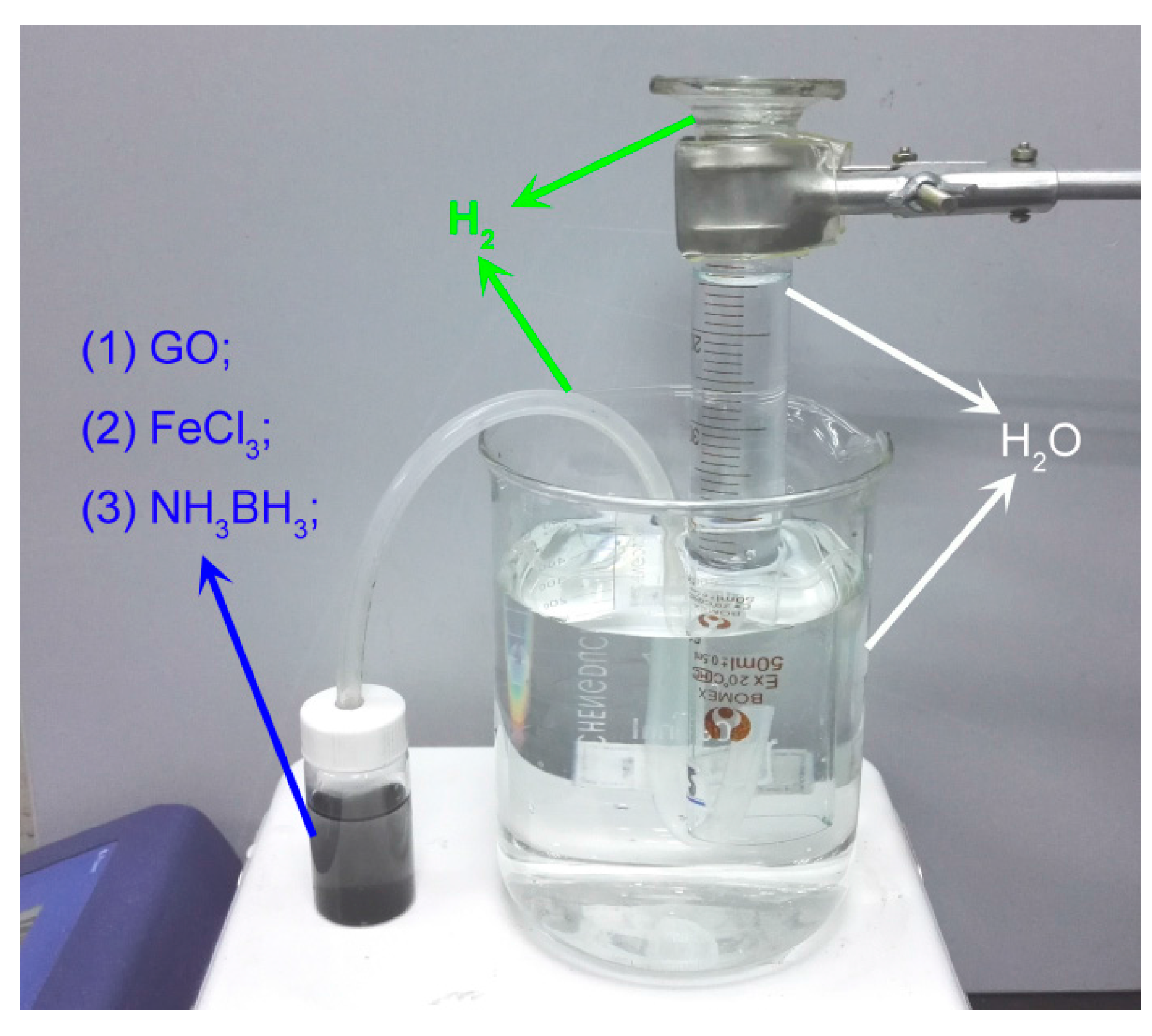
3.1. Schematic of Reduction Process
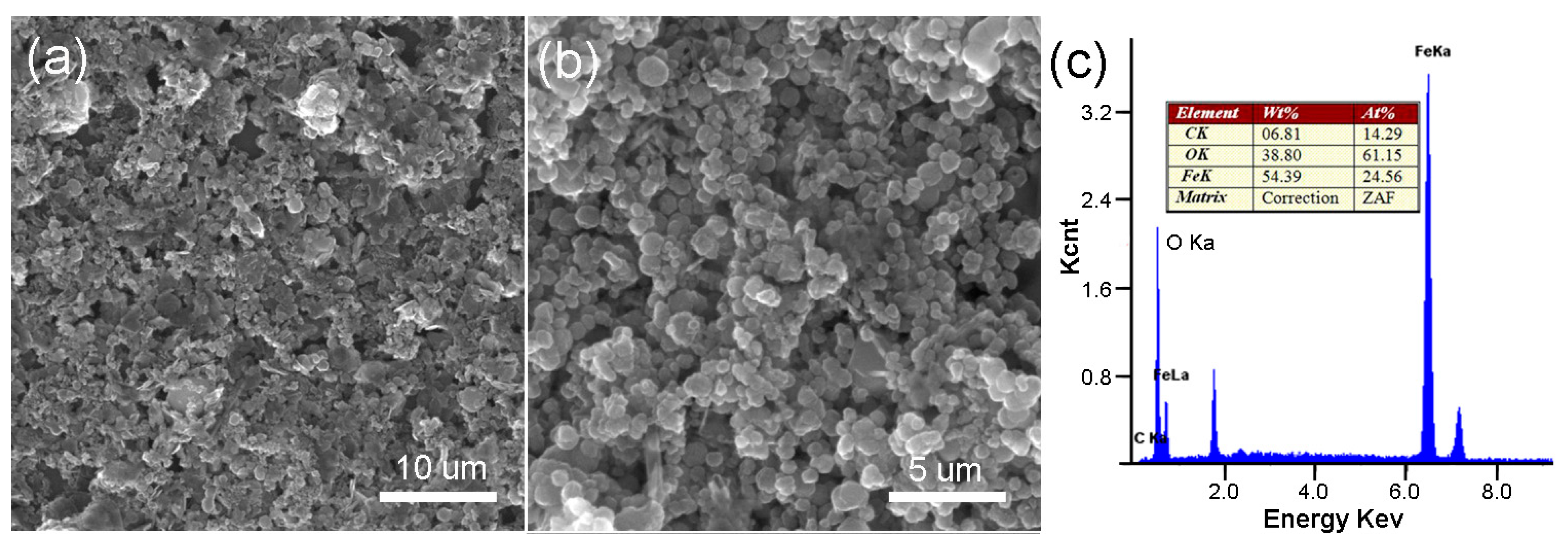
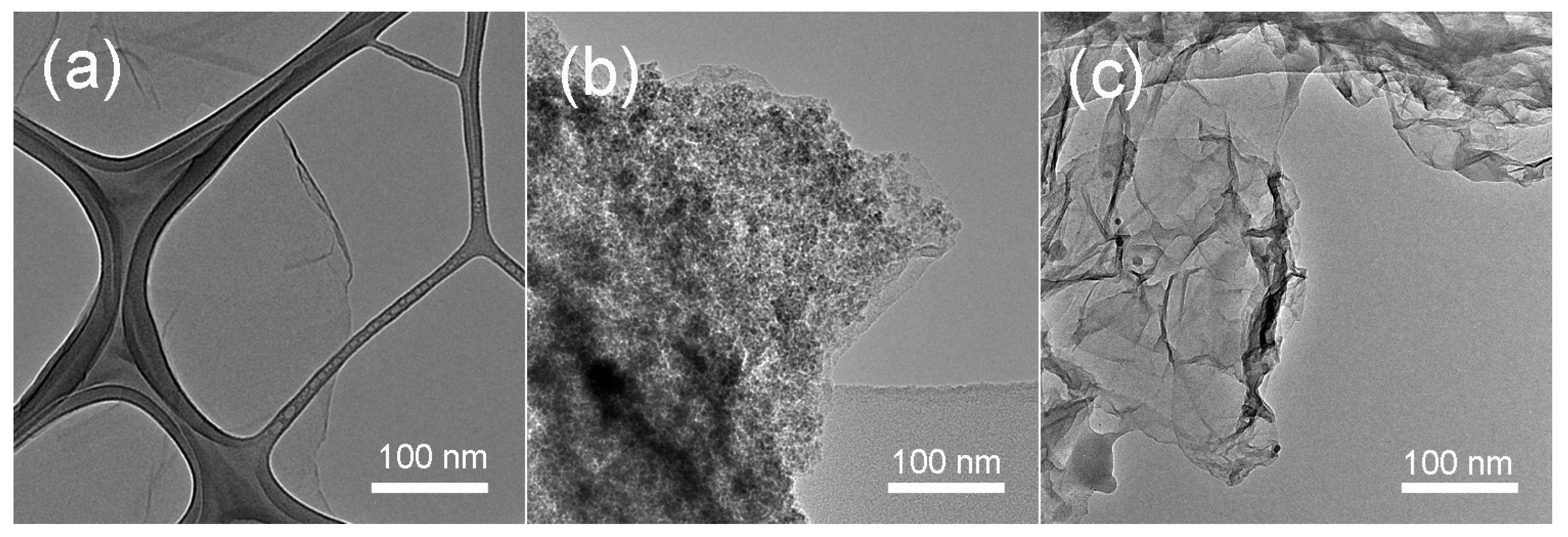
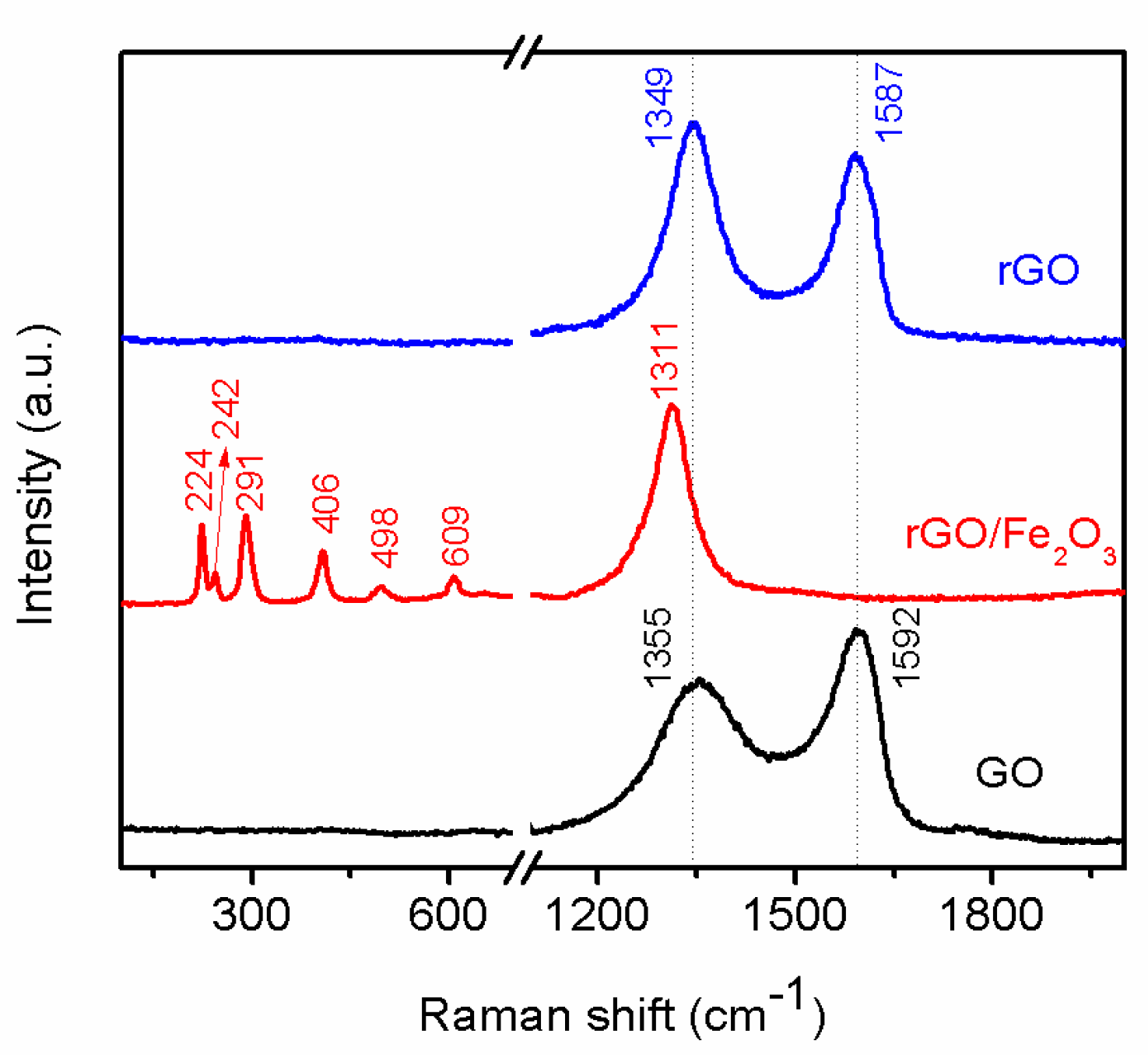
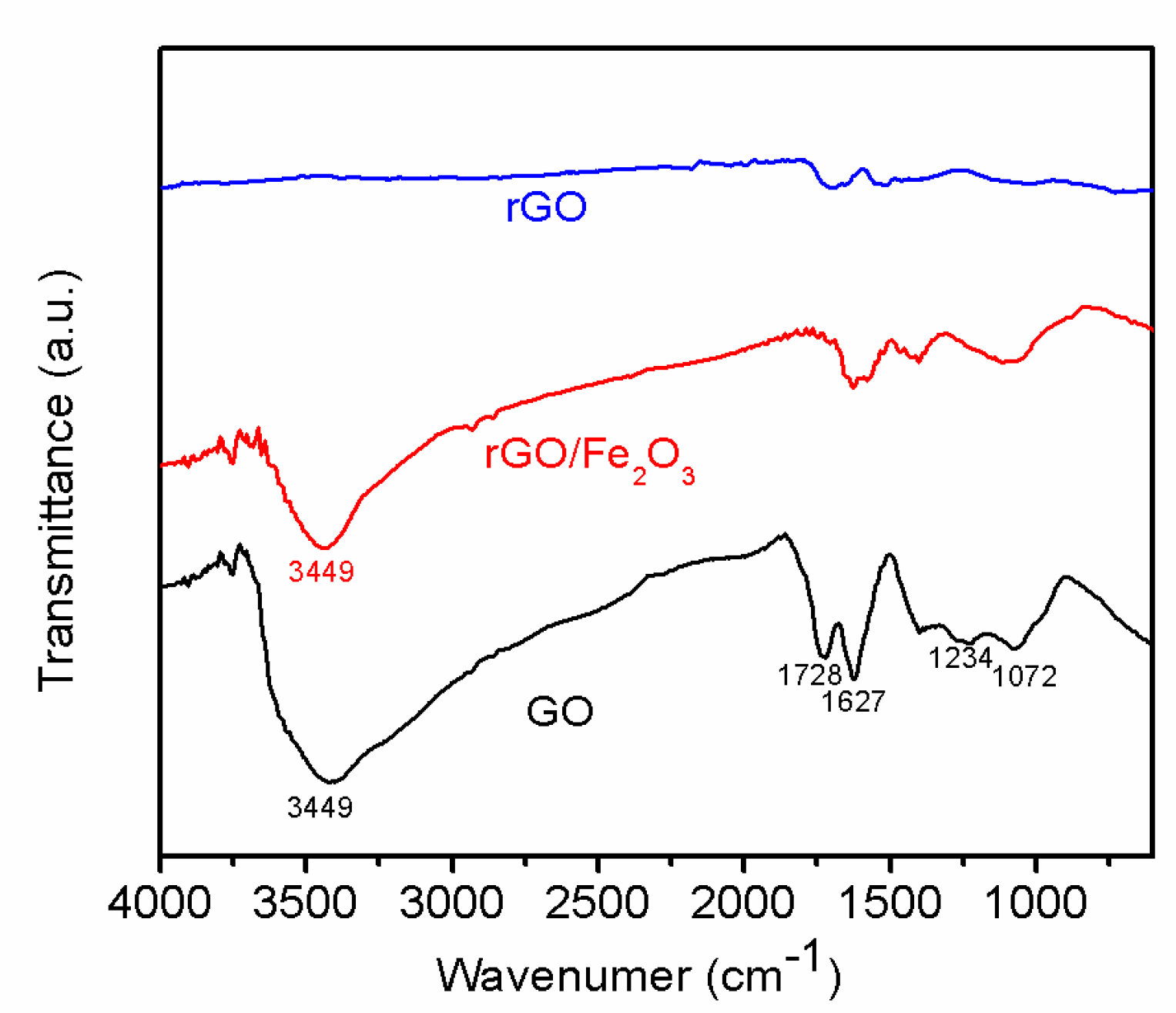
3.2. Structural and Morphological Investigations
3.3. Measure Nascent Hydrogen Reduction Efficiency
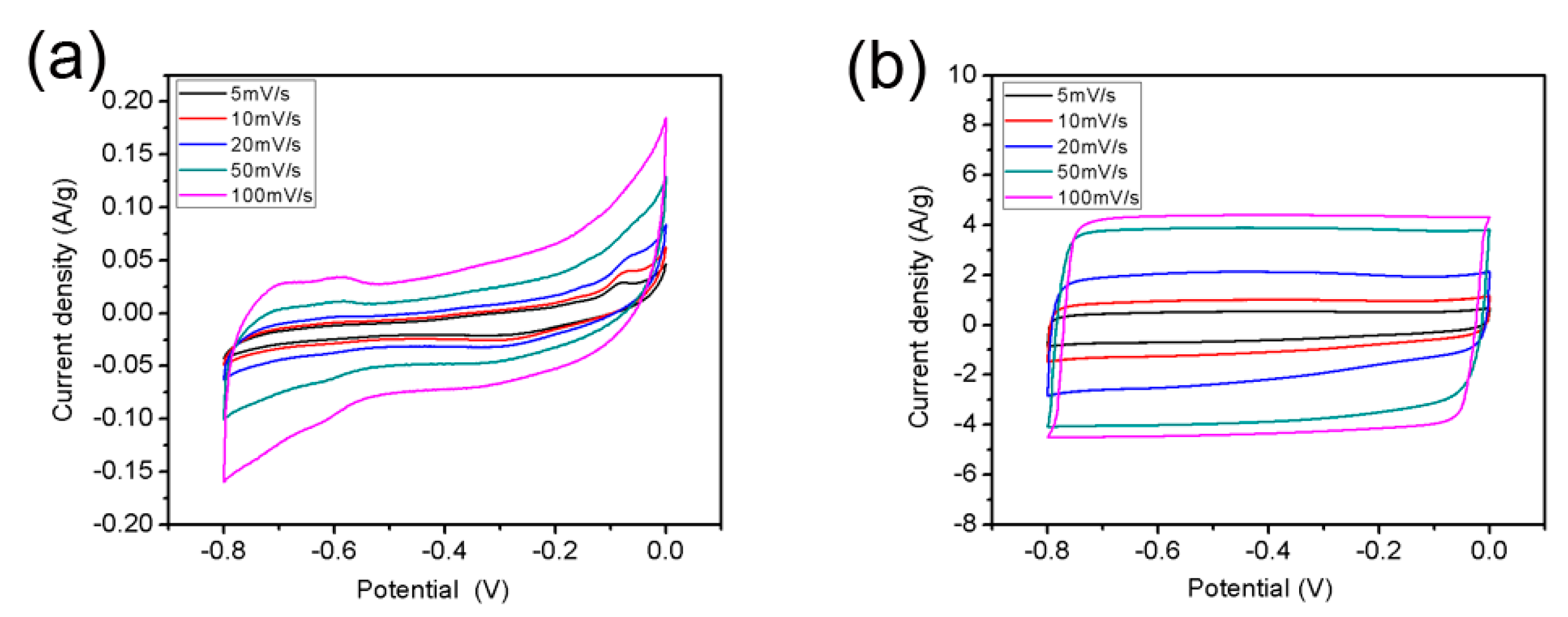
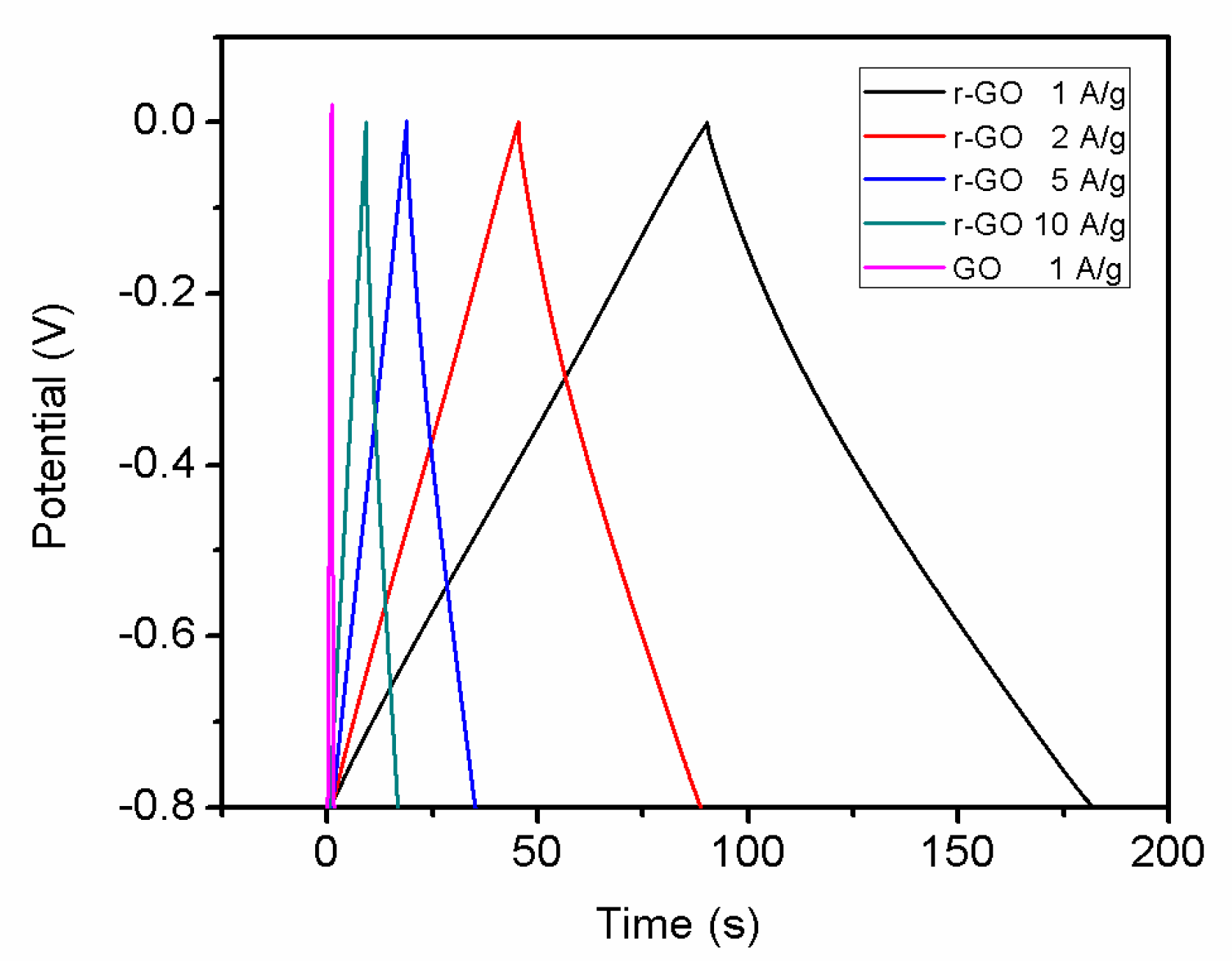
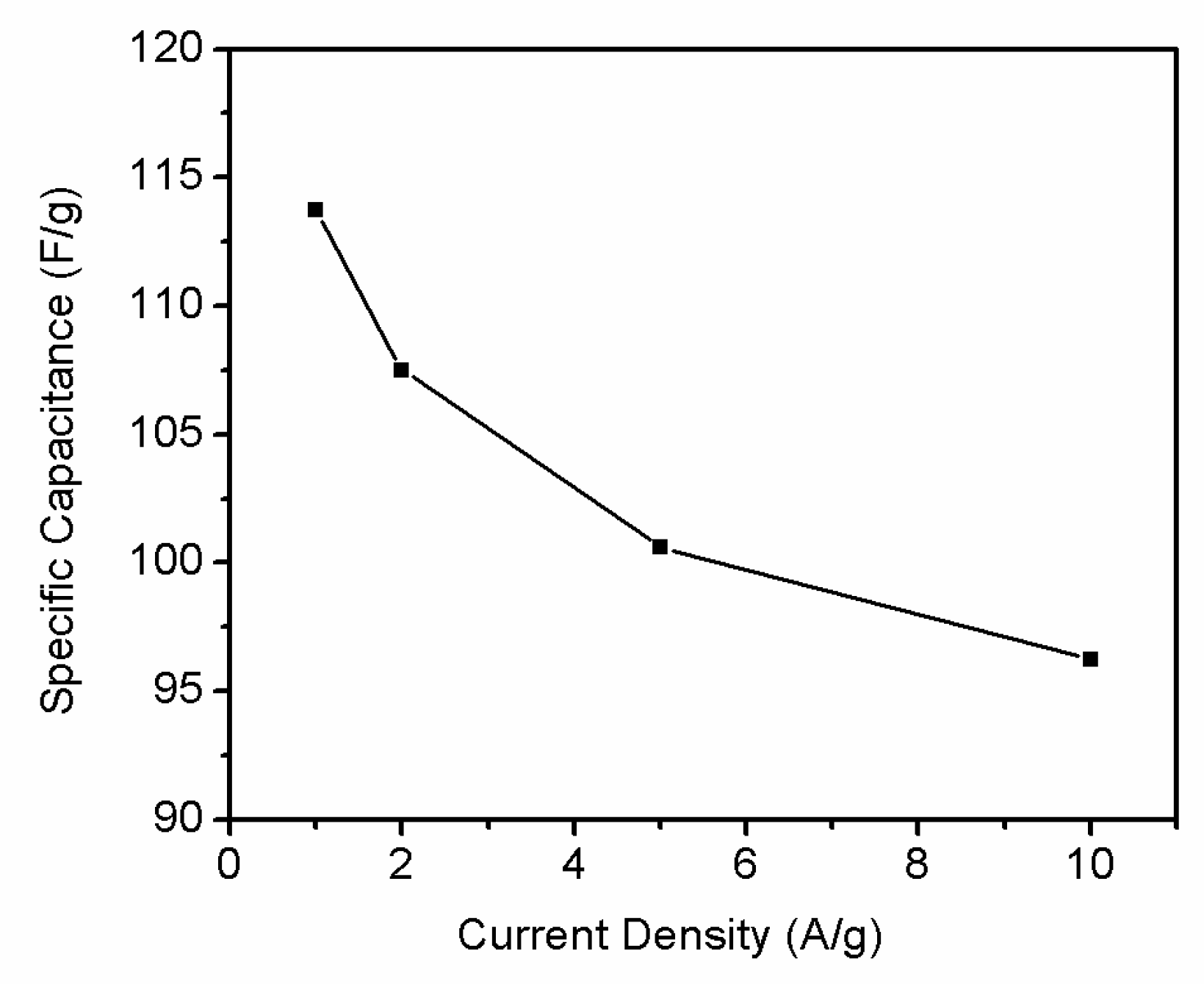
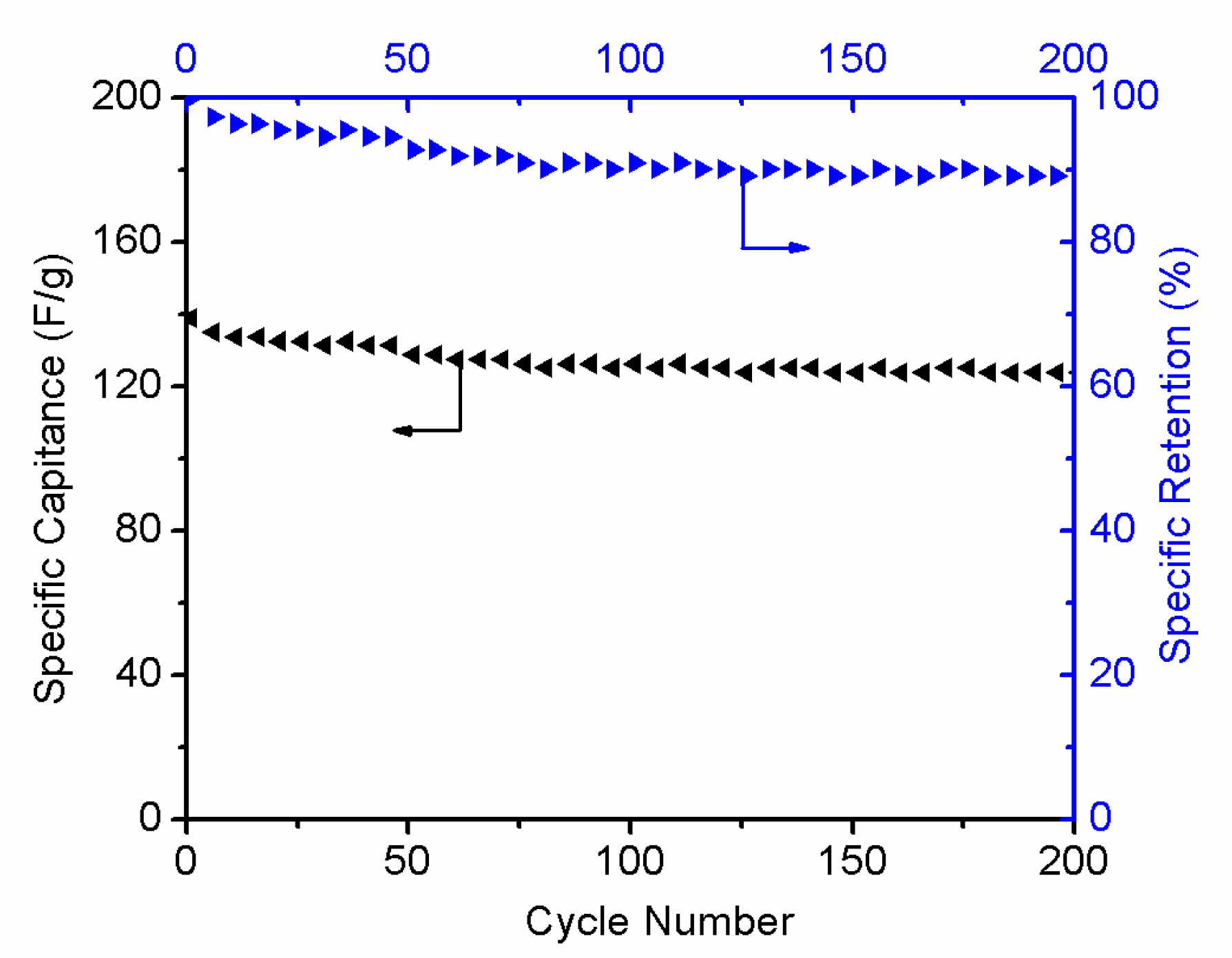
3.4. Electrochemical Properties of r-GO
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wiederoder, M.S.; Nallon, E.C.; Weiss, M.; McGraw, S.K.; Schnee, V.P.; Bright, C.J.; Polcha, M.P.; Paffenroth, R.C.; Uzarski, J.R. Graphene Nanoplatelet-Polymer Chemiresistive Sensor Arrays for the Detection and Discrimination of Chemical Warfare Agent Simulants. ACS Sens. 2017, 2, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.B.; Tai, H.L.; Guo, R.; Yuan, Z.; Liu, C.H.; Su, Y.J.; Chen, Z.; Jiang, Y.D. Excellent ammonia sensing performance of gas sensor based on graphene/titanium dioxide hybrid with improved morphology. Appl. Surf. Sci. 2017, 419, 84–90. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, S.J.; Jang, J.S.; Lee, J.H.; Kim, I.D. Silver Nanowire Embedded Colorless Polyimide Heater for Wearable Chemical Sensors: Improved Reversible Reaction Kinetics of Optically Reduced Graphene Oxide. Small 2016, 12, 5826–5835. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chen, Q.; Zhong, Z.H. Graphene Ambipolar Nanoelectronics for High Noise Rejection Amplification. Nano Lett. 2016, 16, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Yun, K.H.; Chung, Y.C. Graphene Monoxide Bilayer As a High-Performance on/off Switching Media for Nanoelectronics. ACS Appl. Mater. Interfaces 2016, 8, 10477–10482. [Google Scholar] [CrossRef] [PubMed]
- Camilli, L.; Jorgensen, J.H.; Tersoff, J.; Stoot, A.C.; Balog, R.; Cassidy, A.; Sadowski, J.T.; Boggild, P.; Hornekaer, L. Self-assembly of ordered graphene nanodot arrays. Nat. Commun. 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Karahan, H.E.; Zhai, S.L.; Liu, H.W.; Chen, X.C.; Zhou, Z.; Lei, Y.J.; Liu, Z.W.; Chen, Y. Amorphous Bimetallic Oxide-Graphene Hybrids as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn-Air Batteries. Adv. Mater. 2017, 29, 1701410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Wan, S.; Yan, B.; Wang, L.B.; Qian, Y.T. Graphene Encapsulated Fe3O4 Nanospindles as a Superior Anode Material for Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2013, 13, 4364–4369. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Shou, D.; Chen, C.X.; Mao, H.H.; Kong, Y.; Tao, Y.X. Core-shell structured polypyrrole/mesoporous SiO2 nanocomposite capped with graphene quantum dots as gatekeeper for irradiation-controlled release of methotrexate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Zhou, W.; Ying, J.F.; Yang, T.Y.; Gao, Y.M. Thermal Stable Perovskite Solar Cells Improved by ZnO/Graphene Oxide as Electron Transfer Layers. J. Inorg. Mater. 2017, 32, 96–100. [Google Scholar] [CrossRef]
- Xu, Y.H.; Li, J.; Huang, W.X. Porous Graphene Oxide Prepared on Nickel Foam by Electrophoretic Deposition and Thermal Reduction as High-Performance Supercapacitor Electrodes. Materials 2017, 10, 936. [Google Scholar] [CrossRef]
- Zhuo, Q.Q.; Gao, J.; Peng, M.F.; Bai, L.L.; Deng, J.J.; Xia, Y.J.; Ma, Y.Y.; Zhong, J.; Sun, X.H. Large-scale synthesis of graphene by the reduction of graphene oxide at room temperature using metal nanoparticles as catalyst. Carbon 2013, 52, 559–564. [Google Scholar] [CrossRef]
- Zhuo, Q.Q.; Ma, Y.Y.; Gao, J.; Zhang, P.P.; Xia, Y.J.; Tian, Y.M.; Sun, X.X.; Zhong, J.; Sun, X.H. Facile Synthesis of Graphene/Metal Nanoparticle Composites via Self-Catalysis Reduction at Room Temperature. Inorg. Chem. 2013, 52, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.Q.; Zhang, Y.P.; Du, Q.C.; Yan, C. Facile reduction of graphene oxide at room temperature by ammonia borane via salting out effect. J. Colloid Interface Sci. 2015, 457, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Strankowski, M.; Korzeniewski, P.; Strankowska, J.; A., S.A.; Thomas, S. Morphology, Mechanical and Thermal Properties of Thermoplastic Polyurethane Containing Reduced Graphene Oxide and Graphene Nanoplatelets. Materials 2018, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Parviz, D.; Irin, F.; Shah, S.A.; Das, S.; Sweeney, C.B.; Green, M.J. Challenges in Liquid-Phase Exfoliation, Processing, and Assembly of Pristine Graphene. Adv. Mater. 2016, 28, 8796–8818. [Google Scholar] [CrossRef] [PubMed]
- Arao, Y.; Mori, F.; Kubouchi, M. Efficient solvent systems for improving production of few-layer graphene in liquid phase exfoliation. Carbon 2017, 118, 18–24. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.B.; Mendes, R.G.; Wrobel, P.S.; Wlodarski, M.D.; Ta, H.Q.; Zhao, L.; Giebeler, L.; Trzebicka, B.; Gemming, T.; Fu, L.; et al. Self-Terminating Confinement Approach for Large-Area Uniform Monolayer Graphene Directly over Si/SiOX by Chemical Vapor Deposition. ACS Nano 2017, 11, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Wang, H.I.; Teyssandier, J.; Mali, K.S.; Dumslaff, T.; Ivanov, I.; Zhang, W.; Ruffieux, P.; Fasel, R.; Rader, H.J.; et al. Chemical Vapor Deposition Synthesis and Terahertz Photoconductivity of Low-Band-Gap N=9 Armchair Graphene Nanoribbons. J. Am. Chem. Soc. 2017, 139, 3635–3638. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Gyawali, G.; Lee, S.W. Graphene Coating via Chemical Vapor Deposition for Improving Friction and Wear of Gray Cast Iron at Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 32336–32351. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ramabadran, U.; Ryan, G.; Zhou, X.; Farhat, S.; Manciu, F.; Tong, Y.G.; Ayler, R.; Garner, G. Reduced Graphene Oxide on Nickel Foam for Supercapacitor Electrodes. Materials 2017, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Xie, Y.; Zheng, B.; Kou, L.; Gao, C. Polyelectrolyte-Stabilized Graphene Oxide Liquid Crystals against Salt, pH, and Serum. Langmuir 2014, 30, 3715–3722. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.Y.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene Oxide Liquid Crystals. Angew. Chem. Int. Ed. 2011, 50, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Zhou, X.J.; Zhang, J.L.; Wu, H.X.; Yang, H.J.; Zhang, J.Y.; Guo, S.W. Reducing Graphene Oxide via Hydroxylamine: A Simple and Efficient Route to Graphene. J. Phys. Chem. C 2011, 115, 11957–11961. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Lei, Z.B.; Lu, L.; Zhao, X.S. The electrocapacitive properties of graphene oxide reduced by urea. Energy Environ. Sci. 2012, 5, 6391–6399. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing sugar: new functional molecules for the green synthesis of graphene nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010, 46, 1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.H.; Xing, C.Y.; Guo, M.Y.; Xu, F.G.; Wang, X.D.; Gruber, H.; Zhang, B.L.; Tang, J.L. Sodium Citrate: A Universal Reducing Agent for Reduction/Decoration of Graphene Oxide with Au Nanoparticles. Nano Res. 2011, 4, 599–611. [Google Scholar] [CrossRef]
- Fan, Z.J.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.J.; Feng, J.; Ren, Y.M.; Song, L.P.; Wei, F. Facile Synthesis of Graphene Nanosheets via Fe Reduction of Exfoliated Graphite Oxide. ACS Nano 2011, 5, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huang, Y.; Wang, L. A facile synthesis of reduced graphene oxide with Zn powder under acidic condition. Mater. Lett. 2013, 91, 125–128. [Google Scholar] [CrossRef]
- Fan, Z.J.; Wang, K.; Wei, T.; Yan, J.; Song, L.P.; Shao, B. An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder. Carbon 2010, 48, 1686–1689. [Google Scholar] [CrossRef]
- Sofer, Z.; Jankovsky, O.; Simek, P.; Soferova, L.; Sedmidubsky, D.; Pumera, M. Highly hydrogenated graphene via active hydrogen reduction of graphene oxide in the aqueous phase at room temperature. Nanoscale 2014, 6, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.V.; Gagare, P.D. Preparation of ammonia borane in high yield and purity, methanolysis, and regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Mazumder, V.; Metin, O.; Sun, S. Methanolysis of Ammonia Borane by CoPd Nanoparticles. ACS Catal. 2012, 2, 1290–1295. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B-N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Shi, W.; Feng, G.; Lu, Z.-H.; Zhang, X.; Tao, D.; Kong, D.; Chen, X. Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: Highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Power Sources 2014, 257, 293–299. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Indirani, M.; Jagirdar, B.R. First row transition metal ion-assisted ammonia-borane hydrolysis for hydrogen generation. Inorg. Chem. 2008, 47, 7424–7429. [Google Scholar] [CrossRef] [PubMed]
- Akbayrak, S.; Ozkar, S. Ruthenium(0) Nanoparticles Supported on Multiwalled Carbon Nanotube As Highly Active Catalyst for Hydrogen Generation from Ammonia-Borane. ACS Appl. Mater. Inter. 2012, 4, 6302–6310. [Google Scholar] [CrossRef] [PubMed]
- Xi, P.; Chen, F.; Xie, G.; Ma, C.; Liu, H.; Shao, C.; Wang, J.; Xu, Z.; Xu, X.; Zeng, Z. Surfactant free RGO/Pd nanocomposites as highly active heterogeneous catalysts for the hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. Nanoscale 2012, 4, 5597–5601. [Google Scholar] [CrossRef] [PubMed]
- Lapin, N.V.; D’Yankova, N.Y. Hydrogen evolution kinetics during transition metal oxide-catalyzed ammonia borane hydrolysis. Inorg. Mater. 2013, 49, 975–979. [Google Scholar] [CrossRef]
- Hassan, M.F.; Rahman, M.M.; Guo, Z.P.; Chen, Z.X.; Liu, H.K. Solvent-assisted molten salt process: A new route to synthesise alpha-Fe2O3/C nanocomposite and its electrochemical performance in lithium-ion batteries. Electrochim. Acta 2010, 55, 5006–5013. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, Q.; Tang, J.; Sun, J.; Yan, C. High Efficient Reduction of Graphene Oxide via Nascent Hydrogen at Room Temperature. Materials 2018, 11, 340. https://doi.org/10.3390/ma11030340
Zhuo Q, Tang J, Sun J, Yan C. High Efficient Reduction of Graphene Oxide via Nascent Hydrogen at Room Temperature. Materials. 2018; 11(3):340. https://doi.org/10.3390/ma11030340
Chicago/Turabian StyleZhuo, Qiqi, Jijun Tang, Jun Sun, and Chao Yan. 2018. "High Efficient Reduction of Graphene Oxide via Nascent Hydrogen at Room Temperature" Materials 11, no. 3: 340. https://doi.org/10.3390/ma11030340