Comparison of Six Different Silicones In Vitro for Application as Glaucoma Drainage Device
Abstract
:1. Introduction
2. Results
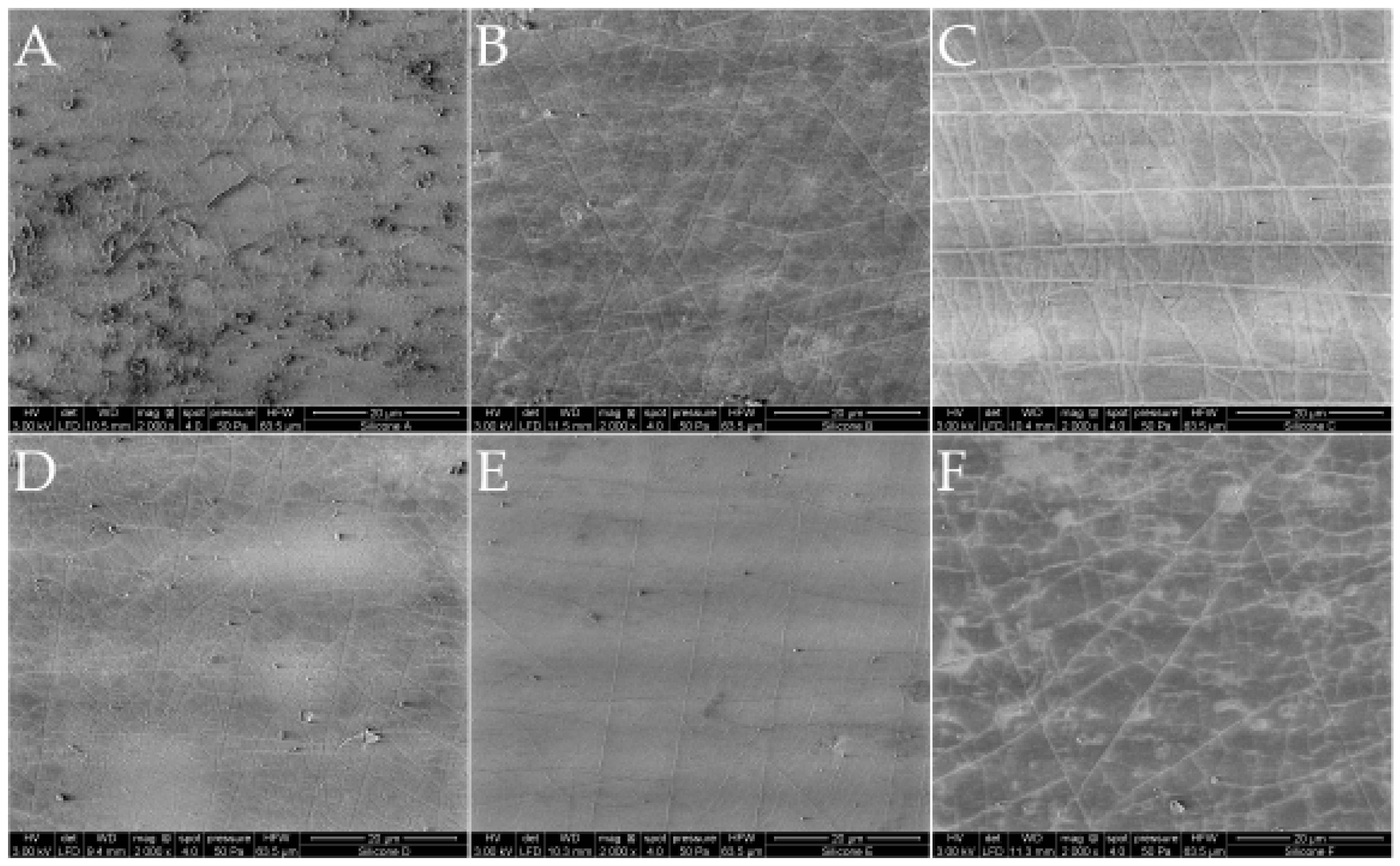
2.1. Silicones
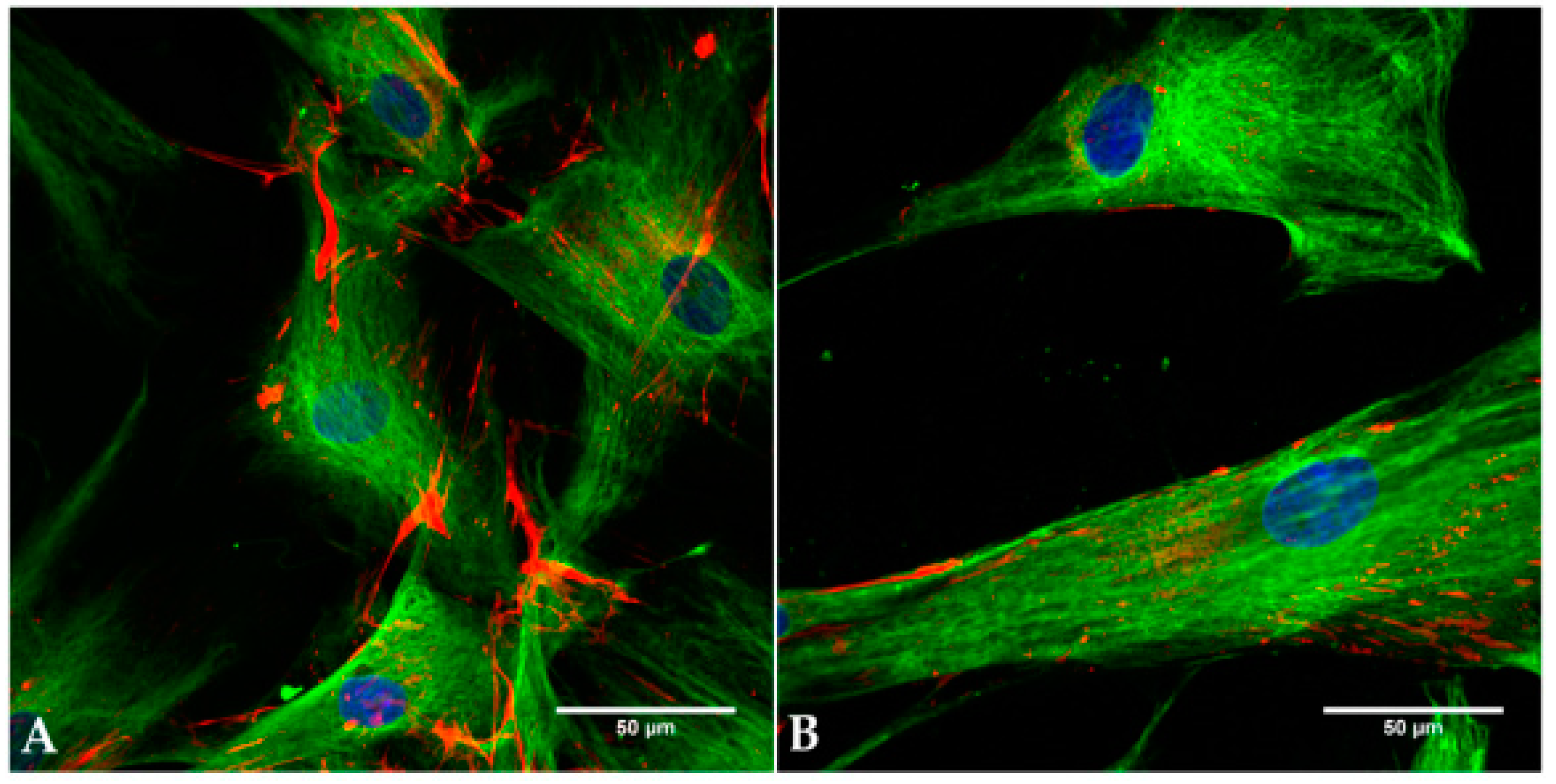
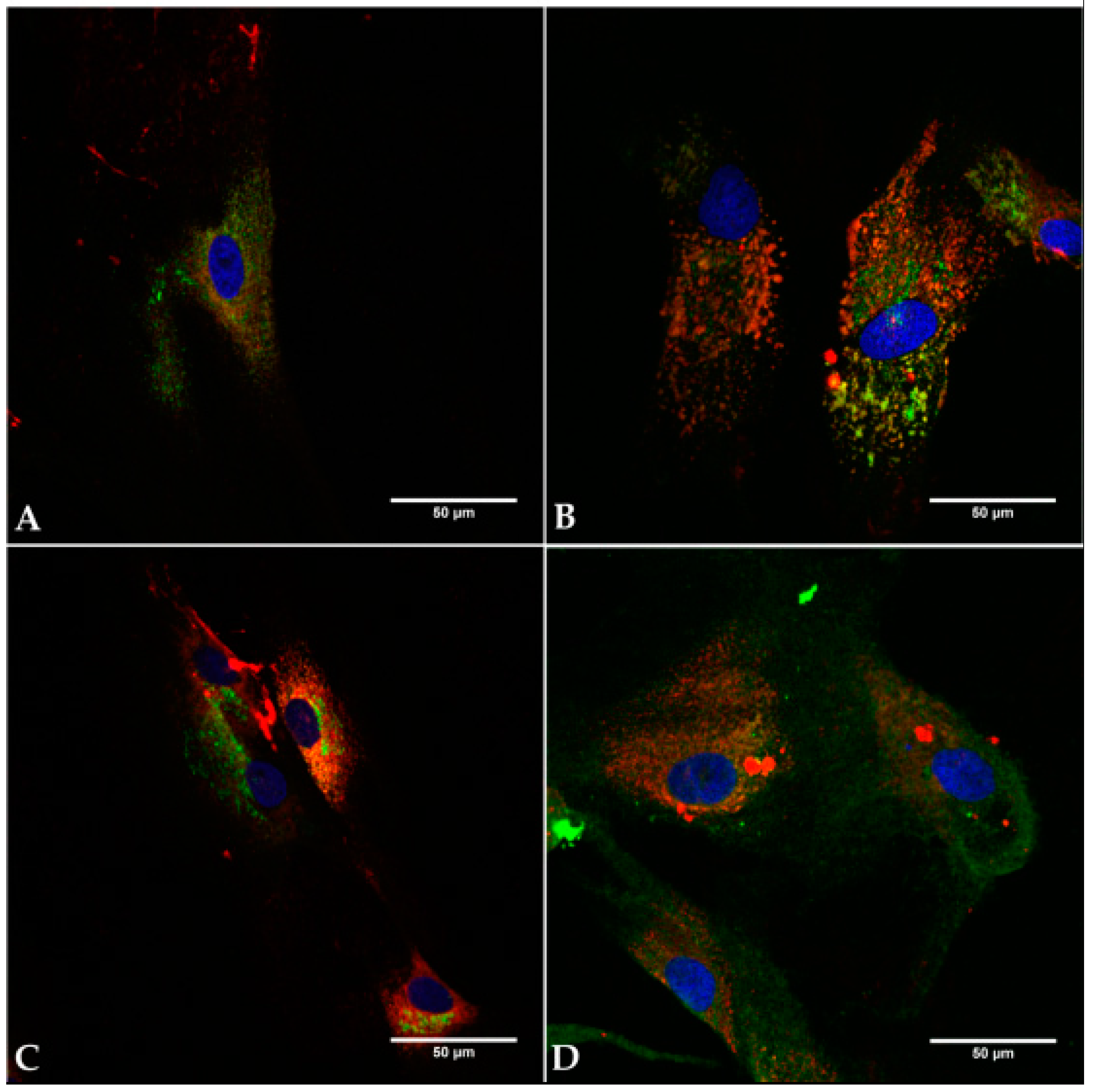
2.2. Cell Identity
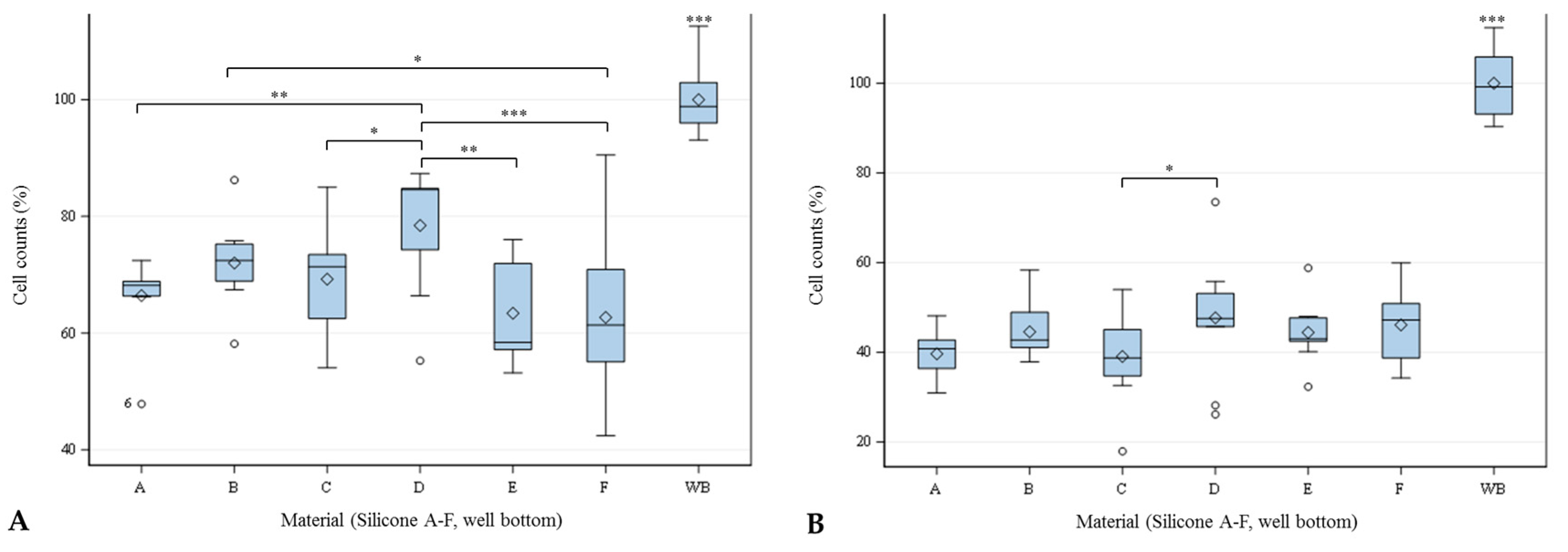
2.3. Cell Count
2.4. Proliferation
2.5. Viability
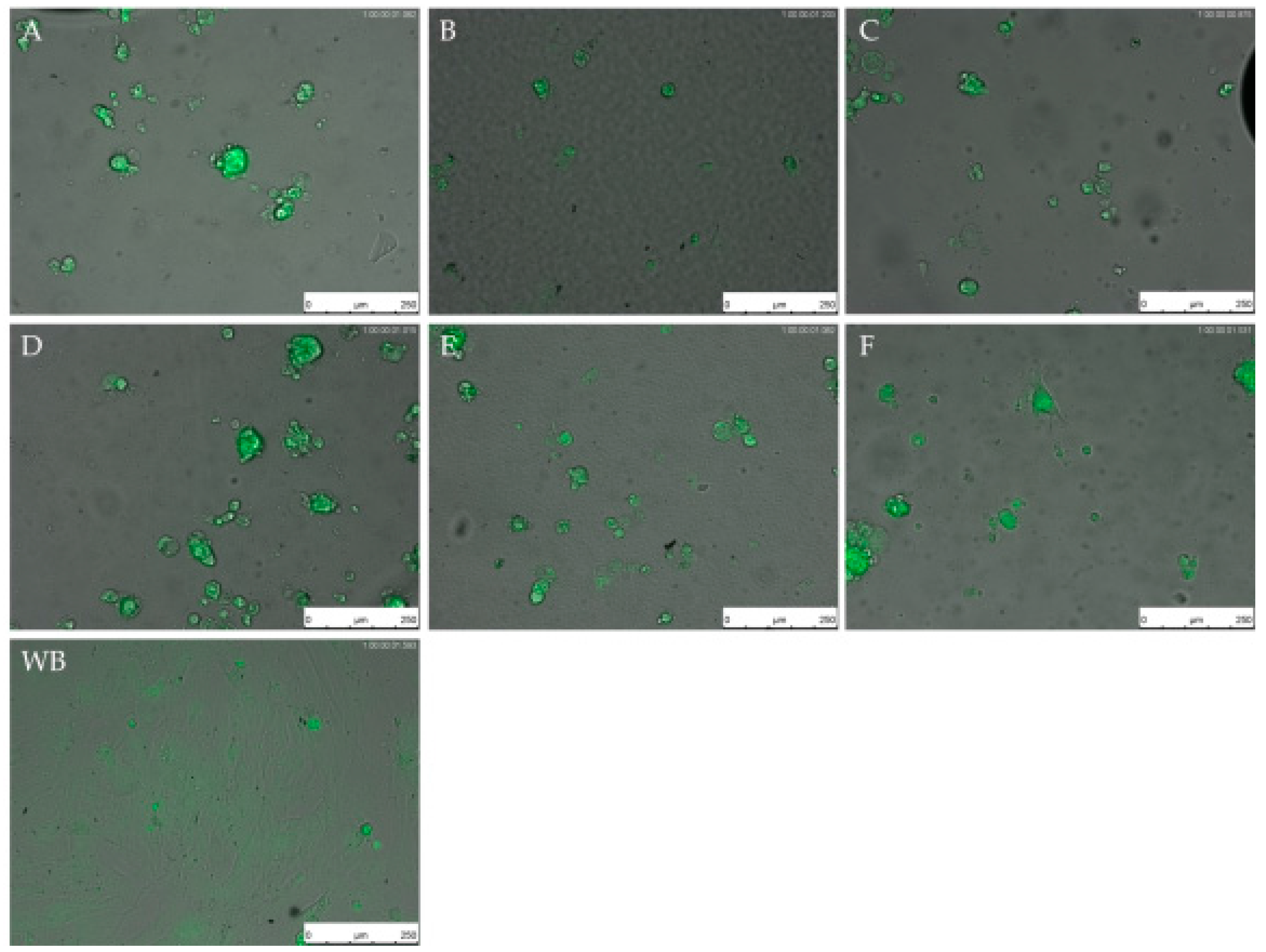
2.6. Live Cell Imaging (LCI)
3. Discussion
4. Materials and Methods
4.1. Silicone
4.1.1. Characterization of the Silicones
Environmental Scanning Electron Microscope (ESEM)
Water Contact Angle
4.1.2. Preparation of the Silicone for In Vitro Analysis
4.2. Cell Culture
4.2.1. Human Sclera Fibroblast (hSF)
4.2.2. Human Tenon’s Fibroblast (hTF)
4.2.3. Immunocytochemical Staining
4.3. Characterization of Cell Behaviour on Silicones
4.3.1. Cell Counting
4.3.2. Staining of hSF and hTF for Cell Division Tracking and Viability
4.3.3. Live Cell Imaging (LCI) of hSF and hTF
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Curtis, J.; Colas, A. Chapter II. 5.18—Medical applications of silicones A2—Ratner, buddy D. In Biomaterials Science, 3rd ed.; Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1106–1116. [Google Scholar]
- Andreopoulos, A.G.; Plytaria, M. Biomedical silicone elastomers as carriers for controlled release. J. Biomater. Appl. 1998, 12, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J. Silicone hydrophobicity and oleophilicity. Silicon 2014, 9, 651–655. [Google Scholar] [CrossRef]
- Ratner, B.D. Chapter II. 1.1—Introduction: Biology and medicine—Key concepts in the use of biomaterials in surgery and medical devices. In Biomaterials Science, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 393–394. [Google Scholar]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Smetana, K., Jr. Cell biology of hydrogels. Biomaterials 1993, 14, 1046–1050. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; Garcia, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A 2003, 66, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Beltran, J.; Rico, P.; Moratal, D.; Song, W.; Mano, J.F.; Salmeron-Sanchez, M. Role of superhydrophobicity in the biological activity of fibronectin at the cell-material interface. Soft Matter 2011, 7, 10803–10811. [Google Scholar] [CrossRef]
- Atlan, M.; Bigerelle, M.; Larreta-garde, V.; Hindié, M.; Hedén, P. Characterization of breast implant surfaces, shapes, and biomechanics: A comparison of high cohesive anatomically shaped textured silicone, breast implants from three different manufacturers. Aesthet. Plast. Surg. 2016, 40, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Dencker, F.; Dreyer, L.; Muller, D.; Zernetsch, H.; Paasche, G.; Sindelar, R.; Glasmacher, B. A silicone fiber coating as approach for the reduction of fibroblast growth on implant electrodes. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 105, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Doucette, L.P.; Rasnitsyn, A.; Seifi, M.; Walter, M.A. The interactions of genes, age, and environment in glaucoma pathogenesis. Surv. Ophthalmol. 2015, 60, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Nickells, R.W.; Howell, G.R.; Soto, I.; John, S.W. Under pressure: Cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 2012, 35, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.H.; Yu, D.Y. Surgical management of glaucoma: A review. Clin. Exp. Ophthalmol. 2012, 40, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Ayyala, R.S.; Duarte, J.L.; Sahiner, N. Glaucoma drainage devices: State of the art. Expert Rev. Med. Devices 2006, 3, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, A.; Brocchini, S.; Khaw, P.T. New developments in the pharmacological modulation of wound healing after glaucoma filtration surgery. Curr. Opin. Pharmacol. 2013, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.S.; Lee, R.K.; Gedde, S.J. Glaucoma drainage implants: A critical comparison of types. Curr. Opin. Ophthalmol. 2006, 17, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ayyala, R.S.; Zurakowski, D.; Monshizadeh, R.; Hong, C.H.; Richards, D.; Layden, W.E.; Hutchinson, B.T.; Bellows, A.R. Comparison of double-plate Molteno and ahmed glaucoma valve in patients with advanced uncontrolled glaucoma. Ophthalmic Surg. Lasers 2002, 33, 94–101. [Google Scholar] [PubMed]
- Ayyala, R.S.; Harman, L.E.; Michelini-Norris, B.; Ondrovic, L.E.; Haller, E.; Margo, C.E.; Stevens, S.X. Comparison of different biomaterials for glaucoma drainage devices. Arch. Ophthalmol. 1999, 117, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Ayyala, R.S.; Michelini-Norris, B.; Flores, A.; Haller, E.; Margo, C.E. Comparison of different biomaterials for glaucoma drainage devices: Part 2. Arch. Ophthalmol. 2000, 118, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Netland, P.A.; Costa, V.P.; Shiroma, L.; Khan, B.; Ahmed, I.I. Comparison of polypropylene and silicone AHMED glaucoma valves. Ophthalmology 2006, 113, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, T.; Lobler, M.; Kastner, C.; Stachs, O.; Wree, A.; Sternberg, K.; Schmitz, K.P.; Guthoff, R. Different fibroblast subpopulations of the eye: A therapeutic target to prevent postoperative fibrosis in glaucoma therapy. Exp. Eye Res. 2012, 100, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.L. Collagen type I and III synthesis by tenon’s capsule fibroblasts in culture: Individual patient characteristics and response to mitomycin C, 5-fluorouracil, and ascorbic acid. Trans. Am. Ophthalmol. Soc. 1999, 97, 513–543. [Google Scholar] [PubMed]
- Mietz, H.; Arnold, G.; Kirchhof, B.; Diestelhorst, M.; Krieglstein, G.K. Histopathology of episcleral fibrosis after trabeculectomy with and without mitomycin C. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Khaw, P.T.; Ward, S.; Porter, A.; Grierson, I.; Hitchings, R.A.; Rice, N.S. The long-term effects of 5-fluorouracil and sodium butyrate on human tenon’s fibroblasts. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2043–2052. [Google Scholar]
- Choritz, L.; Grub, J.; Wegner, M.; Pfeiffer, N.; Thieme, H. Paclitaxel inhibits growth, migration and collagen production of human tenon’s fibroblasts—Potential use in drug-eluting glaucoma drainage devices. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Mondino, B.J.; Mayer, F.J. Scleral fibroblasts. Human leukocyte antigen expression and complement production. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2412–2419. [Google Scholar]
- Jobling, A.I.; Nguyen, M.; Gentle, A.; McBrien, N.A. Isoform-specific changes in scleral transforming growth factor-β expression and the regulation of collagen synthesis during myopia progression. J. Biol. Chem. 2004, 279, 18121–18126. [Google Scholar] [CrossRef] [PubMed]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; van Wijnen, A.J. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.R.; Witkowski, M.; Plas, E.; Pflueger, H. Heparin coating reduces encrustation of ureteral stents: A preliminary report. Int. J. Antimicrob. Agents 2002, 19, 507–510. [Google Scholar] [CrossRef]
- Barrows, T. Degradable implant materials: A review of synthetic absorbable polymers and their applications. Clin. Mater. 1986, 1, 233–257. [Google Scholar] [CrossRef]
- Molteno, A.C.; Fucik, M.; Dempster, A.G.; Bevin, T.H. Otago glaucoma surgery outcome study: Factors controlling capsule fibrosis around molteno implants with histopathological correlation. Ophthalmology 2003, 110, 2198–2206. [Google Scholar] [CrossRef]
- Hong, C.H.; Arosemena, A.; Zurakowski, D.; Ayyala, R.S. Glaucoma drainage devices: A systematic literature review and current controversies. Surv. Ophthalmol. 2005, 50, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Schlie-Wolter, S.; Ngezahayo, A.; Chichkov, B.N. The selective role of ecm components on cell adhesion, morphology, proliferation and communication in vitro. Exp. Cell Res. 2013, 319, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S. Cell and protein adhesion studies in glaucoma drainage device development. The agfid project team. Br. J. Ophthalmol. 1999, 83, 1168–1171. [Google Scholar] [PubMed]
- Dietlein, T.S.; Jordan, J.; Lueke, C.; Krieglstein, G.K. Modern concepts in antiglaucomatous implant surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1653. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.A.; Hollander, D.A.; Juster, R.P.; Lee, L.C. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: Long-term outcomes. Am. J. Ophthalmol. 2008, 146, 276–284.e272. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Ikada, Y. Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J. Colloid Interface Sci. 1993, 155, 334–339. [Google Scholar] [CrossRef]
- Tzoneva, R.; Faucheux, N.; Groth, T. Wettability of substrata controls cell-substrate and cell-cell adhesions. Biochim. Biophys. Acta 2007, 1770, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Brasil, M.V.; Rockwood, E.J.; Smith, S.D. Comparison of silicone and polypropylene ahmed glaucoma valve implants. J. Glaucoma 2007, 16, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Nguyen, A.; Coleman, A.L.; Caprioli, J. Comparison of safety and efficacy between silicone and polypropylene ahmed glaucoma valves in refractory glaucoma. Ophthalmology 2005, 112, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.T.; Rainaldi, G.; Indovina, P.L. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Hematology 2000, 36, 75–87. [Google Scholar] [CrossRef]
- Jain, R.; von Recum, A.F. Fibroblast attachment to smooth and microtextured pet and thin CP-TI films. J. Biomed. Mater. Res. A 2004, 68, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Oden, A.; Wennerberg, A.; Hultenby, K.; Arvidson, K. The influence of surface topography of ceramic abutments on the attachment and proliferation of human oral fibroblasts. Biomaterials 2005, 26, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Meredith, D.O.; Eschbach, L.; Riehle, M.O.; Curtis, A.S.; Richards, R.G. Microtopography of metal surfaces influence fibroblast growth by modifying cell shape, cytoskeleton, and adhesion. J. Orthop. Res. 2007, 25, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Altankov, G.; Grinnell, F.; Groth, T. Studies on the biocompatibility of materials: Fibroblast reorganization of substratum-bound fibronectin on surfaces varying in wettability. J. Biomed. Mater. Res. 1996, 30, 385–391. [Google Scholar] [CrossRef]
- Curtis, A.S.; Wilkinson, C.D. Reactions of cells to topography. J. Biomater. Sci. Polym. Ed. 1998, 9, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Tranquillo, R.T. Self-organization of tissue-equivalents: The nature and role of contact guidance. Biochem. Soc. Symp. 1999, 65, 27–42. [Google Scholar] [PubMed]
- Evans, M.D.; Steele, J.G. Polymer surface chemistry and a novel attachment mechanism in corneal epithelial cells. J. Biomed. Mater. Res. 1998, 40, 621–630. [Google Scholar] [CrossRef]
- Choritz, L.; Koynov, K.; Renieri, G.; Barton, K.; Pfeiffer, N.; Thieme, H. Surface topographies of glaucoma drainage devices and their influence on human tenon fibroblast adhesion. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4047–4053. [Google Scholar] [CrossRef] [PubMed]
- Valimaki, J.; Uusitalo, H. Immunohistochemical analysis of extracellular matrix bleb capsules of functioning and non-functioning glaucoma drainage implants. Acta Ophthalmol. 2014, 92, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Good, R.J. Contact angle, wetting, and adhesion: A critical review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. [Google Scholar] [CrossRef]
- Matena, J.; Petersen, S.; Gieseke, M.; Teske, M.; Beyerbach, M.; Kampmann, A.; Murua Escobar, H.; Gellrich, N.C.; Haferkamp, H.; Nolte, I. Comparison of selective laser melted titanium and magnesium implants coated with PCL. Int. J. Mol. Sci. 2015, 16, 13287–13301. [Google Scholar] [CrossRef] [PubMed]

| Silicone | Water Contact Angle Mean ± SD [°] |
|---|---|
| A | 119 ± 7 |
| B | 117 ± 3 |
| C | 122 ± 4 |
| D | 100 ± 9 |
| E | 103 ± 9 |
| F | 118 ± 10 |
| Material | A | B | C | D | E | F | WB | |
|---|---|---|---|---|---|---|---|---|
| Viability (%) | hSF mean ± SD | 87.46 ± 5.10 | 87.76 ± 3.98 | 84.07 ± 9.84 | 83.14 ± 4.19 | 84.48 ± 9.84 | 90.43 ± 3.10 | 90.08 ± 3.51 |
| hTF mean ± SD | 91.76 ± 2.72 | 90.03 ± 6.62 | 92.50 ± 1.92 | 91.21 ± 2.58 | 91.21 ± 2.56 | 90.96 ± 1.91 | 91.61 ± 1.63 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windhövel, C.; Harder, L.; Bach, J.-P.; Teske, M.; Grabow, N.; Eickner, T.; Hinze, U.; Chichkov, B.; Nolte, I. Comparison of Six Different Silicones In Vitro for Application as Glaucoma Drainage Device. Materials 2018, 11, 341. https://doi.org/10.3390/ma11030341
Windhövel C, Harder L, Bach J-P, Teske M, Grabow N, Eickner T, Hinze U, Chichkov B, Nolte I. Comparison of Six Different Silicones In Vitro for Application as Glaucoma Drainage Device. Materials. 2018; 11(3):341. https://doi.org/10.3390/ma11030341
Chicago/Turabian StyleWindhövel, Claudia, Lisa Harder, Jan-Peter Bach, Michael Teske, Niels Grabow, Thomas Eickner, Ulf Hinze, Boris Chichkov, and Ingo Nolte. 2018. "Comparison of Six Different Silicones In Vitro for Application as Glaucoma Drainage Device" Materials 11, no. 3: 341. https://doi.org/10.3390/ma11030341





