Enhancement of Inverted Polymer Solar Cells Performances Using Cetyltrimethylammonium-Bromide Modified ZnO
Abstract
:1. Introduction
2. Materials and Methods
2.1. ZnO Precursor Solution Preparation
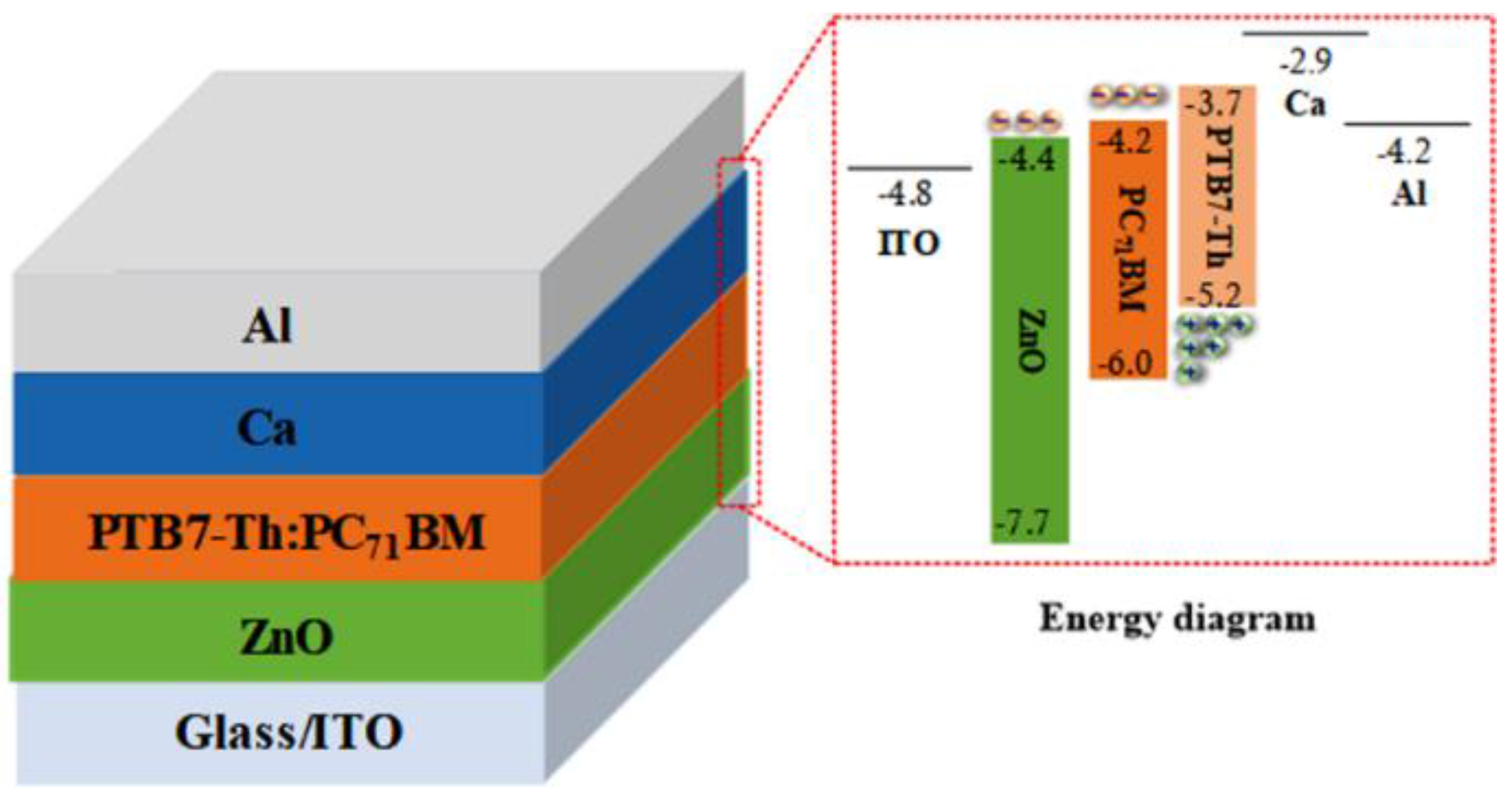
2.2. Fabrication of BHJ PSCs
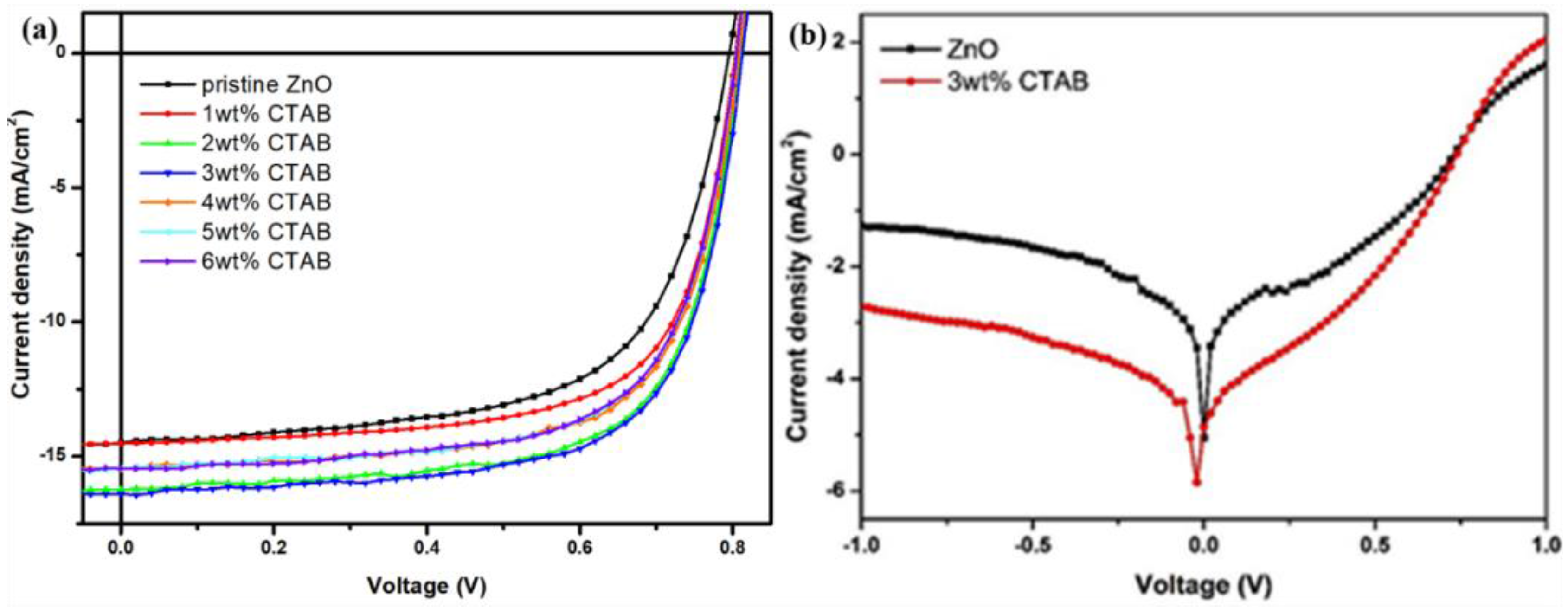
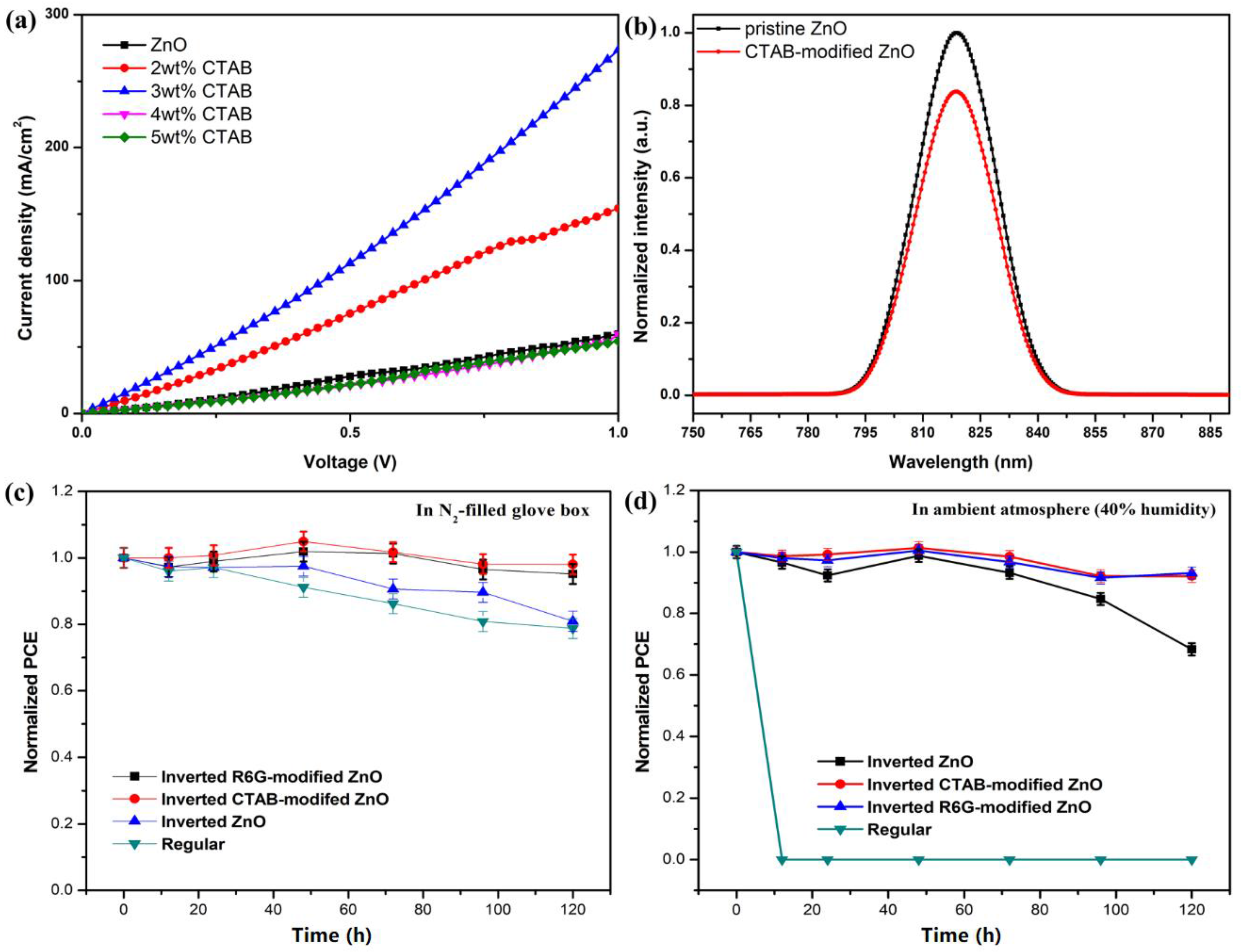
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudi, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Santos, D.A.D.; das, J.L.B.; gdlund, M.L.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.-Y.; Tsang, S.-W.; Zhou, J.; Verly, P.G.; Lu, J.; Beaupré, S.; Leclerc, M.; Tao, Y. High-efficiency inverted solar cells based on a low bandgap polymer with excellent air stability. Sol. Energy Mater. Sol. Cells 2012, 96, 155–159. [Google Scholar] [CrossRef]
- Service, R.F. Outlook brightens for plastic solar cells. Science 2011, 332, 293. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Cui, C.; Li, Y.Q.; Zhou, L.; Ou, Q.D.; Li, C.; Li, Y.; Tang, J.X. Single-junction polymer solar cells exceeding 10% power conversion efficiency. Adv. Mater. 2015, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Baı́a, I.; Quintela, M.; Mendes, L.; Nunes, P.; Martins, R. Performances exhibited by large area ITO layers produced by r.f. Magnetron sputtering. Thin Solid Films 1999, 337, 171–175. [Google Scholar] [CrossRef]
- Krebs, F.C.; Norrman, K. Analysis of the failure mechanism for a stable organic photovoltaic during 10000 h of testing. Prog. Photovolt. 2007, 15, 697–712. [Google Scholar] [CrossRef]
- De Jong, M.P.; van Ijzendoorn, L.J.; de Voigt, M.J.A. Stability of the interface between indium-tin-oxide and poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) in polymer light-emitting diodes. Appl. Phys. Lett. 2000, 77, 2255–2257. [Google Scholar] [CrossRef]
- Greczynski, G.; Kugler, T.; Keil, M.; Osikowicz, W.; Fahlman, M.; Salaneck, W.R. Photoelectron spectroscopy of thin films of PEDOT–PSS conjugated polymer blend: A mini-review and some new results. J. Electron Spectros. Relat. Phenomena 2001, 121, 1–17. [Google Scholar] [CrossRef]
- Wong, K.W.; Yip, H.L.; Luo, Y.; Wong, K.Y.; Lau, W.M.; Low, K.H.; Chow, H.F.; Gao, Z.Q.; Yeung, W.L.; Chang, C.C. Blocking reactions between indium-tin oxide and poly(3,4-ethylene dioxythiophene): Poly(styrene sulphonate) with a self-assembly monolayer. Appl. Phys. Lett. 2002, 80, 2788–2790. [Google Scholar] [CrossRef]
- Şahin, Y.; Alem, S.; de Bettignies, R.; Nunzi, J.-M. Development of air stable polymer solar cells using an inverted gold on top anode structure. Thin Solid Films 2005, 476, 340–343. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Krebs, F.C.; Gevorgyan, S.A.; Alstrup, J. A roll-to-roll process to flexible polymer solar cells: Model studies, manufacture and operational stability studies. J. Mater. Chem. 2009, 19, 5442–5451. [Google Scholar] [CrossRef]
- Gaspar, D.; Pereira, L.; Gehrke, K.; Galler, B.; Fortunato, E.; Martins, R. High mobility hydrogenated zinc oxide thin films. Sol. Energy Mater. Sol. Cells 2017, 163, 255–262. [Google Scholar] [CrossRef]
- Lyubchyk, A.; Vicente, A.; Soule, B.; Alves, P.U.; Mateus, T.; Mendes, M.J.; Águas, H.; Fortunato, E.; Martins, R. Mapping the electrical properties of ZnO based transparent conductive oxides grown at room temperature and improved by controlled postdeposition annealing. Adv. Electron. Mater. 2016, 2, 1500287. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. High efficiency double heterojunction polymer photovoltaic cells using highly ordered TiO2 nanotube arrays. Appl. Phys. Lett. 2007, 91, 152111. [Google Scholar] [CrossRef]
- Steim, R.; Choulis, S.A.; Schilinsky, P.; Brabec, C.J. Interface modification for highly efficient organic photovoltaics. Appl. Phys. Lett. 2008, 92, 093303. [Google Scholar] [CrossRef]
- White, M.S.; Olson, D.C.; Shaheen, S.E.; Kopidakis, N.; Ginley, D.S. Inverted bulk-heterojunction organic photovoltaic device using a solution-derived zno underlayer. Appl. Phys. Lett. 2006, 89, 143517. [Google Scholar] [CrossRef]
- Yu, B.Y.; Tsai, A.; Tsai, S.P.; Wong, K.T.; Yang, Y.; Chu, C.W.; Shyue, J.J. Efficient inverted solar cells using TiO2 nanotube arrays. Nanotechnology 2008, 19, 255202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.H.; Lee, H.H.; Lee, K.; Ma, W.; Gong, X.; Heeger, A.J. New architecture for high-efficiency polymer photovoltaic cells using solution-based titanium oxide as an optical spacer. Adv. Mater. 2006, 18, 572–576. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.Y.; Park, S.H.; Kim, S.H.; Cho, S.; Heeger, A.J. Air-stable polymer electronic devices. Adv. Mater. 2007, 19, 2445–2449. [Google Scholar] [CrossRef]
- Yang, D.; Fu, P.; Zhang, F.; Wang, N.; Zhang, J.; Li, C. High efficiency inverted polymer solar cells with room-temperature titanium oxide/polyethylenimine films as electron transport layers. J. Mater. Chem. A 2014, 2, 17281–17285. [Google Scholar] [CrossRef]
- Yeonsong, M. Enhancement of photovoltaic characteristics using a pedot interlayer in TiO2/mehppv heterojunction devices. Sol. Energy Mater. Sol. Cells 2005, 85, 31–39. [Google Scholar] [CrossRef]
- Beek, W.J.E.; Wienk, M.M.; Janssen, R.A.J. Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv. Mater. 2004, 16, 1009–1013. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheun, H.; Potscavage, J.W.J.; Fuentes-Hernandez, C.; Kim, S.-J.; Kippelen, B. Inverted organic solar cells with ito electrodes modified with an ultrathin Al2O3 buffer layer deposited by atomic layer deposition. J. Mater. Chem. 2010, 20, 6189–6194. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Lee, K.-T.; Huang, W.-K.; Siao, H.-Y.; Chang, Y.-C. High-performance, air-stable, low-temperature processed semitransparent perovskite solar cells enabled by atomic layer deposition. Chem. Mater. 2015, 27, 5122–5130. [Google Scholar] [CrossRef]
- Sun, Z.-P.; Liu, L.; Zhang, L.; Jia, D.-Z. Rapid synthesis of ZnO nano-rods by one-step, room-temperature, solid-state reaction and their gas-sensing properties. Nanotechnology 2006, 17, 2266–2270. [Google Scholar] [CrossRef]
- Jo, S.B.; Lee, J.H.; Sim, M.; Kim, M.; Park, J.H.; Choi, Y.S.; Kim, Y.; Ihn, S.-G.; Cho, K. High performance organic photovoltaic cells using polymer-hybridized ZnO nanocrystals as a cathode interlayer. Adv. Energy Mater. 2011, 1, 690–698. [Google Scholar] [CrossRef]
- Liu, J.; Shao, S.; Meng, B.; Fang, G.; Xie, Z.; Wang, L.; Li, X. Enhancement of inverted polymer solar cells with solution-processed ZnO-TiOx composite as cathode buffer layer. Appl. Phys. Lett. 2012, 100, 213906. [Google Scholar] [CrossRef]
- Lee, Y.J.; Wang, J.; Cheng, S.R.; Hsu, J.W. Solution processed ZnO hybrid nanocomposite with tailored work function for improved electron transport layer in organic photovoltaic devices. ACS Appl. Mater. Interfaces 2013, 5, 9128–9133. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zheng, K.; Pullerits, T.; Zhang, F. Enhanced performance of inverted polymer solar cells by using poly(ethylene oxide)-modified ZnO as an electron transport layer. ACS Appl. Mater. Interfaces 2013, 5, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Jagadamma, L.K.; Abdelsamie, M.; El Labban, A.; Aresu, E.; Ngongang Ndjawa, G.O.; Anjum, D.H.; Cha, D.; Beaujuge, P.M.; Amassian, A. Efficient inverted bulk-heterojunction solar cells from low-temperature processing of amorphous zno buffer layers. J. Mater. Chem. A 2014, 2, 13321–13331. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Shao, S.; Deng, Y.; Meng, B.; Xie, Z.; Geng, Y.; Wang, L.; Zhang, F. Printable highly conductive conjugated polymer sensitized zno ncs as cathode interfacial layer for efficient polymer solar cells. ACS Appl. Mater. Interfaces 2014, 6, 8237–8245. [Google Scholar] [CrossRef] [PubMed]
- Kamalasanan, M.N.; Chandra, S. Sol-gel synthesis of ZnO thin films. Thin Solid Films 1996, 288, 112–115. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Sun, X.W.; Jiang, C.Y.; Lo, G.Q.; Zhao, D.W.; Kwong, D.L. An inverted organic solar cell employing a sol-gel derived ZnO electron selective layer and thermal evaporated MoO3 hole selective layer. Appl. Phys. Lett. 2008, 93, 221107. [Google Scholar] [CrossRef]
- Krebs, F.C.; Thomann, Y.; Thomann, R.; Andreasen, J.W. A simple nanostructured polymer/ ZnO hybrid solar cell-preparation and operation in air. Nanotechnology 2008, 19, 424013. [Google Scholar] [CrossRef] [PubMed]
- Courtright, B.A.; Jenekhe, S.A. Polyethylenimine interfacial layers in inverted organic photovoltaic devices: Effects of ethoxylation and molecular weight on efficiency and temporal stability. ACS Appl. Mater. Interfaces 2015, 7, 26167–26175. [Google Scholar] [CrossRef] [PubMed]
- Lampande, R.; Kim, G.W.; Pode, R.; Kwon, J.H. Effectiveness of a polyvinylpyrrolidone interlayer on a zinc oxide film for interfacial modification in inverted polymer solar cells. RSC Adv. 2014, 4, 49855–49860. [Google Scholar] [CrossRef]
- Woo, S.; Hyun Kim, W.; Kim, H.; Yi, Y.; Lyu, H.-K.; Kim, Y. 8.9% single-stack inverted polymer solar cells with electron-rich polymer nanolayer-modified inorganic electron-collecting buffer layers. Adv. Energy Mater. 2014, 4, 1301692. [Google Scholar] [CrossRef]
- Liao, S.H.; Jhuo, H.J.; Cheng, Y.S.; Chen, S.A. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-TH) for high performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-M.; Leu, C.-Y. Conjugated polyelectrolyte and zinc oxide stacked structure as an interlayer in highly efficient and stable organic photovoltaic cells. J. Mater. Chem. A 2013, 1, 6446–6451. [Google Scholar] [CrossRef]
- Yu, W.; Huang, L.; Yang, D.; Fu, P.; Zhou, L.; Zhang, J.; Li, C. Efficiency exceeding 10% for inverted polymer solar cells with a ZnO/ionic liquid combined cathode interfacial layer. J. Mater. Chem. A 2015, 3, 10660–10665. [Google Scholar] [CrossRef]
- Chen, H.C.; Lin, S.W.; Jiang, J.M.; Su, Y.W.; Wei, K.H. Solution-processed zinc oxide/polyethylenimine nanocomposites as tunable electron transport layers for highly efficient bulk heterojunction polymer solar cells. ACS Appl. Mater. Interfaces 2015, 7, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.H.; Jhuo, H.J.; Yeh, P.N.; Cheng, Y.S.; Li, Y.L.; Lee, Y.H.; Sharma, S.; Chen, S.A. Single junction inverted polymer solar cell reaching power conversion efficiency 10.31% by employing dual-doped zinc oxide nano-film as cathode interlayer. Sci. Rep. 2014, 4, 6813. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Gutacker, A.; Sun, Y.; Wu, H.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A.J.; Bazan, G.C. Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J. Am. Chem. Soc. 2011, 133, 8416–8419. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Cheng, Y.-J.; Li, P.-J.; Chen, C.-H.; Dubosc, M.; Liang, R.-M.; Hsu, C.-S. Highly efficient and stable inverted polymer solar cells integrated with a cross-linked fullerene material as an interlayer. J. Am. Chem. Soc. 2010, 132, 4887–4893. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, Q.D.; Xiao, J.; Xue, Q.; Li, H.W.; Guan, Z.; Yip, H.L.; Tsang, S.W. Decomposition of organometal halide perovskite films on zinc oxide nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19986–19993. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chin, C.Y.; Chen, T.Y.; Hsieh, S.N.; Lee, C.H.; Guo, T.F.; Jen, A.K.Y.; Wen, T.C. Enhanced performance of polymer solar cells using solution-processed tetra-n-alkyl ammonium bromides as electron extraction layers. J. Mater. Chem. A 2013, 1, 2582–2587. [Google Scholar] [CrossRef]


| EEL | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (AVG) (%) * | RS (Ω) |
|---|---|---|---|---|---|
| Pristine ZnO | 0.80 | 14.49 | 63.03 | 7.31 | 145.59 |
| 1 wt % CTAB | 0.81 | 14.52 | 67.66 | 7.93 | 139.34 |
| 2 wt % CTAB | 0.82 | 16.23 | 67.65 | 8.99 | 102.94 |
| 3 wt % CTAB | 0.82 | 16.38 | 67.51 | 9.07 | 105.90 |
| 4 wt % CTAB | 0.81 | 15.47 | 67.62 | 8.48 | 148.26 |
| 5 wt % CTAB | 0.81 | 15.45 | 66.97 | 8.36 | 138.75 |
| 6 wt % CTAB | 0.81 | 15.44 | 66.82 | 8.34 | 140.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-K.; Sivashanmugan, K.; Guo, T.-F.; Wen, T.-C. Enhancement of Inverted Polymer Solar Cells Performances Using Cetyltrimethylammonium-Bromide Modified ZnO. Materials 2018, 11, 378. https://doi.org/10.3390/ma11030378
Wu C-K, Sivashanmugan K, Guo T-F, Wen T-C. Enhancement of Inverted Polymer Solar Cells Performances Using Cetyltrimethylammonium-Bromide Modified ZnO. Materials. 2018; 11(3):378. https://doi.org/10.3390/ma11030378
Chicago/Turabian StyleWu, Chung-Kai, Kundan Sivashanmugan, Tzung-Fang Guo, and Ten-Chin Wen. 2018. "Enhancement of Inverted Polymer Solar Cells Performances Using Cetyltrimethylammonium-Bromide Modified ZnO" Materials 11, no. 3: 378. https://doi.org/10.3390/ma11030378




