1. Introduction
Polymeric fibres have been widely explored as scaffolds in tissue engineering applications and regenerative medicine. They offer the potential to mimic the architecture of extracellular matrix (ECM), which comprises a fibrous network [
1,
2]. A scaffold ideally satisfies requirements including a porous structure with interconnected pores suitable for cell growth and exchange of nutrients, biocompatibility, and mechanical properties similar to the substituted tissue [
3,
4,
5]. Furthermore, scaffold structure dictates cell growth and matrix deposition for tissue formation [
6,
7,
8,
9]. Therefore, it is important for a scaffold design to mimic the structure and properties of the native tissue.
Ultra-fine fibres can be fabricated using electrospinning, which is versatile and efficient for producing non-woven networks with fibre diameters ranging from 3 nm to 5 µm [
10]. This technique has been broadly explored for numerous engineering purposes, including filtration, protective clothing, optical electronics, solar sails, light sails, mirrors for use in space, pesticides, reinforced composites, and biomedical devices [
10,
11,
12,
13]. Wound dressings, drug delivery systems, tissue engineering, and structural elements in artificial organs are some examples of electrospun fibres in medical applications. Using electrospinning, fibrous structures can be tailored to possess unique characteristics, such as high surface area, extreme length up to kilometres in magnitude, and alignment on a molecular level [
14]. Hence, the development of fibrous scaffolds for tissue engineering could benefit from the electrospinning technique.
Recent studies on electrospinning for tissue engineering application included the use of electrospun fibres combined with other materials. Composite of hydrogel/electrospun collagen scaffold was fabricated to mimic native extracellular matrix for meniscus tissue regeneration [
15]. Electrospun meshes of poly(lactic-co-glycolic acid) and nanohydroxyapatite were modified using poly(allylamine hydrochloride) and poly(sodium 4-styrenesulfonate) as polyelectrolytes through layer-by-layer assembly, followed by peptides incorporation, to stimulate bone healing [
16]. Layer-by-layer coating of chitosan and collagen was also introduced onto co-electrospun PCL/cellulose acetate nanofibrous matrix for wound healing application [
17]. Alternating process of PCL electrospinning and inkjet printing of chondrocytes suspended in a fibrin–collagen hydrogel was developed to fabricate a construct for cartilage tissue engineering [
18].
In the process of product development, including that of biomedical products and devices, quality by design and manufacturing process takes an important role. This approach was recommended by the FDA (Food and Drug Administration) and ISO 13485 (international standard of quality management systems for medical devices) [
19,
20]. Quality is defined as product ability to meet customer satisfaction and these requirements of quality need to be translated into specification [
21]. Quality characteristics of a product are dependent on multivariate processing variables. For this, a designed experiment offers a strategy to simultaneously investigate multiple variables defining process or product quality. This approach is obviously more efficient compared to a one-factor-at-a-time strategy [
22]. There are several techniques in the design of experiments, including factorial, robust parameter design (Taguchi’s method), and the response surface method (RSM) [
22]. This study will be focused on the latter.
Response surface methodology is a collection of statistical design and numerical optimization techniques used to optimize a process and product design, and has become the core of industrial experimentation [
23]. Gaining advantage from computer technology and software development, RSM provides techniques for reducing variance and for process improvement [
24]. RSM offers potential benefits compared to other optimization techniques, for example shorter computational modelling and ability to suggest straightforward optimization with comparably high accuracy [
25]. Using this method, which mostly is using either first-order or second-order polynomial models for function estimation, an empirical relationship between independent variables and one or more response variables is obtained [
26]. Users of the RSM approach have broadened, from chemicals, foods, and manufacturing, to biological, biomedical, and biopharmaceutical. It has even been considered as the standard for optimization experiments, both in laboratory and industrial settings [
27]. The application of RSM on process and product optimization has been reported in literature. RSM was used with multicriteria decision analysis to optimize the production of vancomycin nanoparticles to achieve desired particle size and encapsulation efficiency [
27]. On optical fibre coating, RSM was utilized to build prediction model and subsequently optimize the process, resulting in the improved contraction rate of the outer coating [
28]. In the area of biotechnology, RSM was applied to optimize product formulation and operating conditions in many cases, for example to find the important components for cells medium and their optimum amount to improve microbial transglutaminase (MTGase) activity [
19].
In this study, a scaffold for acetabular labrum implant was developed from electrospun polycaprolactone (PCL) fibres and the properties emphasized were mechanical properties and fibre diameter. This device is proposed to aid recovery in labral injury, as well as an alternative treatment for labral reconstruction. Electrospinning is a process that involves multivariate factors determining the properties of resultant fibres. In general, they can be grouped into three parameters: solution properties, processing variables, and ambient parameters [
12,
29]. Solution properties include viscosity, conductivity, surface tension, molecular weight of the polymer, and dielectric constant. Control factors comprise flow rate, electric field, tip to collector distance, needle tip design, and collector material and geometry. Ambient parameters, such as temperature, humidity, and air velocity may also influence the result. Combinations of these factors determine fibre diameter, uniformity (beading formation), and alignment [
7,
12,
30,
31,
32,
33]. Simultaneously, mechanical properties will also be affected.
In investigating electrospun fibre properties with respect to the multivariate nature of electrospinning, the RSM approach has been applied in numerous electrospinning studies of various materials [
26,
32,
34,
35,
36,
37,
38,
39,
40,
41]. These investigations demonstrated that this technique could develop a predictive model along with confirmation of validity. Furthermore, optimum settings to achieve the desired output or response can be obtained [
34,
37]. Mostly, fibre diameter was the response under investigation. Although these studies were conducted using different materials and experimental conditions, they appeared to be in good agreement, in which the most influential factors for fibre diameter are material content or concentration [
26,
32,
34,
36,
37,
38,
39,
40] and voltage [
26,
32,
34,
35,
36,
37,
40]. Only a few studies examined the effect on mechanical properties. Optimization was also developed only for a single response. When different materials and experimental conditions are involved, the results obtained are usually specific to the case under study. For different cases, particular designs of experiments need to be developed.
This present study explored the use of RSM in PCL electrospinning to investigate the effect of parameter settings on fibres properties. Parameters under investigation were solution concentration, flow rate, collector to needle distance, solution temperature, and mandrel rotation speed. Furthermore, this paper will report the use of RSM to find optimum parameter setting combinations to simultaneously achieve desired multiple responses. To the author’s knowledge, there are currently no literature reports on optimization of electrospinning parameter for multiple responses. Two responses representing quality of fibrous scaffolds were examined: fibre diameter and elastic modulus. Optimization then followed to achieve the desired quality, which was defined by minimum fibre diameter and an elastic modulus of 25 MPa. The smallest diameter is preferred since it can provide a higher surface area, allowing more binding sites for cell adhesion [
42]. A target value of 25 MPa for elastic modulus was preferred so as to match the mechanical properties of human acetabular labrum [
43].
3. Discussion
The relationship between a series of electrospinning parameters and their outcomes has been investigated and modelled using RSM, along with factors that impact output characteristics. For fibre diameter, solution concentration was the only significant factor. Secondly, solution concentration, flow rate, temperature, collector rotation speed, and interaction between concentration and temperature were the influential factors for the fibre elastic modulus. The fibre diameter model showed relatively low predicted R2 (31.77%), suggesting that it should be exploited more carefully for predicting future data, by considering possible sources of variance. However, the predicted R2 (78.20%) for fibre stiffness was reasonably high and could be used more confidently.
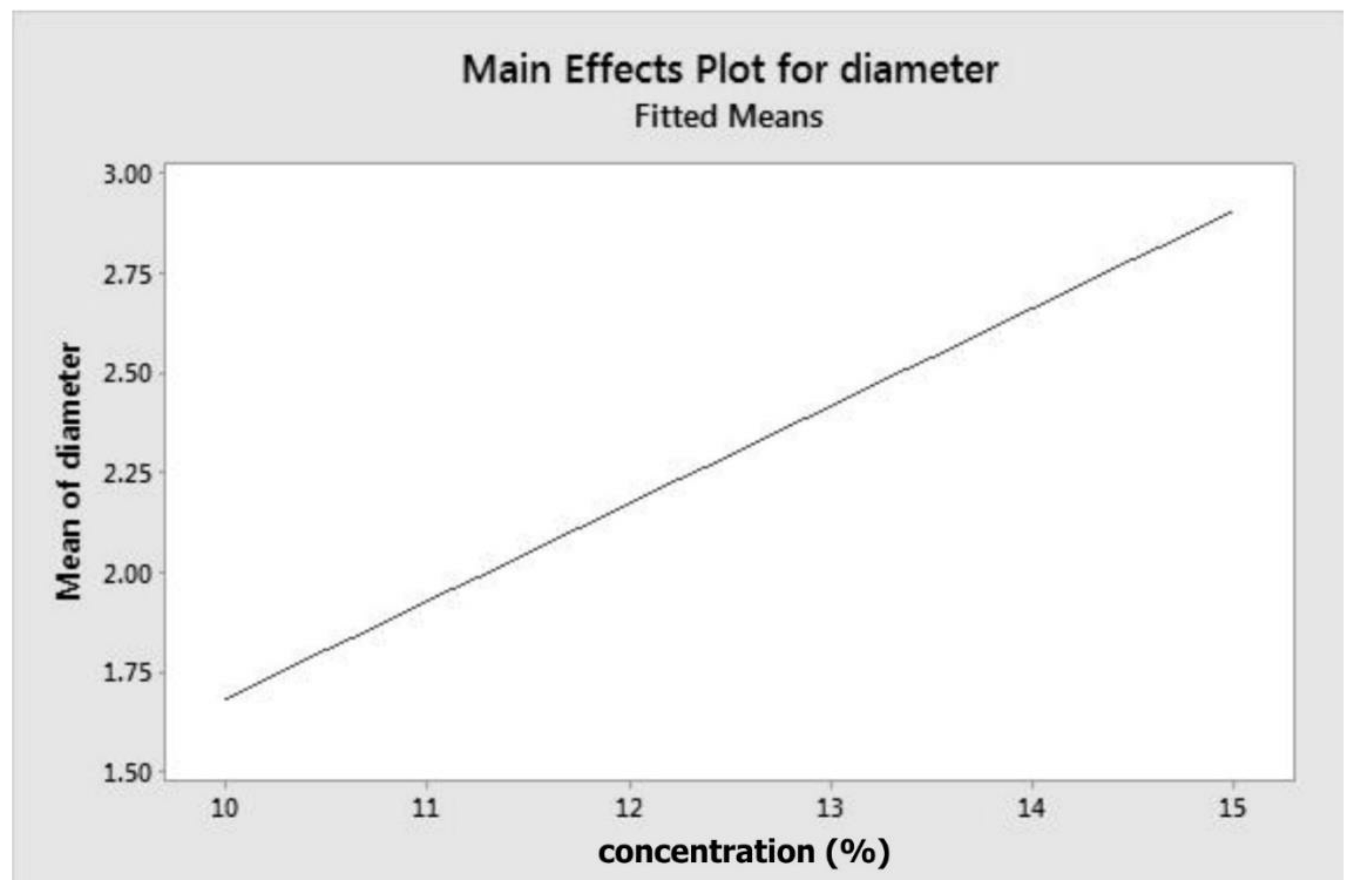
Solution concentration and fibre diameter appeared to have a positive correlation. The increase in fibre thickness following the increasing concentration was documented in the literature, both in electrospun PCL and other polymers [
26,
30,
32,
34,
38,
39,
40,
41,
45,
48,
49,
50,
51,
52,
53]. It proved to be one of the most influential factors regarding fibre diameter [
34]. Higher solution concentration led to more significant chain entanglement [
32]. Consequently, it possessed higher viscoelasticity to resist elongation from electrostatic forces. At high concentration, viscoelastic force overcame surface tension, contributing to thicker fibre formation but less beading or droplets [
41,
48,
54]. The relation between concentration and fibre diameter could be either quadratic or linear [
41,
49]. Additionally, there was also a certain concentration limit above which fibres could not be formed at all [
51,
53].
Regarding mechanical properties, the positive impact of solution concentration was also confirmed by several studies [
26,
35,
39]. The higher modulus obtained was associated with the aligned polymer chain along the fibre axis [
26]. Polymer solutions with lower concentration might lack effective chain entanglements, which are required for the extension of main chain segments without slippage and molecular relaxation effects [
55]. Furthermore, mechanical properties of polymer and electrospun polymer fibres were related to chain ordering or crystallinity [
56,
57,
58,
59].
Investigations of temperature effect on elastic modulus appear to be rarely reported compared to those on fibre morphology. The elevation of temperature generally reduced fibre diameter, through molecule expansion, reduction in chain entanglements, viscosity, and surface tension, as well as increased elongation [
12,
60,
61,
62,
63,
64,
65]. High thermal application could also decrease fibre crystallinity by accelerating solvent evaporation, thus prohibiting chain ordering [
62]. As mechanical properties were correlated to chain entanglement and alignment, it is possible that raising temperature can reduce fibre modulus of elasticity [
26,
55].
In PCL electrospinning, temperature showed interdependency with concentration on molecular orientation. It was reported that, in all concentrations, molecular alignment was improved from a temperature of 25 to 35 °C, but was then decreased after further increase to 40 °C. Increased temperature helped chain relaxation, thus reducing molecular orientation and crystallinity [
64]. In another study, the drawing process of gravity-spun PCL fibre at room temperature involved breakdown and unfolding of crystalline units, as well as extension of amorphous segments [
55]. This extension was eased by the low glass transition temperature (T
g) of PCL, which enables high chain mobility at room temperature. This probably explains why, in this present study, concentration had almost no effect on fibre elasticity at the higher temperature (45 °C), as the chain was already relaxed, thus there was no increase in crystallinity. In electrospinning at room temperature, the chain mobility was probably already facilitated along with slower solvent evaporation, thus inducing improved crystallinity and modulus compared to the higher temperature.
It is also likely that the interaction between temperature and concentration was related to viscosity. Temperature and concentration may have opposite effects on it. Increasing temperature would reduce viscosity, while higher concentration would increase viscosity [
55]. Moreover, viscosity was also dependent on temperature, based on Arrhenius-type activation energy [
64]. According to the equation, there was a stronger influence of temperature on solution viscosity at higher concentration, as well as a stronger effect of concentration on viscosity at lower temperature. On the other hand, mechanical properties of electrospun fibre were positively correlated to solution concentration [
26,
35,
39]. This further confirms the interaction found in this study. As depicted in
Figure 3 and
Figure 4, the difference in fibre modulus at different temperatures was more pronounced at higher concentration, whereas the increase in fibre modulus due to concentration was higher at the lower temperature. This suggests that there might be a correlation between solution viscosity and fibre modulus.
The corresponding increase of fibre modulus with respect to flow rate can be seen to be associated with the increasing shear rate. In the case of dry-spinning of PCL, higher shear stress and shear rate improved chain orientation, which corresponded to the higher mechanical properties, until a critical level was reached [
39]. Meanwhile, the increase of fibre modulus following higher speed of the rotating collector was also confirmed, in which fibre orientation and point bonding had a role [
66].
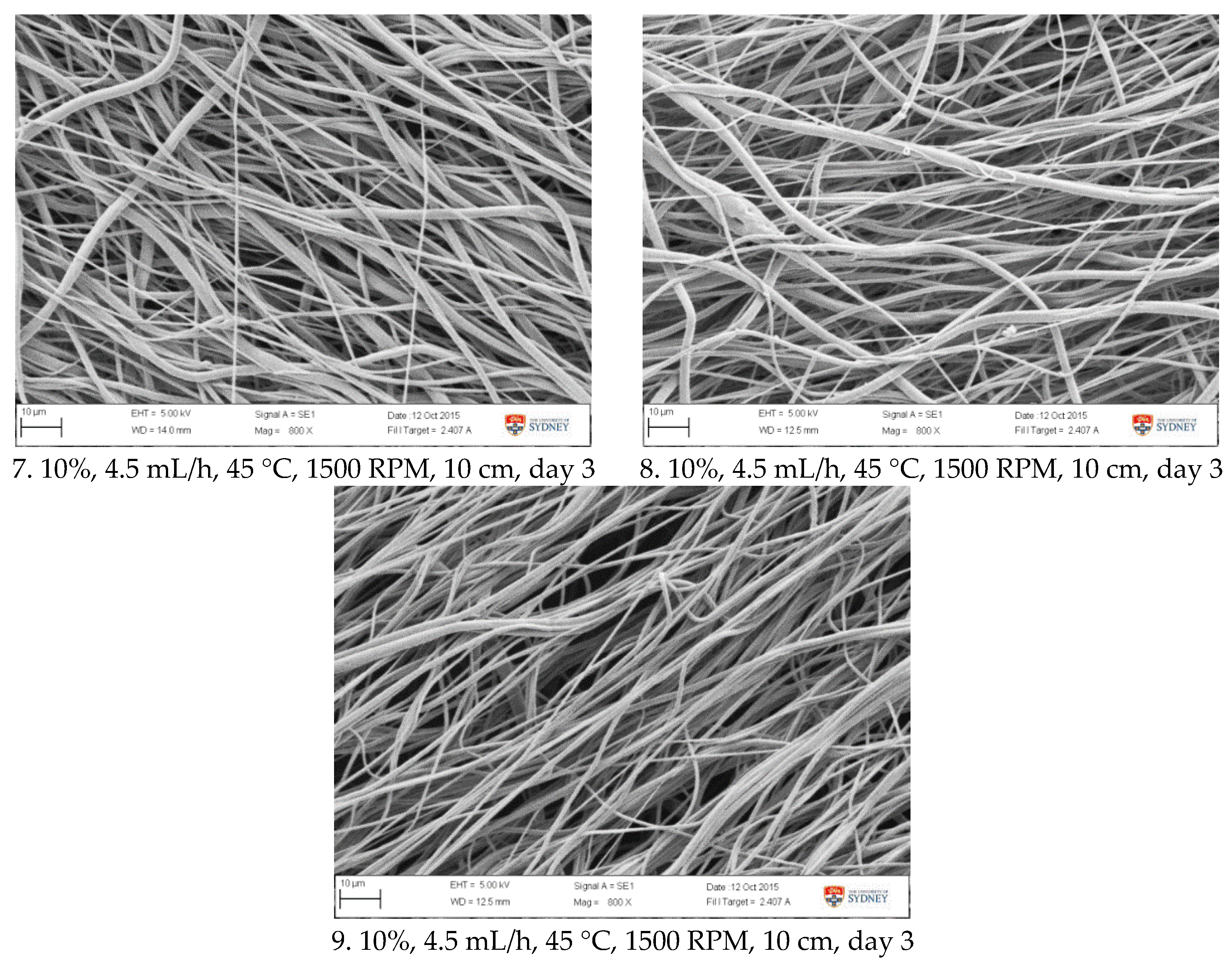
Applying RSM and optimization procedure, an optimised fibrous PCL biomaterial suitable for synthetic acetabular labrum scaffold has been developed. It had an elastic modulus of 25 MPa with and average fibre diameter of about 1.5 µm. The modulus of the obtained fibre was able to mimic that of acetabular labrum [
43], and therefore was potentially suitable as a scaffold to aid labral recovery. Fibril thickness obtained was the smallest possible to be produced using this system, and yet was still capable of accommodating the target value of the elastic modulus. This fibrous morphology can potentially provide a microenvironment that supports cell growth. SEM images (
Figure 8) showed that the fibres were reasonably well aligned while providing a sufficient pore volume. Uniformly arranged fibres are reportedly more favourable for cell alignment, proliferation, and extracellular matrix production [
33,
67]. Meanwhile, pore volume between fibres can facilitate cell infiltration. For the broader context, RSM is also applicable for the development of scaffolds for tissue engineering applications. Once the ideal scaffold parameters have been specified, the various electrospinning parameters can be studied and optimized to achieve a scaffold with the desired properties, in a relatively efficient and straightforward manner.