Electric and Hydraulic Properties of Carbon Felt Immersed in Different Dielectric Liquids
Abstract
:1. Introduction
2. Methodology of Experimental Research
2.1. Characteristics of CF
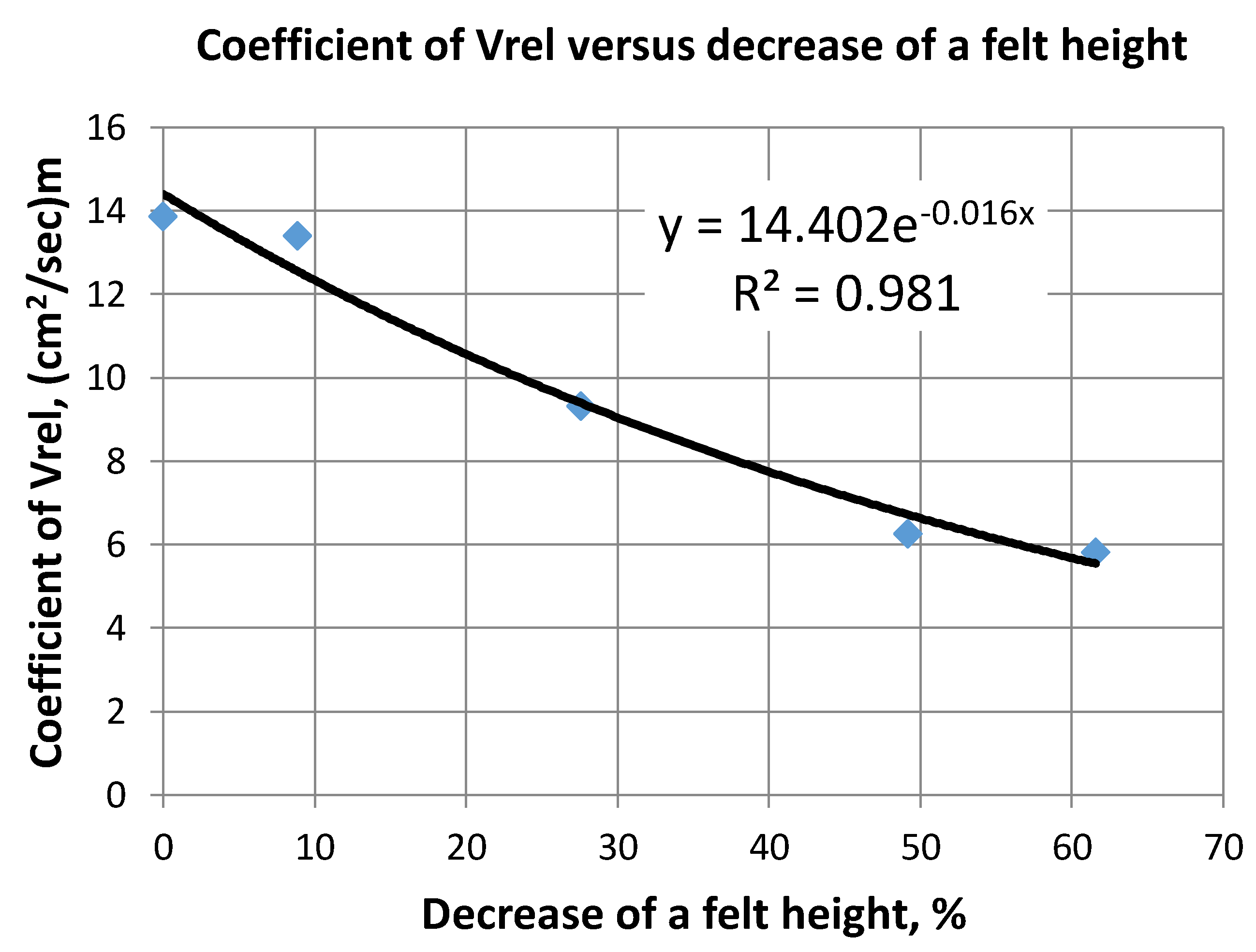
2.2. Carbon Felt Hydrodynamic Permeability
2.3. Electrical Conductivity
3. Conclusions
- CF material is widely used for electrodes in different electrochemical applications. All of them can be characterized by the presence of electrode reactions in the electrolyte flow. Therefore, the knowledge of electrical and hydraulic properties of CF electrodes has enormous importance for development and exploitation of these electrochemical facilities. New vanadium redox batteries, electrostatic desalination units, and the like could not be optimally designed without such information.
- The CF framework is represented by a complex pattern of stochastically bundled thin carbon fibers, which to some extent is similar to the porous media, for which pure theoretical approaches have sufficiently low efficiency. Therefore, assessment of hydraulic and electrical CF parameters was carried out in an experimental trial, ensuring good quantitative analytical approximation of obtained results.
- Hydraulic parameters for each porous medium can be determined by permeability or hydraulic resistivity. Both determine the requirements for pumping equipment and define the pumping specifications for providing electrolyte flow to electrodes. Therefore, they determine the hydraulic efficiency of flow cells with CF electrodes.
- It should be emphasized that hydraulic resistance (as opposed to permeability) was tested using water due to safety reasons. However, for other liquids (including different electrolyte solutions), the hydraulic resistance or permeability could be recalculated to consider their actual density and viscosity.
- Hydraulic resistance was found to be linearly proportional to the applied pressure (water head) and drops proportionally to the area of a compressed felt. A special approximating expression for the determination electrolyte flow through differently compression factors of CF was established. This analytical representation can improve the development of further electrochemical applications.
- The electrical (electronic) conductivity of CF plays a crucial role for electrons to reach the surface of the electrodes and to participate in reactions. Also, electrical resistivity (the inverse of electrical conductivity) plays a significant role in generating electric losses, which are converted to heat, causing temperature problems. In addition, these losses decrease the electrical efficiency of the device.
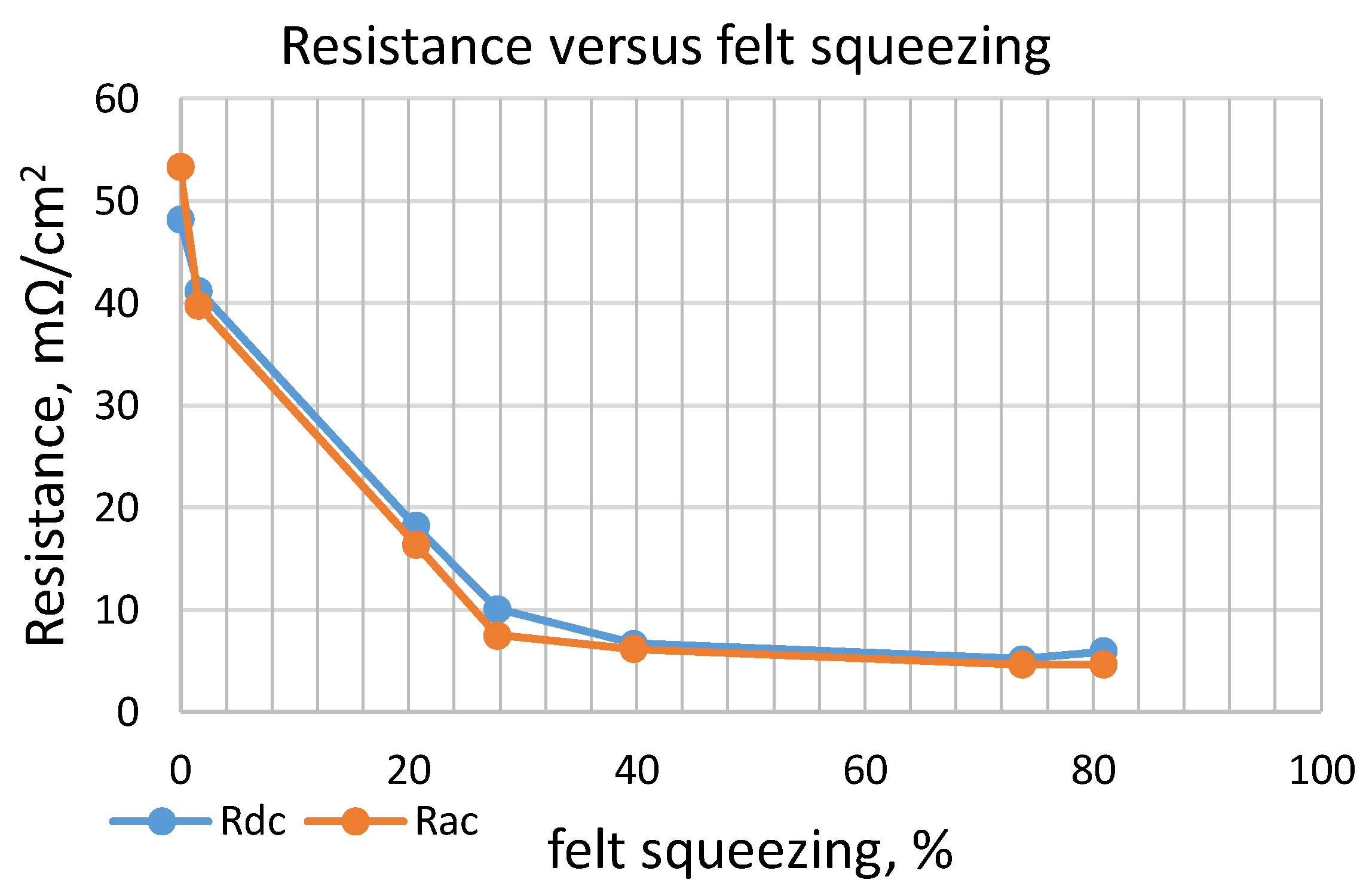
- Electronic resistivity was measured by a special device, applying for this purpose DC and AC currents with different frequencies (100 Hz, 120 Hz, 1 kHz, and 10 kHz). Taking into account the requirement to verify the influence of dielectric liquid parameters on CF conductivity, four sets of tests were performed. Resistance was measured in dry conditions, and also with the CF immersed in different non-conducting (dielectric) liquids: glycerol, alcohol, and cyclohexane. Usage of dielectric liquids instead of real electrolytes was justified in order to prevent the influence of electrolyte ionic conductivity on the measurement results.
- It was observed that electrical resistivity was diminished during felt compression, having a non-linear relationship to volume decrease similar to the negative exponential function. In the initial stage of volume decrease, the resistance drops quickly. However, after 60–80% of its initial value, additional compression has a negligible effect on conductivity.
- Electrical conductivity moderately depends on liquid permittivity properties. The dielectric constant (ε), among other liquid parameters, obviously has a major influence on the quantity of interconnection between carbon filaments and electrodes. An increase in ε causes a slight improvement in CF conductivity.
- It seems to be important to continue the present work for finding solid theoretical explanations of all observed experimental data.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Fialkov, A. Carbon application in chemical power sources. Rus. J. Electrochem. 2000, 36, 345–366. [Google Scholar] [CrossRef]
- Gözmen, B.; Oturan, M.A.; Oturan, N.; Erbatur, O. Indirect electrochemical treatment of bisphenol A in water via electrochemically generated Fenton’s reagent. Environ. Sci. Technol. 2003, 37, 3716–3723. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, J.; Bonete, P.; Exposito, E.; Montiel, V.; Aldaz, A.; Torregrosa-Maciá, R. Characterization of a carbon felt electrode: Structural and physical properties. J. Mater. Chem. 1999, 9, 419–426. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.; Zhao, P.; Yi, B. A comparative study of carbon felt and activated carbon based electrodes for sodium polysulfide/bromine redox flow battery. Electrochim. Acta 2006, 51, 6304–6312. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X. Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery. Electrochim. Acta 2007, 52, 6755–6762. [Google Scholar] [CrossRef]
- Li, X.; Huang, K.; Liu, S.; Ning, T.; Chen, L. Characteristics of graphite felt electrode electrochemically oxidized for vanadium redox battery application. Trans. Nonferrous Met. Soc. China 2007, 17, 195–199. [Google Scholar] [CrossRef]
- Joerissen, L.; Garche, J.; Fabjan, C.; Tomazic, G. Possible use of vanadium redox-flow batteries for energy storage in small grids and stand-alone photovoltaic systems. J. Power Sources 2004, 127, 98–104. [Google Scholar] [CrossRef]
- Yue, L.; Li, W.; Sun, F.; Zhao, L.; Xing, L. Highly hydroxylated carbon fibers as electrode materials of all-vanadium redox flow battery. Carbon 2010, 48, 3079–3090. [Google Scholar] [CrossRef]
- Rahman, F.; Skyllas-Kazacos, M. Vanadium redox battery: Positive half-cell electrolyte studies. J. Power Sources 2009, 189, 1212–1219. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, H.; Zhou, H.; Chen, J.; Gao, S.; Yi, B. Characteristics and performance of 10 kW class all-vanadium redox-flow battery stack. J. Power Sources 2006, 162, 1416–1420. [Google Scholar] [CrossRef]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–162. [Google Scholar] [CrossRef]
- You, D.; Zhang, H.; Chen, J. A simple model for the vanadium redox battery. Electrochim. Acta 2009, 54, 6827–6836. [Google Scholar] [CrossRef]
- Rezk, K.; Forsberg, J.; Nilsson, L.; Berghel, J. Characterizing flow resistance in 3-dimensional disordered fibrous structures based on forchheimer coefficients for a wide range of reynolds numbers. Appl. Math. Model. 2016, 40, 8898–8911. [Google Scholar] [CrossRef]
- Oh, K.; Won, S.; Ju, H. Numerical study of the effects of carbon felt electrode compression in all-vanadium redox flow batteries. Electrochim. Acta 2015, 181, 13–23. [Google Scholar] [CrossRef]
- Chen, L.; He, Y.; Tao, W.; Zelenay, P.; Mukundan, R.; Kang, Q. Pore-scale study of multiphase reactive transport in fibrous electrodes of vanadium redox flow batteries. Electrochim. Acta 2017, 248, 425–439. [Google Scholar] [CrossRef]
- Shou, D.; Fan, J.; Ding, F. A difference-fractal model for the permeability of fibrous porous media. Phys. Lett. A 2010, 374, 1201–1204. [Google Scholar] [CrossRef]
- Shou, D.; Fan, J.; Ding, F. Hydraulic permeability of fibrous porous media. Int. J. Heat Mass Transf. 2011, 54, 4009–4018. [Google Scholar] [CrossRef]
- Xiao, F.; Yin, X. Geometry models of porous media based on voronoi tessellations and their porosity–permeability relations. Comput. Math. Appl. 2016, 72, 328–348. [Google Scholar] [CrossRef]
- Chugh, R.; Chung, D.D.L. Flexible graphite as a heating element. Carbon 2002, 40, 2285–2289. [Google Scholar] [CrossRef]
- Lee, M.; Chen, L.; He, Z.; Yang, S. The development of a heterogeneous composite bipolar plate of a proton exchange membrane fuel cell. J. Electrochem. Energy Convers. Storage 2005, 2, 14–19. [Google Scholar] [CrossRef]
- Qian, P.; Zhang, H.; Chen, J.; Wen, Y.; Luo, Q.; Liu, Z.; You, D.; Yi, B. A novel electrode-bipolar plate assembly for vanadium redox flow battery applications. J. Power Sources 2008, 175, 613–620. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Walsh, F.C.; Nava, J.L.; de León, C.P. Graphite felt as a versatile electrode material: Properties, reaction environment, performance and applications. Electrochim. Acta 2017, 258, 1115–1139. [Google Scholar] [CrossRef]
- Chang, T.; Zhang, J.; Fuh, Y. Electrical, mechanical and morphological properties of compressed carbon felt electrodes in vanadium redox flow battery. J. Power Sources 2014, 245, 66–75. [Google Scholar] [CrossRef]
- Choe, J.; Kim, K.H.; Lee, D.G. Corrugated carbon/epoxy composite bipolar plate for vanadium redox flow batteries. Compos. Struct. 2015, 119, 534–542. [Google Scholar] [CrossRef]
- Available online: http://www.graphite-eng.com (accessed on 3 July 2017).
- Averbukh, M.; Pozin, A.; Sukoriansky, S. Electrolyte Pumping Optimization in Already Manufactured Vanadium Redox Battery Based on Experimentally Determined Electrical and Hydrodynamic Losses. J. Energy Eng. 2016, 143, 04016050. [Google Scholar] [CrossRef]
- Langlois, S.; Coeuret, F. Flow-through and flow-by porous electrodes of nickel foam. I. Material characterization. J. Appl. Electrochem. 1989, 19, 43–50. [Google Scholar] [CrossRef]








| Fiber Diameter, µm | Average | Standard Deviation |
|---|---|---|
| 19.2 | 1.66 | |
| Felt density, (kg/m3), | 88 | - |
| Carbon density, (kg/m3), | 1954 | - |
| Porosity, (%), θ | 95.5 | - |
| Relative carbon volume, p.u. (%), V | 0.045 (4.5%) | - |
| Specific felt surface, (m−1), S | 9.8 × 103 | - |
| Parameter | Liquid | ||
|---|---|---|---|
| Glycerol | Alcohol | Cyclohexane | |
| Density, (g/cm3) (25 °C) | 1.26 | 0.789 | 0.8 |
| Dielectric constant, ε, (p.u.), (0.57 MHz, 25 °C) | ~42.5 | ~21.6 | ~2.02 |
| Electrical conductivity, ((Ω·cm)−1), 25 °C | 5 × 10−8 | ~1 × 10−6 | <5 × 10−9 |
| Viscosity, (Pa·s), 20 °C (30 °C) | 141 (61.2) | ~0.11 | 0.61 |
| Resistance | h, (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 6.2 | 6.1 | 4.9 | 4.45 | 3.7 | 1.55 | 1.1 | ||
| Volume Decrease, (%) | ||||||||
| 0 | 2 | 21 | 28 | 40 | 74 | 81 | ||
| Rdc (mΩ·cm2) | 48.184 | 41.2 | 18.2 | 10.100 | 6.672 | 5.189 | 5.930 | |
| Rac, (mΩ/cm2) | 100, (Hz) | 54.855 | 39.8 | 16.3 | 6.820 | 5.930 | 4.374 | 4.374 |
| 120, (Hz) | 54.633 | 38.9 | 16.6 | 7.042 | 6.079 | 4.522 | 4.299 | |
| 1, (kHz) | 54.633 | 40.1 | 15.9 | 6.894 | 6.375 | 4.522 | 4.225 | |
| 10, (kHz) | 49.296 | 38.7 | 14.9 | 6.746 | 5.930 | 4.670 | 4.299 | |
| Rac average, (mΩ·cm2) | 53.354 | 39.740 | 16.38 | 7.520 | 6.197 | 4.655 | 4.626 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kossenko, A.; Lugovskoy, S.; Averbukh, M. Electric and Hydraulic Properties of Carbon Felt Immersed in Different Dielectric Liquids. Materials 2018, 11, 650. https://doi.org/10.3390/ma11040650
Kossenko A, Lugovskoy S, Averbukh M. Electric and Hydraulic Properties of Carbon Felt Immersed in Different Dielectric Liquids. Materials. 2018; 11(4):650. https://doi.org/10.3390/ma11040650
Chicago/Turabian StyleKossenko, Alexey, Svetlana Lugovskoy, and Moshe Averbukh. 2018. "Electric and Hydraulic Properties of Carbon Felt Immersed in Different Dielectric Liquids" Materials 11, no. 4: 650. https://doi.org/10.3390/ma11040650





