Preparation of Tea Tree Oil/Poly(styrene-butyl methacrylate) Microspheres with Sustained Release and Anti-Bacterial Properties
Abstract
:1. Introduction
2. Results and Discussion
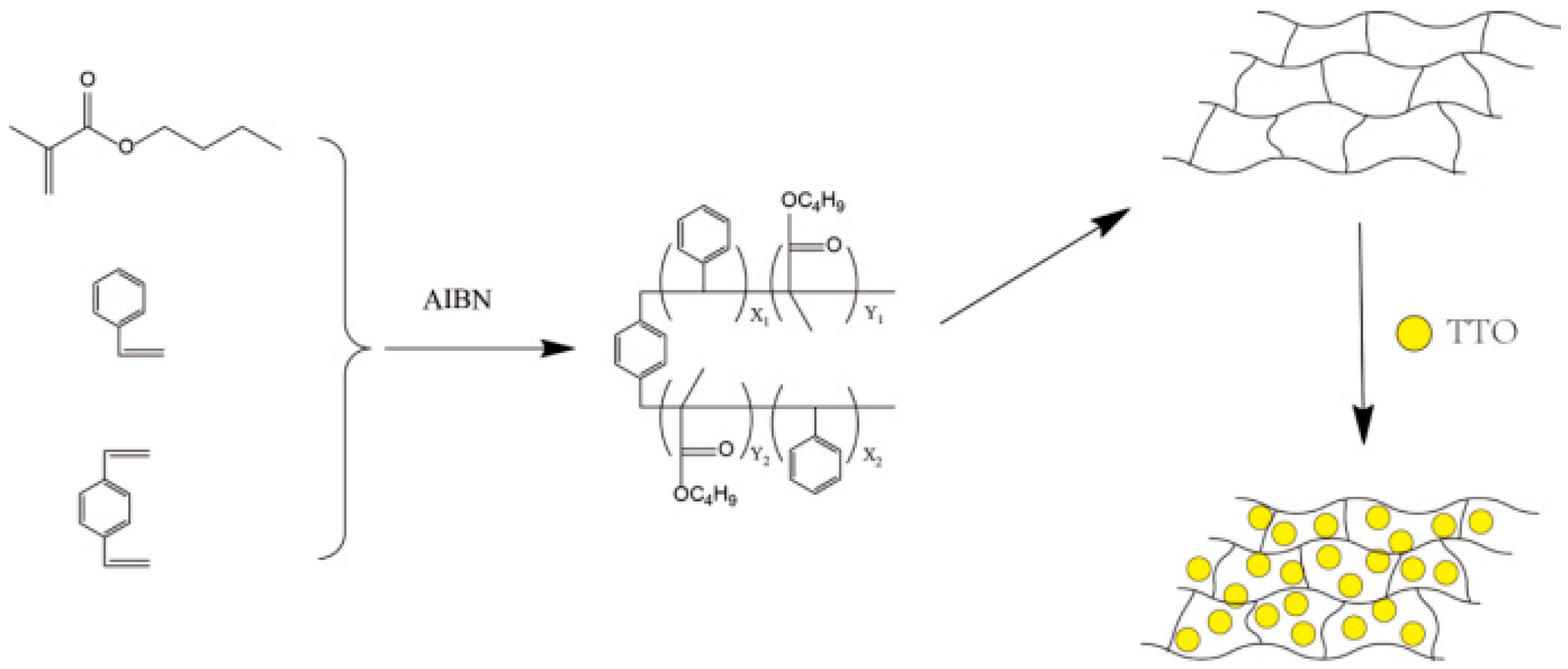
2.1. Structure and Morphology Analysis
2.2. Thermal Stability Analysis
2.3. Oil Absorption Performance Analysis
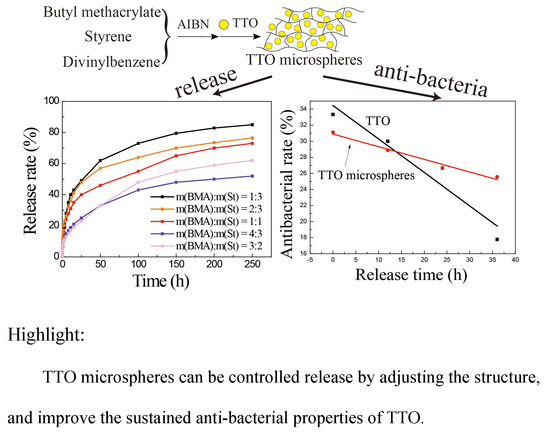
2.4. Sustained-Release Performance Analysis
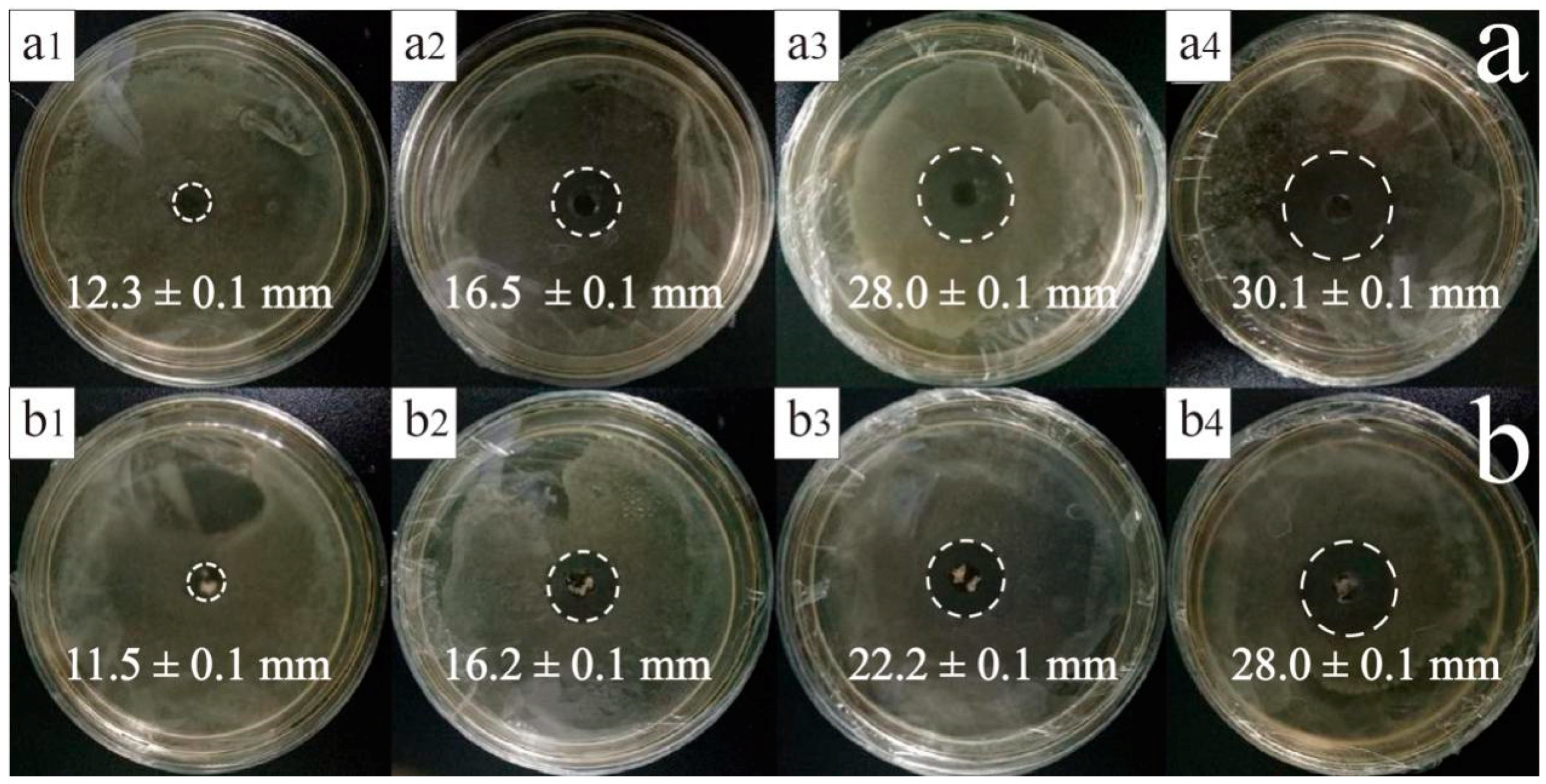
2.5. Antimicrobial Activity Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of TTO Microspheres
3.3. Structural Characterization of TTO Microspheres
3.4. Performance Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mazzarrino, G.; Paparella, A.; Chaves-López, C.; Faberi, A.; Sergi, M.; Sigismondi, C.; Compagnone, D.; Serio, A. Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food Control 2015, 50, 794–803. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sadekuzzaman, M.; Mizan, M.F.R.; Kim, H.; Yang, S.; Ha, S. Activity of thyme and tea tree essential oils against selected foodborne pathogens in biofilms on abiotic surfaces. LWT 2018, 89, 134–139. [Google Scholar] [CrossRef]
- Hernández, H.; Fraňková, A.; Sýkora, T.; Klouček, P.; Kouřimská, L.; Kučerová, I.; Banout, J. The effect of oregano essential oil on microbial load and sensory attributes of dried meat. J. Sci. Food Agric. 2017, 97, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zang, Y.; Cui, Q.; Gai, Q.; Jiao, J.; Wang, W.; Zu, Y.; Fu, Y. The preservative potential of Amomum tsaoko e ssential oil against E. coil, its antibacterial property and mode of action. Food Control 2017, 75, 236–245. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Li, X.; Li, H.; Cui, L.; He, D. Microcapsules biologically prepared using Perilla frutescens (L.) Britt. essential oil and their use for extension of fruit shelf life. J. Sci. Food Agric. 2018, 98, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Frankova, A.; Smid, J.; Bernardos, A.; Finkousova, A.; Marsik, P.; Novotny, D.; Legarová, V.; Pulkrabek, J.; Kloucek, P. The antifungal activity of essential oils in combination with warm air flow against postharvest phytopathogenic fungi in apples. Food Control 2016, 68, 62–68. [Google Scholar] [CrossRef]
- Kloucek, P.; Smid, J.; Frankova, A.; Kokoska, L.; Valterova, I.; Pavela, R. Fast screening method for assessment of antimicrobial activity of essential oils in vapor phase. Food Res. Int. 2012, 47, 161–165. [Google Scholar] [CrossRef]
- De Groot, A.C.; Schmidt, E. Tea tree oil: Contact allergy and chemical composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Arancia, G.; Molinari, A.; Salvatore, G.; Mondello, F. Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Sokolik, C.G.; Ben-Shabat-Binyamini, R.; Gedanken, A.; Lellouche, J. Proteinaceous microspheres as a delivery system for carvacrol and thymol in antibacterial applications. Ultrason. Sonochem. 2018, 41, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. J. Antimicrob. Chemother. 2002, 50, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca genus as antimicrobial agents: From farm to pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef] [PubMed]
- Dettwiller, P.; Spelman, T.; Carson, C.F.; Davey, R.C.; Thomas, J.; Baby, K.E.; Cooper, G.M.; Kyle, G.; Naunton, M.; Hammer, K.A.; et al. Therapeutic potential of tea tree oil for scabies. Am. J. Trop. Med. Hyg. 2016, 94, 258–266. [Google Scholar] [CrossRef]
- Rieder, B.O. Consumer exposure to certain ingredients of cosmetic products: The case for tea tree oil. Food Chem. Toxicol. 2017, 108, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Campelo, P.H.; Sanches, E.A.; de Barros Fernandes, R.V.; Botrel, D.A.; Borges, S.V. Stability of lime essential oil microparticles produced with protein- carbohydrate blends. Food Res. Int. 2018, 105, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The combined effects of tea tree oil and hot air treatment on the quality and sensory characteristics and decay of strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Lin, L. Plasma-treated poly(ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohyd. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Ho, T.M.; Bhandari, B.R. Encapsulation of tea tree oil by amorphous beta-cyclodextrin powder. Food Chem. 2017, 221, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, Y.; Zhou, J.; Xie, Y.; Wu, H.; Yuan, T.; Yang, Z. Facile fabrication of tea tree oil-loaded antibacterial microcapsules by complex coacervation of sodium alginate/quaternary ammonium salt of chitosan. RSC Adv. 2016, 6, 13032–13039. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, M.; Ge, X.; Wang, K.; Zhang, X. Preparation of high oil-absorptive uniform gel with controllable oil-absorbency by radiation. RSC Adv. 2017, 7, 31519–31524. [Google Scholar] [CrossRef]
- Du, Y.; Fang, P.; Chen, J.; Hou, X. Synthesis of reusable macroporous St/BMA copolymer resin and its absorbency to organic solvent and oil. Polym. Adv. Technol. 2016, 27, 393–403. [Google Scholar] [CrossRef]
- Ocak, B.R.; Gülümser, G.; Balo Lu, E. Microencapsulation of Melaleuca alternifolia (Tea Tree) Oil by Using Simple Coacervation Method. J. Essent. Oil Res. 2011, 23, 58. [Google Scholar] [CrossRef]
- Tian, G.; Wang, W.; Zhu, Y.; Zong, L.; Kang, Y.; Wang, A. Carbon/attapulgite composites as recycled palm oil-decoloring and dye adsorbents. Materials 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; Arndt, K.F. Swelling of high oil-absorptive network based on 1-octene and isodecyl acrylate copolymers. J. Polym. Res. 2005, 12, 77–88. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Y.; Lin, J.; Lin, S. Study on starch-graft-acrylamide/mineral powder superabsorbent composite. Polymer 2003, 44, 6513–6520. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, L.; Sun, B. Model study of CO2 absorption in aqueous amine solution enhanced by nanoparticles. Int. J. Greenh. Gas Control 2017, 60, 51–58. [Google Scholar] [CrossRef]
- Wu, C.; An, X.; Gao, S.; Su, L. Self-assembly of cuprous oxide nanoparticles supported on reduced graphene oxide and their enhanced performance for catalytic reduction of nitrophenols. RSC Adv. 2015, 5, 71259–71267. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buria, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharmaceut. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Y.; Zhou, H.; Zhou, X.; Gong, S.; Xu, H. Synthesis and characterization of chlorpyrifos/copper(II) schiff base mesoporous silica with pH sensitivity for pesticide sustained release. J. Agric. Food Chem. 2016, 64, 8095–8102. [Google Scholar] [CrossRef] [PubMed]
- Huayao, C.; Yueshun, L.; Hongjun, Z.; Xinhua, Z.; Sheng, G.; Hua, X. Highly efficient alginate sodium encapsulated chlorpyrifos/copper(II) Schiff base mesoporous silica sustained release system with pH and ion response for pesticide delivery. RSC Adv. 2016, 6, 114714–114721. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Truong, V.K.; Wang, F.; Pushpamali, W.A.A.; Wang, J.Y.; Ellis, A.V.; Berndt, C.C.; Crawford, R.J.; Ivanova, E.P. Plasma-enhanced synthesis of bioactive polymeric coatings from monoterpene alcohols: A combined experimental and theoretical study. Biomacromolecules 2010, 11, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Pegalajar-Jurado, A.; Easton, C.; Styan, K.; McArthur, S. Antibacterial activity studies of plasma polymerised cineole films. J. Mater. Chem. B 2014, 2, 4993–5002. [Google Scholar] [CrossRef]
- Kulawardana, E.U.; Neckers, D.C. Photoresponsive oil sorbers. J. Polym. Sci. Chem. 2010, 48, 55–62. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Chen, H.; Tan, Z.; Fan, L. Synthesis and Properties of High Oil-Absorbing Resins with Long Chain by High Internal Phase Emulsions as Template. Sep. Sci. Technol. 2014, 49, 2518–2524. [Google Scholar] [CrossRef]
- Lee, D.; Cohen, R.E.; Rubner, M.F. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir 2005, 21, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polum. 2017, 117, 106–111. [Google Scholar] [CrossRef]







| Functional Group | Wavelength (cm−1) | ||
|---|---|---|---|
| TTO | P(St-BMA) Microspheres | TTO Microspheres | |
| O–H stretching vibration | 3478. | - | 3447 |
| C–H (benzene ring) stretching vibration | - | 2926, 1450 | 2926, 1452 |
| C–H (C=C) stretching vibration | 2965 | - | 2965 |
| carbonyl stretching vibration | - | 1729 | 1729 |
| C–C (benzene ring) stretching vibration | - | 1600 | 1600 |
| C–O (ester) stretching vibration | 1166 | 1174 | 1174 |
| C–O (tertiary alcohol in terpenes) stretching vibration | 1127 | - | 1127 |
| C=C stretching vibration | 902 | - | 910 |
| C–H (benzene ring) plane bending vibration | - | 763, 688 | 763, 689 |
| Kinetic Model | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | |
|---|---|---|---|---|---|
| Sample | r2 | n | |||
| mSt:mBMA = 2:8 | 0.7599 | 0.8863 | 0.9288 | 0.9733 | 0.3418 |
| mSt:mBMA = 4:6 | 0.7365 | 0.8963 | 0.9221 | 0.9759 | 0.4446 |
| mSt:mBMA = 6:4 | 0.7094 | 0.8487 | 0.9018 | 0.9718 | 0.4191 |
| mSt:mBMA = 8:2 | 0.8049 | 0.8687 | 0.9567 | 0.9937 | 0.3275 |
| mSt:mBMA = 10:0 | 0.8782 | 0.9383 | 0.9844 | 0.9886 | 0.4005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.; Chen, H.; Zhou, H.; Zhou, X.; Xu, H. Preparation of Tea Tree Oil/Poly(styrene-butyl methacrylate) Microspheres with Sustained Release and Anti-Bacterial Properties. Materials 2018, 11, 710. https://doi.org/10.3390/ma11050710
Lin G, Chen H, Zhou H, Zhou X, Xu H. Preparation of Tea Tree Oil/Poly(styrene-butyl methacrylate) Microspheres with Sustained Release and Anti-Bacterial Properties. Materials. 2018; 11(5):710. https://doi.org/10.3390/ma11050710
Chicago/Turabian StyleLin, Guanquan, Huayao Chen, Hongjun Zhou, Xinhua Zhou, and Hua Xu. 2018. "Preparation of Tea Tree Oil/Poly(styrene-butyl methacrylate) Microspheres with Sustained Release and Anti-Bacterial Properties" Materials 11, no. 5: 710. https://doi.org/10.3390/ma11050710