Void Formation/Elimination and Viscoelastic Response of Polyphenylsilsesquioxane Monolith
Abstract
:1. Introduction
2. Materials and Methods
3. Results and discussion
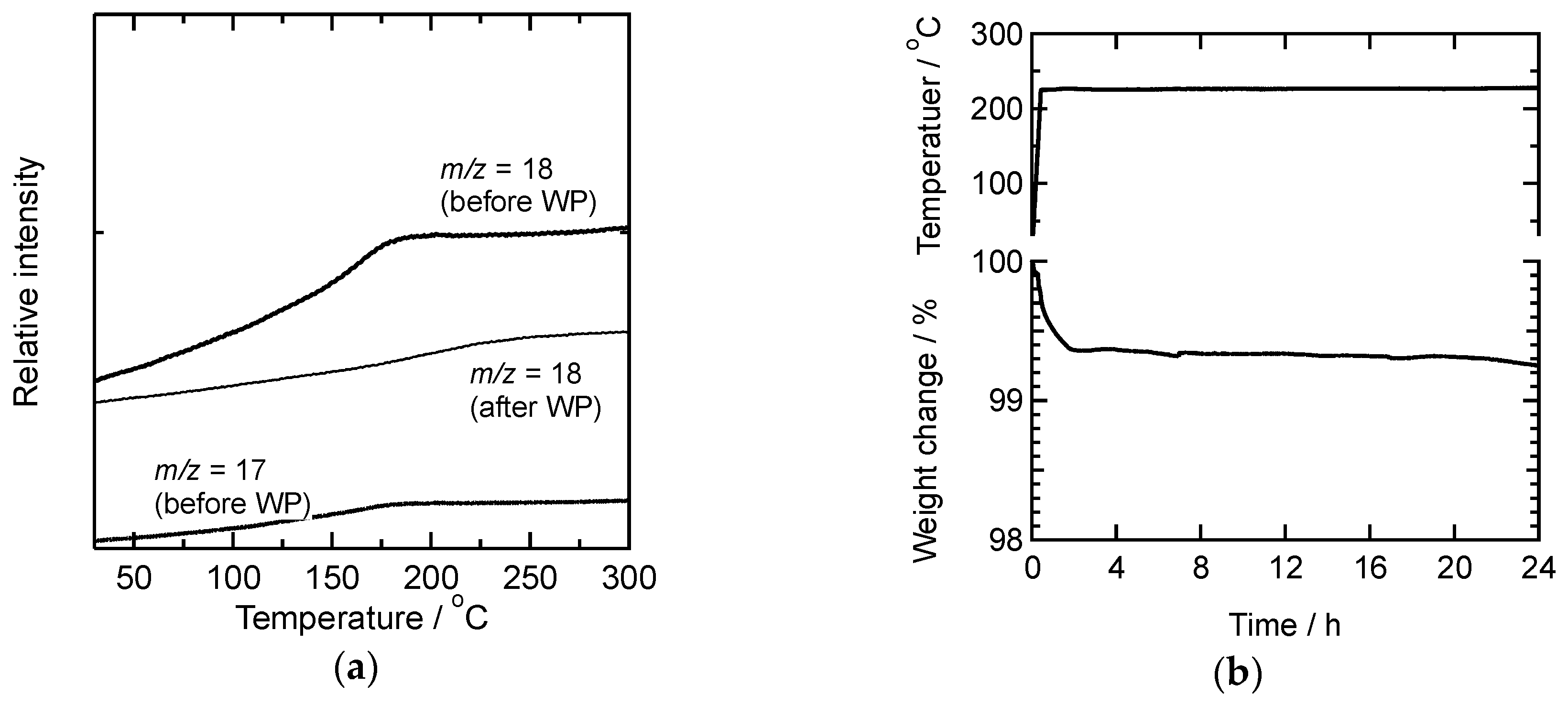
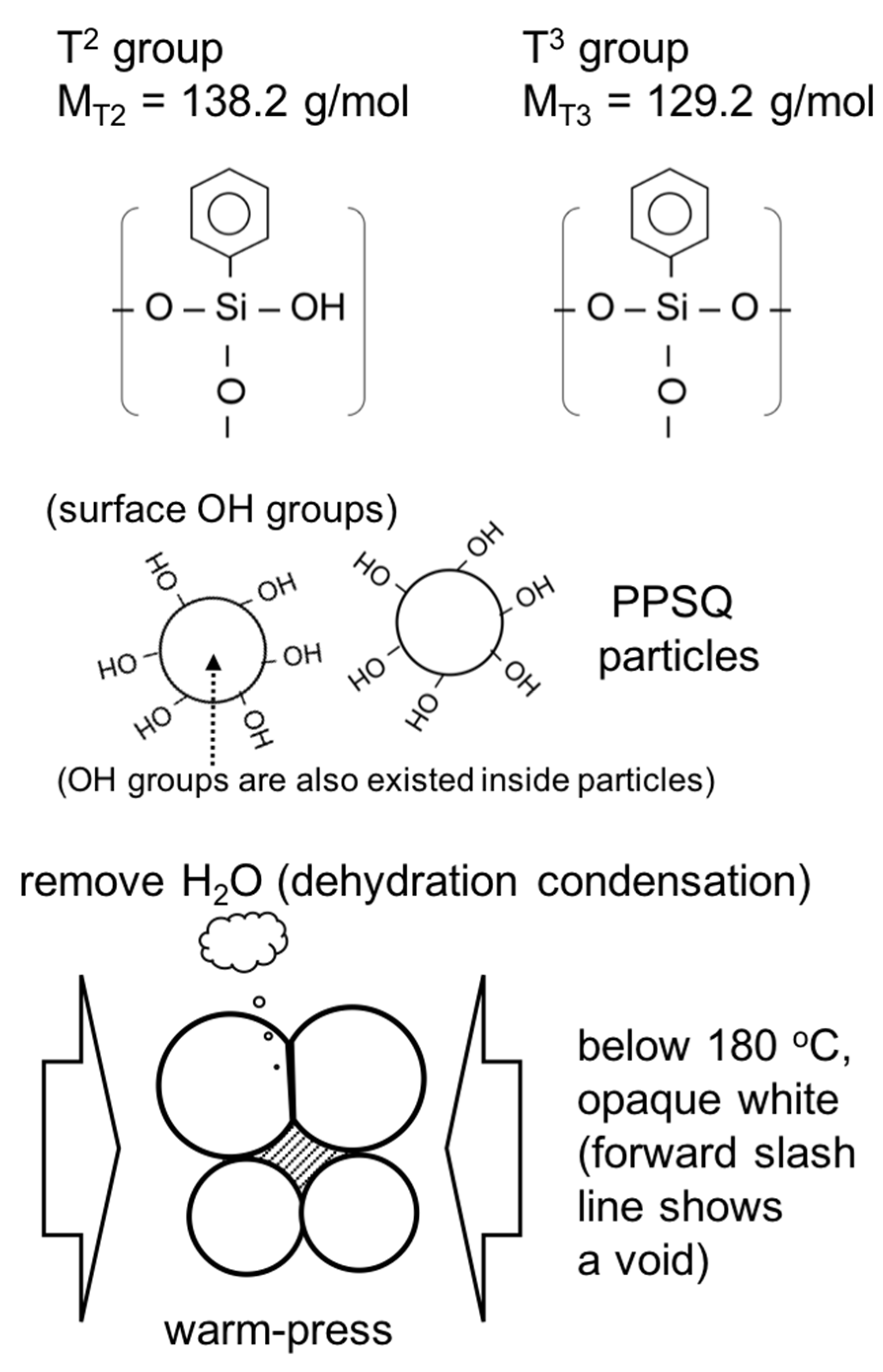
3.1. Structure and Degree of PhSiO3/2 Polymerization via Warm-Pressing
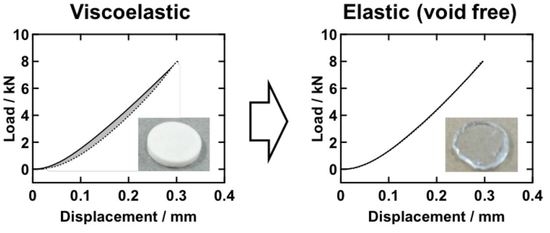
3.2. Loading-Unloading Hysteresis and Void Formation
3.3. AC Insulating Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, J.F., Jr.; Vogt, L.H., Jr.; Katchman, A.; Eustance, J.W.; Kiser, K.M.; Krantz, K.W. Double chain polymers of phenylsisesquioxane. J. Am. Chem. Soc. 1960, 82, 6194–6195. [Google Scholar] [CrossRef]
- Hah, H.J.; Kim, J.S.; Jeon, B.J.; Koo, S.M.; Lee, Y.E. Simple preparation of monodisperse hollow silica particles without using templates. Chem. Comm. 2003, 14, 1712–1713. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, C.H.; Kim, D.K. Formation and characterization of monodisperse, spherical organo-silica powders from organo-alkoxysilane-water system. J. Am. Ceram. Soc. 1998, 81, 1184–1188. [Google Scholar] [CrossRef]
- Ma, C.; Kimura, Y. Preparation of nano-particles of poly(phenylsilsesquioxane)s by emulsion polycondensation of phenylsilanetriol formed in aqueous solution. Polym. J. 2002, 34, 709–713. [Google Scholar] [CrossRef]
- Arkhireeva, A.; Hay, J.N. Synthesis of sub-200 nm silsesquioxane particles using a modified Stober sol-gel route. J. Mater. Chem. 2003, 13, 3122–3127. [Google Scholar] [CrossRef]
- Bermundo, J.P.; Ishikawa, Y.; Yamazaki, H.; Nonaka, Y.; Uraoka, Y. Highly reliable polysilsesquioxane passivation layer for a-InGaZnO thin-film transistors. ESC J. Solid State Sci. Technol. 2014, 3, Q16–Q19. [Google Scholar] [CrossRef]
- Bermundo, J.P.; Ishikawa, Y.; Fujii, M.N.; Nonaka, T.; Ishihara, R.; Ikenoue, H.; Uraoka, Y. Effect of excimer laser annealing on a-InGaZnO thin-film transistors passivated by solution-processed hybrid passivation layers. J. Phys. D Appl. Phys. 2016, 49, 035102. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, L.; Toghiani, H.; Daulton, T.L.; Koyama, K.; Pittman, C.U. Viscoelastic and mechanical properties of epoxy/multifunctional polyhedral oligomeric silsesquioxane nanocomposites and epoxy/ladderlike polyphenylsilsesquioxane blends. Macromolecules 2001, 34, 8686–8693. [Google Scholar] [CrossRef]
- Delvallé, C.; Meur, M.L.; Reeth, I.V. Personal care applications for phenylsilsesquioxane resins. IP.com J. 2016, 17, 1–17. [Google Scholar]
- Shibata, S.; Yamane, M.; Kamada, K.; Ohta, K.; Sasaki, K.; Masuhara, H. Laser emission from dye-doped organic-inorganic particles of microcavity structure. J. Sol-Gel Sci. Technol. 1997, 8, 959–964. [Google Scholar] [CrossRef]
- Biyachenko, A.N.; Yalymov, A.I.; Dronova, M.S.; Korlyukov, A.A.; Vologzhanina, A.V.; Eskova, M.A.; Jerome, L.; Larionova, J.; Guari, Y.; Forobatovskii, P.V.; et al. Family of polynuclear nickel cagelike phenylsilsesquioxanes; features of periodic networks and magnetic properties. Inorg. Chem. 2017, 56, 12751–12763. [Google Scholar] [CrossRef] [PubMed]
- Biyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Korlyukov, A.A.; Shul’pina, L.S.; Arkhipov, D.E.; Shubina, E.S.; Levisky, M.M.; Kirilin, A.D.; Shul’pin, G.B. Binclear cage-like copper(II) silsesquioxane(“cooling tower”)-It’s high catalytic activity in the oxidation of benzene and alcohols. Eur. J. Inorg. Chem. 2013, 30, 5240–5246. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Dong, X.-H.; Yu, X.; Guo, K.; Su, H.; Yue, K.; Wesdemiotis, C.; Cheng, S.Z.D; Zhang, W.-B. Giant gemini surafactants based on polystyrene-hydrophilic polyhedral oligomeric silsesquioxane shape amphiphiles: Sequential “click” chemistry and solution self-assembly. Chem. Sci. 2013, 4, 1345–1352. [Google Scholar] [CrossRef]
- Takahashi, K.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Substituent effects on the glass transition phenomena of polyorganosilsesquioxane particles prepared by two-step acid-base catalyzed sol-gel process. J. Ceram. Soc. Jpn. 2011, 119, 173–179. [Google Scholar] [CrossRef]
- Takahashi, K.; Tadanaga, K.; Matsuda, A.; Hayashi, A.; Tatsumisago, M. Effects of phenyltriethoxysilane concentration in starting solutions on thermal properties of polyphenylsilsesquioxane particles prepared by a two-step acid-base catalyzed sol-gel process. J. Ceram. Soc. Jpn. 2007, 115, 131–135. [Google Scholar] [CrossRef]
- Daiko, Y.; Katagiri, K.; Shimoike, K.; Sakai, M.; Matsuda, A. Structures and electrical properties of core-shell composite electrolyte with multi-heterointerfaces. Solid State Ion. 2007, 178, 621–625. [Google Scholar] [CrossRef]
- Daiko, Y.; Sakamoto, H.; Katagiri, K.; Muto, H.; Sakai, M.; Matsuda, A. Deposition of ultrathin Nation layers on sol-gel-derived phenylsilsesquioxane particles via layer-by-layer assembly. J. Electrochem. Soc. 2008, 155, B479–B482. [Google Scholar] [CrossRef]
- Daiko, Y.; Sakakibara, S.; Sakamoto, H.; Katagiri, K.; Muto, H.; Sakai, M.; Matsuda, A. Formation of a high conductivity fuel cell electrolyte by pressing diphenylsiloxane-based inorganic-organic hybrid particles. J. Am. Ceram. Soc. 2009, 92, S185–S188. [Google Scholar] [CrossRef]
- Kozuka, H. On ceramic thin film formation from gels: evolution of stress, cracks and radiative striations. J. Ceram. Soc. Jpn. 2003, 111, 624–632. [Google Scholar] [CrossRef]
- Kozuka, H. Stress evolution on gel-to-ceramic thin film conversion. J. Sol-Gel Sci. Technol. 2006, 40, 287–297. [Google Scholar] [CrossRef]
- Kozuka, H.; Takenaka, S.; Tokita, H.; Okubayashi, M. PVP-assisted sol-gel deposition of single layer ferroelectric thin films over submicron or micron in thickness. J. Eur. Ceram. Soc. 2004, 24, 1585–1588. [Google Scholar] [CrossRef]
- Katagiri, K.; Hasegawa, K.; Matsuda, A.; Tatsumisago, M.; Minami, T. Preparation of transparent thick films by electrophoretic sol-gel deposition using phenyltriethoxysilane-derived particles. J. Am. Ceram. Soc. 1998, 81, 2501–2503. [Google Scholar] [CrossRef]
- Takahashi, K.; Tadanaga, K.; Matsuda, A.; Hayashi, A.; Tatsumisago, M. Formation of convex shaped poly(phenylsilsesquioxane) macropatterns on indium tin oxide substrates with hydrophobic-phydrophilic patterns using the electrophoretic sol-gel deposition method. J. Mater. Res. 2006, 21, 1255–1260. [Google Scholar] [CrossRef]
- Shammas, N.Y.A. Present problems of power module packaging technology. Microelectron. Reliab. 2003, 43, 519–527. [Google Scholar] [CrossRef]
- Fukuda, K.; Okamoto, D.; Okamoto, M.; Tadayoshi, D.; Mizushima, T.; Takenaka, K.; Fujisawa, H.; Harada, S.; Tanaka, Y.; Yonezawa, Y.; et al. Development of ultrahigh-voltage SiC devices. IEEE Trans. Electron Dev. 2015, 62, 396–404. [Google Scholar] [CrossRef]
- Mimura, K.; Nakamura, Y.; Masaki, M.; Nishimura, T. Development of resin insulated material with high thermal conductivity and application to the power module. J. Photopolym. Sci. Technol. 2015, 28, 169–173. [Google Scholar] [CrossRef]
- Edmond, J.A.; Das, K.; Davis, R.F. Electrical properties of ion-implanted p-n junction diodes in β-SiC. J. Appl. Phys. 1988, 63, 922–929. [Google Scholar] [CrossRef]
- Chow, T.P.; Ramungui, N.; Ghezzo, M. Recent advances in high-voltage SiC power devices. High-Temp. Electron. Mater. Dev. Sens. Conf. 1998, 55–67. [Google Scholar]
- Bai, J.G.; Yin, J.; Zhang, Z.; Lu, G.-Q.; van Wyk, J.D. High-temperature operation of SiC power devices by low-temperature sintered silver die-attachment. IEEE Trans. Adv. Pack. 2007, 30, 506–510. [Google Scholar] [CrossRef]
- Palmour, J.W.; Edmond, J.A.; Kong, H.S.; Carter, C.H., Jr. 6H-silicon carbide devices and applications. Phys. B 1993, 185, 461–465. [Google Scholar] [CrossRef]
- Dissado, L.A.; Sweeney, P.J.J. Physical mode for breakdown structures in solid dielectrics. Phys. Rev. B 1993, 48, 16261–16268. [Google Scholar] [CrossRef]
- Illias, H.A.; Tunio, M.A.; Mokhlis, H.; Chen, G.; Bakar, A.H.A. Determination of partial discharge time lag in void using physical model approach. IEEE Tran. Dielectr. Electr. Insul. 2015, 22, 463–471. [Google Scholar] [CrossRef]
- Mishra, D.; Satapathy, A. An experimental investigation on the effect of particle size on the thermal properties and void content of solid glass microsphere filled epoxy composites. Mater. Sci. Eng. 2016, 115, 012011. [Google Scholar] [CrossRef]
- Varna, J.; Joffe, R.; Berglund, L.A.; Lundström, T.S. Effect of voids on failure mechanisms in RTM laminates. Comp. Sci. Technol. 1995, 53, 241–249. [Google Scholar] [CrossRef]
- Chowdhury, K.A.; Talreja, R.; Benzerga, A.A. Effects of manufacturing-induced voids on local failure in polymer-based composites. J. Eng. Mater. Technol. 2008, 130, 021010. [Google Scholar] [CrossRef]
- Ueda, T.; Helfen, L.; Morgeneyer, T.F. In situ laminography study of three-dimensional individual void shape evolution at crack initiation and comparison with Gurson-Tvergaard-Needleman-type simulations. Acta Mater. 2014, 78, 254–270. [Google Scholar] [CrossRef]
- Adriaensens, P.; Pollaris, A.; Vanderzande, D.; Gelan, J. Critical analysis of network defects in cross-linked isobutylene-based elastomers by NMR imaging. Macromolecules 1992, 32, 4692–4699. [Google Scholar] [CrossRef]
- Findley, W.N.; Lai, J.S.; Onaran, K. Linear viscoelastic constitutive equations. In Creep and Relaxation of Nonlinear Viscoelastic Materials; Dover Publications: New York, NY, USA, 1976. [Google Scholar]
- Mohanty, A.; Srivastava, V.K. Dielectric breakdown performance of alumina/epoxy resin nanocomposites under high voltage application. Mater. Des. 2013, 47, 711–716. [Google Scholar] [CrossRef]
- Boggs, S.A. Partial Discharge. III. Cavity-induced PD in solid dielectrics. IEEE Electr. Insul. Mag. 2002, 6, 11–16. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daiko, Y.; Oda, Y.; Honda, S.; Iwamoto, Y. Void Formation/Elimination and Viscoelastic Response of Polyphenylsilsesquioxane Monolith. Materials 2018, 11, 846. https://doi.org/10.3390/ma11050846
Daiko Y, Oda Y, Honda S, Iwamoto Y. Void Formation/Elimination and Viscoelastic Response of Polyphenylsilsesquioxane Monolith. Materials. 2018; 11(5):846. https://doi.org/10.3390/ma11050846
Chicago/Turabian StyleDaiko, Yusuke, Yuki Oda, Sawao Honda, and Yuji Iwamoto. 2018. "Void Formation/Elimination and Viscoelastic Response of Polyphenylsilsesquioxane Monolith" Materials 11, no. 5: 846. https://doi.org/10.3390/ma11050846