Preparation and Performance Analysis of Modified Sodium Acetate Trihydrate
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Instruments
2.3. Experiment Methods
2.3.1. Time-Temperature Curve
2.3.2. Analysis of Parameters
3. Results and Discussion
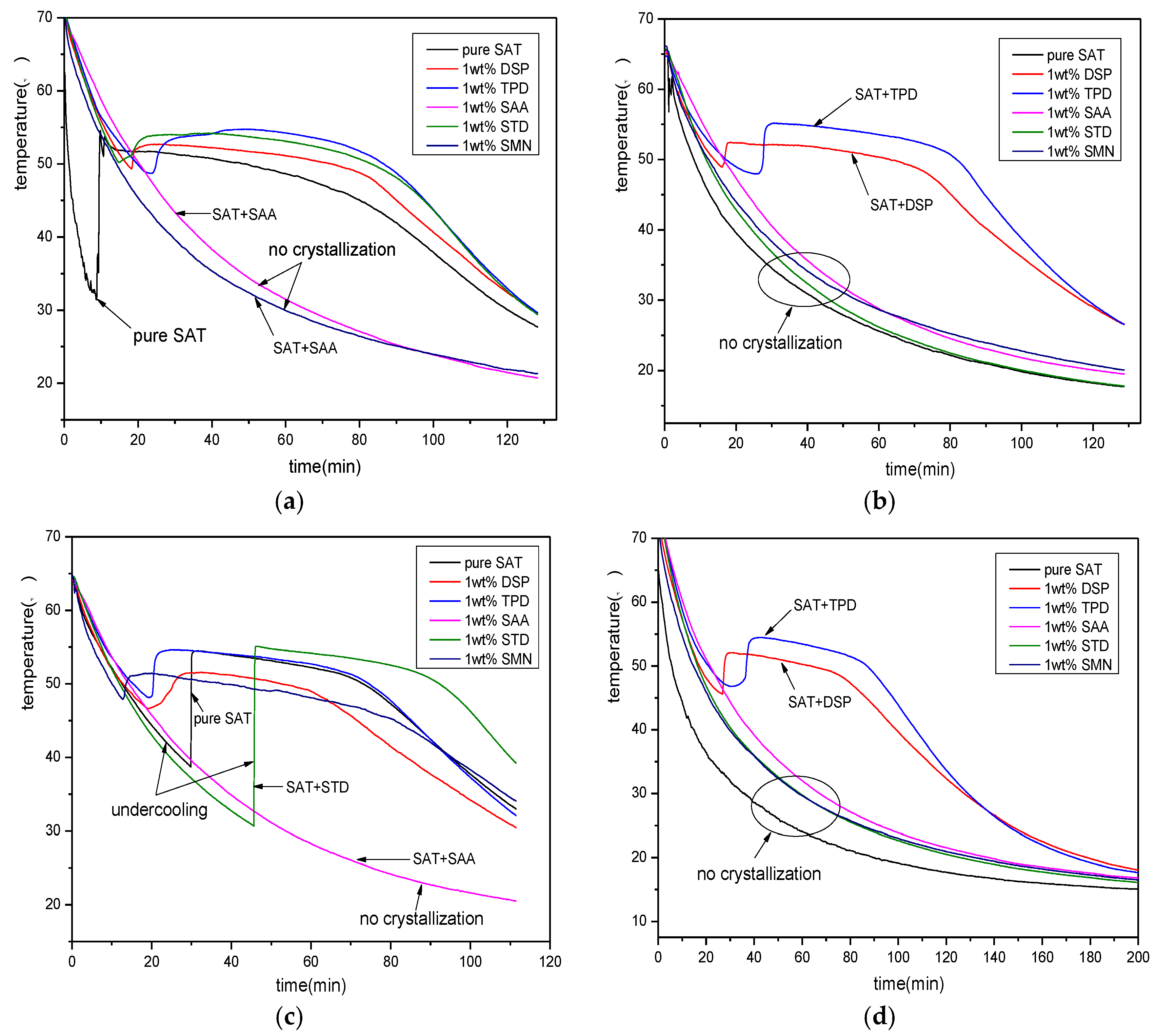
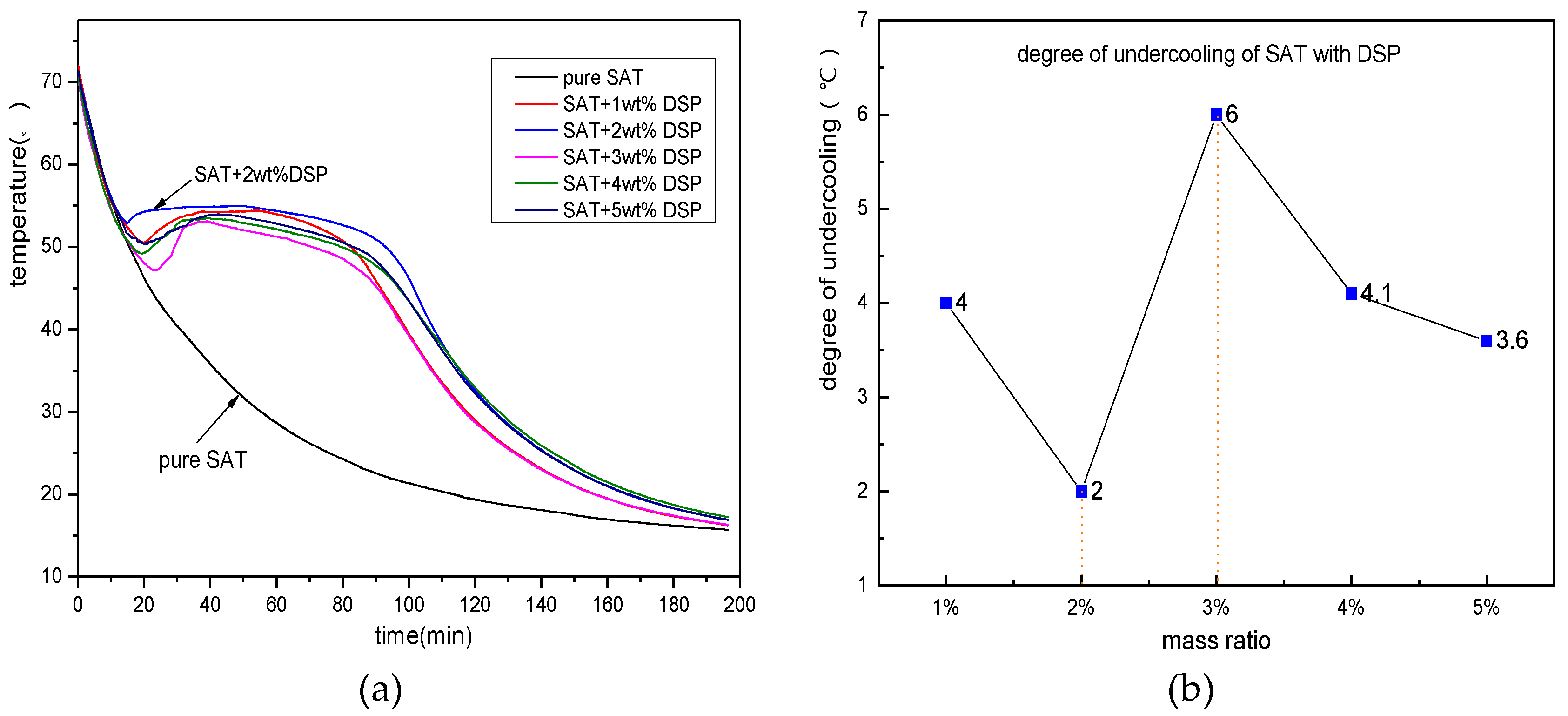
3.1. Screening of Nucleating Agents
3.2. The Selection of Thickeners
3.3. The Interaction of Nucleating Agents and Thickeners
3.4. Analysis of DSC
3.5. Analysis of Thermal Conductivity
3.6. Analysis of pH Tests
3.7. Analysis of the Volume Change Rate and Solid-Liquid Density
- —liquid density;
- ms—sample mass;
- Vl—liquid volume;
- —solid density;
- Vs—solid volume;
- Vo—the volume of silicone oil in the measuring cylinder;
- ΔV—volume change rate of solid and liquid;
- Va—The sum of the volume of solid phase change material and silicone oil.
4. Conclusions
- (1)
- DSP is an effective nucleating agent to SAT, which can effectively suppress the degree of undercooling of SAT. When the DSP’s addition content is 2 wt %, the degree of undercooling of SAT can be reduced to 2 K.
- (2)
- Based on thickening agent screening experiments, it is found that XG can be used as an effective thickener for SAT. It can effectively solve the problem of phase separation of pure SAT. When the mass content of XG is between 1 and 1.5 wt %, thickening and dispersing effects are optimal and there is no stratification. With the combined effect of thickener and nucleating agents, the degree of undercooling of modified SAT can be reduced to within 1.5 K. The recommended mass composition of modified SAT is 96.5 wt % SAT, 2 wt % DSP, 1.5 wt % XG.
- (3)
- Through the comparative tests of parameters, it is found that the thickener is the main factor that affects the performance of modified SAT. After thickeners are added to pure SAT, its latent heat, solid/liquid volume expansion rate, solid/liquid density and thermal conductivity all decrease accordingly. Therefore, in actual applications, the mass content of XG is between 1 and 1.5 wt %.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Brancato, V.; Frazzica, A.; Sapienza, A.; Freni, A. Identification and characterization of promising phase change materials for solar cooling applications. Sol. Energy Mater. Sol. Cells 2017, 160, 225–232. [Google Scholar] [CrossRef]
- Kapsalis, V.; Karamanis, D. Solar thermal energy storage and heat pumps with phase change materials. Appl. Therm. Eng. 2016, 99, 1212–1224. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao C, Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Guo, L.; Li, X.; Wang, H.; Chen, S.; Zhou, Y.; Li, J. Salt hydrate based phase change materials for thermal energy storage—A review. Energy Storage Sci. Technol. 2017, 6, 623–632. [Google Scholar]
- Kenfack, F.; Bauer, M. Innovative Phase Change Material (PCM) for Heat Storage for Industrial Applications. Energy Procedia 2014, 46, 310–316. [Google Scholar] [CrossRef]
- Günther, E.; Mehling, H.; Werner, M. Melting and nucleation temperatures of three salt hydrate phase change materials under static pressures up to 800 MPa. J. Phys. D Appl. Phys. 2007, 40, 4636. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Z.; Memon, S.A.; Bao, X.; Cui, H. Preparation and Supercooling Modification of Salt Hydrate Phase Change Materials Based on CaCl2·2H2O/CaCl2. Materials 2017, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, D.J.; Fan, X.Y.; Zhou, X.; Chen, Z.S. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Sol. Energy Mater. Sol. Cells 2011, 95, 2645–2649. [Google Scholar] [CrossRef]
- Johansen, J.B.; Dannemand, M.; Kong, W.; Fan, J.; Dragsted, J.; Furbo, S. Thermal Conductivity Enhancement of Sodium Acetate Trihydrate by Adding Graphite Powder and the Effect on Stability of Supercooling. Energy Procedia 2015, 70, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Xiang, Y. Experimental investigations on stable supercooling performance of sodium acetate trihydrate PCM for thermal storage. Sol. Energy 2017, 155, 1261–1272. [Google Scholar] [CrossRef]
- Wada, T.; Yamamoto, R. Studies on salt hydrate for latent heat storage. I. Crystal nucleation of sodium acetate trihydrate catalyzed by tetrasodium pyrophosphate decahydrate. Bull. Chem. Soc. Jpn. 1982, 55, 3603–3606. [Google Scholar] [CrossRef]
- Wada, T.; Kimura, F.; Yamamoto, R. Studies on salt hydrate for latent heat storage, II. Eutectic mixture of pseudo-binary system CH3CO2Na·3H2O-CO(NH2)2. Bull. Chem. Soc. Jpn. 1983, 56, 1223–1226. [Google Scholar] [CrossRef]
- Wada, T.; Yamamoto, R.; Matsuo, Y. Heat storage capacity of sodium acetate trihydrate during thermal cycling. Sol. Energy 1984, 33, 373–375. [Google Scholar] [CrossRef]
- Wada, T.; Matsuo, Y. Studies on salt hydrates for latent heat storge. VI. Preheating effect on crystallization of sodium acetate trihydrate from aqueous solution with a small amount of disodium hydrogenphosphate. Bull. Chem. Soc. Jpn. 1984, 57, 561–563. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Svensson, G.; Hiebler, S.; Mehling, H. Thermal performance of sodium acetate trihydrate thickened with different materials as phase change energy storage material. Appl. Therm. Eng. 2003, 23, 1697–1704. [Google Scholar] [CrossRef]
- Cui, W.; Yuan, Y.; Sun, L.; Cao, X.; Yang, X. Experimental studies on the supercooling and melting/freezing characteristics of nano-copper/sodium acetate trihydrate composite phase change materials. Renew. Energy 2016, 99, 1029–1037. [Google Scholar] [CrossRef]
- Mao, J.; Dong, X.; Hou, P. Preparation research of novel composite phase change materials based on sodium acetate trihydrate. Appl. Therm. Eng. 2017, 118, 817–825. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, P.; Wang, Z. Study on heat storage performance of Ch3co2·3H2O. Hebei Acad. Sci. 1988, 2, 21–29. [Google Scholar]








| Material Name | Solid Thermal Conductivity (W/m·K) | Average Value (W/m·K) | Liquid Thermal Conductivity (W/m·K) | Average Value (W/m·K) |
|---|---|---|---|---|
| SAT | 0.8945 | 0.9015 | 1.51 | 1.51 |
| 0.9130 | 1.53 | |||
| 0.8971 | 1.49 | |||
| SAT + DSP + XG | 0.6895 | 0.6818 | 1.21 | 1.21 |
| 0.6885 | 1.18 | |||
| 0.6675 | 1.25 |
| Material Name | Vl (mL) | Va (mL) | Vo (mL) | ms (g) | ΔV | |
|---|---|---|---|---|---|---|
| 21.0 | 38.0 | 20 | 30 | 16.7% | ||
| SAT | 21.5 | 38.5 | 20 | 30 | 16.2% | 1.636/1.406 |
| 21.5 | 38.5 | 20 | 30 | 16.2% | ||
| 23.5 | 41.5 | 20 | 30 | 9.3% | ||
| SAT/DSP/XG | 24.0 | 41.5 | 20 | 30 | 11.6% | 1.406/1.268 |
| 23.5 | 41.0 | 20 | 30 | 11.9% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, W.; Zhang, X.; Muthoka, M.J.; Han, X. Preparation and Performance Analysis of Modified Sodium Acetate Trihydrate. Materials 2018, 11, 1016. https://doi.org/10.3390/ma11061016
Hua W, Zhang X, Muthoka MJ, Han X. Preparation and Performance Analysis of Modified Sodium Acetate Trihydrate. Materials. 2018; 11(6):1016. https://doi.org/10.3390/ma11061016
Chicago/Turabian StyleHua, Weisan, Xuelai Zhang, Munyalo Jotham Muthoka, and Xingchao Han. 2018. "Preparation and Performance Analysis of Modified Sodium Acetate Trihydrate" Materials 11, no. 6: 1016. https://doi.org/10.3390/ma11061016




