An Investigation of the Wear on Silicon Surface at High Humidity
Abstract
:1. Introduction
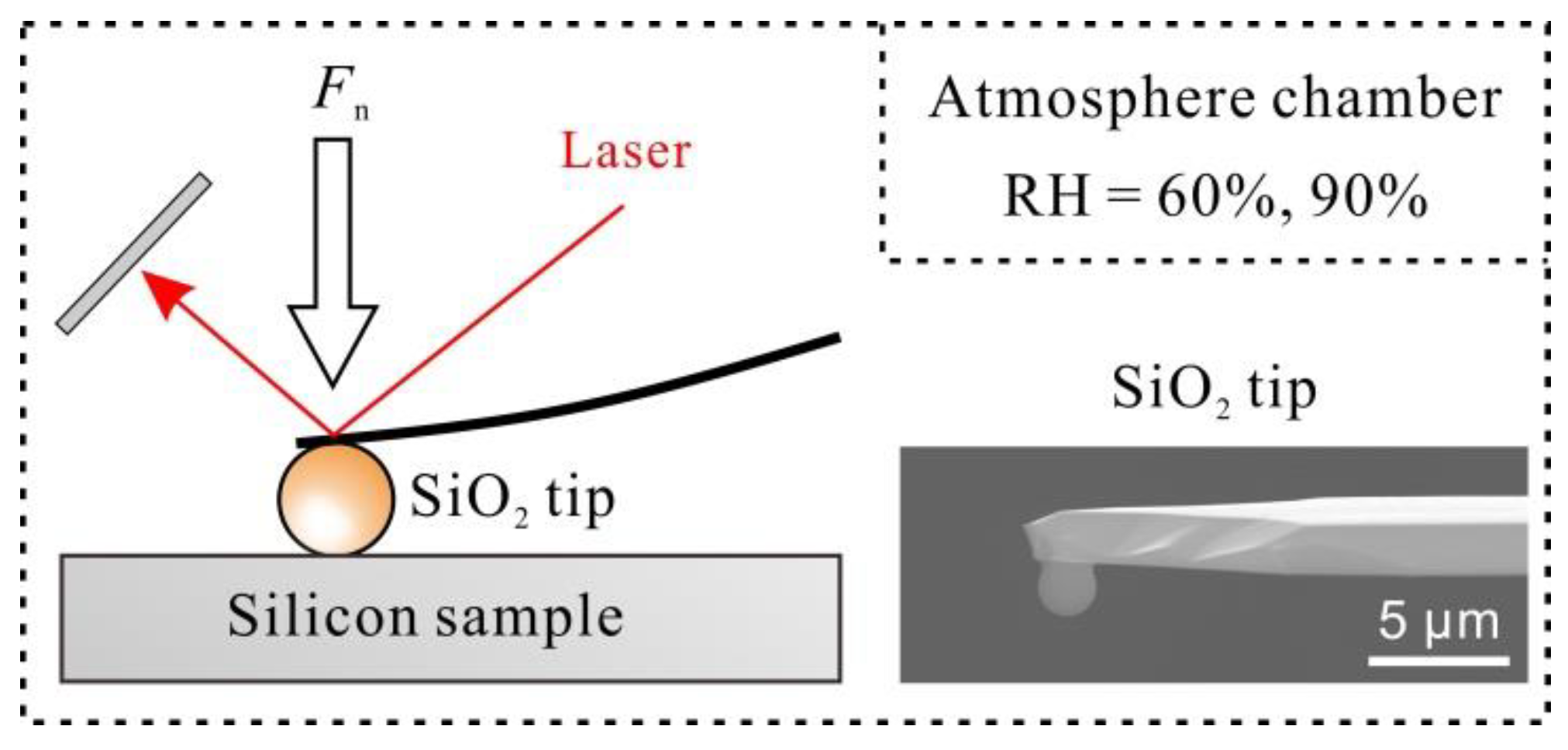
2. Materials and Methods
3. Results
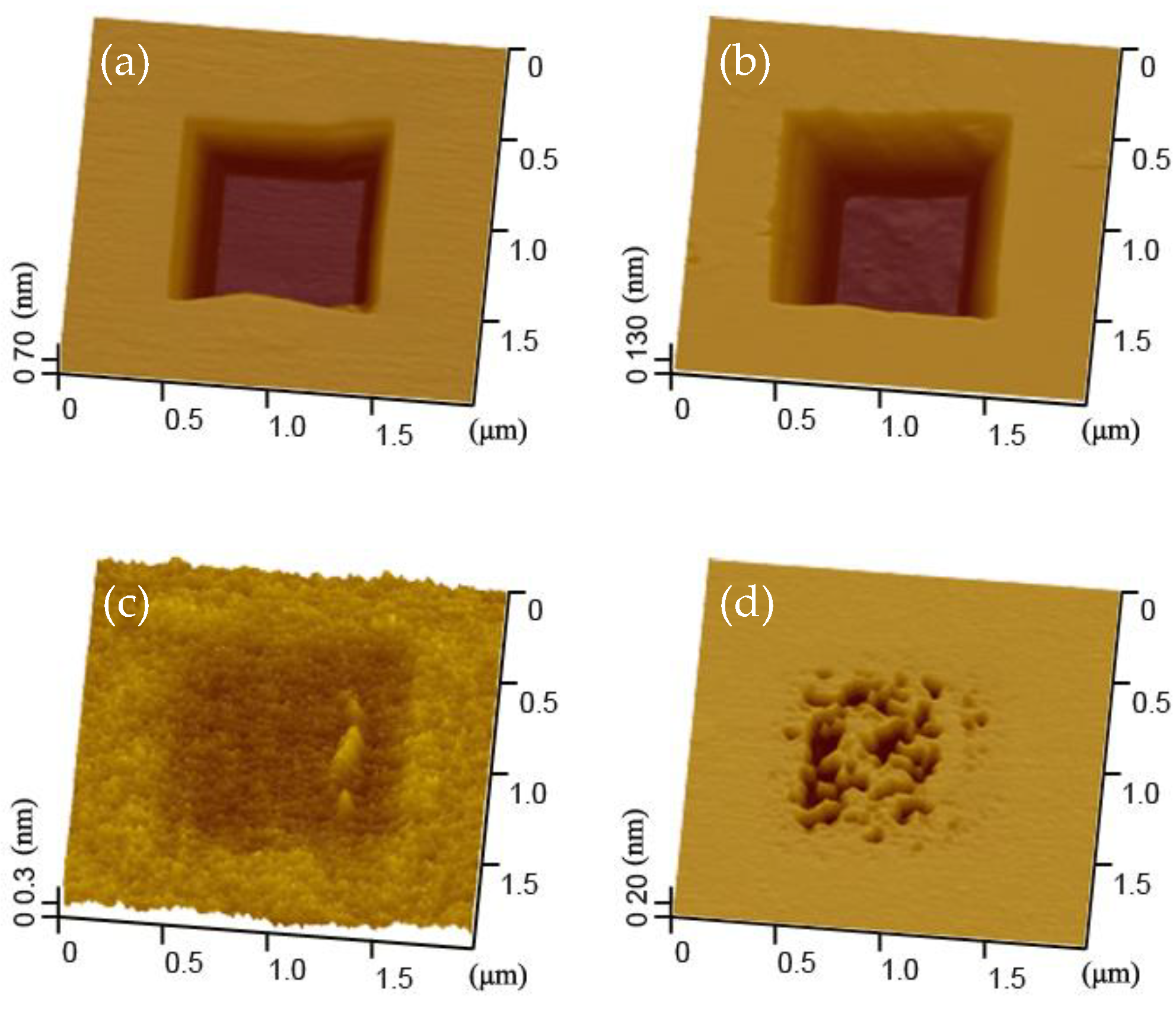
3.1. Wear of Silicon at 60% RH and 90% RH
3.2. Evolution of the Wear Scar in KOH Solution
4. Discussion
4.1. Effect of Adhesion and Friction Force on the Wear Behaviors of Si/SiO2 Pair
4.2. Effect of Water Structure on the Wear Mechanism of Si/SiO2 Pair
5. Conclusions
- The wear of silicon sample was serious and very slight at 60% RH and 90% RH, respectively. Under the given conditions, the wear depth on silicon sample surface was around 3 nm at 60% RH. However, the wear scar was indistinct and the wear depth was close to the roughness of the silicon sample at 90% RH. Based on the repeated wear test at 60% RH, the slight wear at 90% RH was not caused by the wear of SiO2 tip;
- At 60% RH, the native oxide layer was totally removed by the wear tests and the wear scar was etched to a deeper pit after the etching tests. On the other hand, after the etching tests for 16 min, a porous pit with the depth of ~41 nm would evolve from the indistinct wear scar at 90% RH;
- Since the contact pressure (<1.2 GPa) in the present study was much smaller than the critical yield stress of silicon (7.0 GPa), the wear of silicon sample might be dominated by the tribochemistry instead of mechanical interactions. Even so, the tribochemical reactions would be still affected by the mechanical interactions in the form of the dissipated energy;
- Further analysis indicated that the tribochemical reactions were related to the formation of Si-O-Si chemical bond bridges between the interfaces of Si/SiO2 pair. Due to the different water structures at various RHs, the formation of the chemical bond bridges would be facilitated and restrained at 60% RH and 90% RH, respectively. As a result, the dissipated energy and the wear of silicon sample were different at 60% RH and 90% RH.
Author Contributions
Funding
Conflicts of Interest
References
- Malka, D.; Cohen, M.; Turkiewicz, J.; Zalevsky, Z. Optical micro-multi-racetrack resonator filter based on SOI waveguides. Photonics Nanostruct. Fundam. Appl. 2015, 16, 16–23. [Google Scholar] [CrossRef]
- Ben Zaken, B.; Zanzury, T.; Malka, D. An 8-Channel Wavelength MMI Demultiplexer in Slot Waveguide Structures. Materials 2016, 9, 881. [Google Scholar] [CrossRef] [PubMed]
- Katz, O.; Malka, D. Design of novel SOI 1 × 4 optical power splitter using seven horizontally slotted waveguides. Photonics Nanostruct. Fundam. Appl. 2017, 25, 9–13. [Google Scholar] [CrossRef]
- Birleanu, C.; Pustan, M.; Rusu, F.; Dudescu, C.; Muller, R.; Baracu, A. Relative humidity influence on adhesion effect in MEMS flexible structures. Microsyst. Technol. 2018. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, S.-J.; Hsueh, T.-J. Three-Dimensional ZnO Nanostructure Based Gas and Humidity Sensors. J. Nanosci. Nanotechno. 2018, 18, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, B.; Liu, J. Effect of interdigital electrode gap on the performance of SnO2-modified MoS2 capacitive humidity sensor. Sens. Actuators B 2018, 271, 256–263. [Google Scholar] [CrossRef]
- Wang, X.; Kim, S.H.; Chen, C.; Chen, L.; He, H.; Qian, L. Humidity Dependence of Tribochemical Wear of Monocrystalline Silicon. ACS Appl. Mat. Interfaces 2015, 7, 14785–14792. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, S.H.; Yu, B.; Qian, L.; Zhou, Z. Role of Tribochemistry in Nanowear of Single-Crystalline Silicon. ACS Appl. Mat. Interfaces 2012, 4, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, H.; Wang, X.; Kim, S.H.; Qian, L. Tribology of Si/SiO2 in Humid Air: Transition from Severe Chemical Wear to Wearless Behavior at Nanoscale. Langmuir 2015, 31, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiao, C.; Wang, X.; Zhang, P.; Chen, L.; Qi, Y.; Qian, L. Role of water in the tribochemical removal of bare silicon. Appl. Surf. Sci. 2016, 390, 696–702. [Google Scholar] [CrossRef]
- Wang, X.D.; Guo, J.; Chen, C.; Chen, L.; Qian, L.M. A simple method to control nanotribology behaviors of monocrystalline silicon. J. Appl. Phys. 2016, 119, 044304. [Google Scholar] [CrossRef]
- Chung, K.-H.; Kim, D.-E. Fundamental investigation of micro wear rate using an atomic force microscope. Tribol. Lett. 2003, 15, 135–144. [Google Scholar] [CrossRef]
- Asay, D.B.; Hsiao, E.; Kim, S.H. Effects of adsorbate coverage and capillary on nano-asperity friction in atmosphere containing organic vapor. J. Appl. Phys. 2011, 110, 064326. [Google Scholar] [CrossRef]
- Clint, J.H. Adhesion and components of solid surface energies. Curr. Opin. Colloid Interface Sci. 2001, 6, 28–33. [Google Scholar] [CrossRef]
- George, M.; Goddard, D. The characterisation of rough particle contacts by atomic force microscopy. J. Colloid Interface Sci. 2006, 299, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, U.D. A generalized analytical model for the elastic deformation of an adhesive contact between a sphere and a flat surface. J. Colloid Interface Sci. 2003, 261, 99–106. [Google Scholar] [CrossRef]
- Chen, L.; Wen, J.; Zhang, P.; Yu, B.; Chen, C.; Ma, T.; Lu, X.; Kim, S.H.; Qian, L. Nanomanufacturing of silicon surface with a single atomic layer precision via mechanochemical reactions. Nat. Commun. 2018, 9, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E, S.; Zhang, X.; Li, C.; Long, X.; Li, Z.; Ma, S.; Li, Q.; Geng, R.; Lu, W.; Yao, Y. Tribological characteristics of boron nitride nanosheets on silicon wafers obtained by the reaction of MgB2 and NH3. Surf. Coat. Technol. 2018, 340, 36–44. [Google Scholar] [CrossRef]
- Richter, J.; Chrapoński, J. Application of FIB in the preparation of TEM specimens of experimental supercoarse WC-Co.(Ni) composites. Int. J. Refract. Met. Hard Mat. 2018, 75, 163–169. [Google Scholar] [CrossRef]
- Guo, J.; Yu, B.; Wang, X.; Qian, L. Nanofabrication on monocrystalline silicon through friction-induced selective etching of Si3N4 mask. Nanoscale Res. Lett. 2014, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Imoto, R.; Stevens, F.; Langford, S.; Dickinson, J. Atomic force microscopy studies of chemical–mechanical processes on silicon(100) surfaces. Appl. Phys. A 2009, 94, 35–43. [Google Scholar] [CrossRef]
- Niwano, M.; Miura, T.; Kimura, Y.; Tajima, R.; Miyamoto, N. Real-time, in situ infrared study of etching of Si (100) and (111) surfaces in dilute hydrofluoric acid solution. J. Appl. Phys. 1996, 79, 3708–3713. [Google Scholar] [CrossRef]
- Seidel, H.; Csepregi, L.; Heuberger, A.; Baumgärtel, H. Anisotropic etching of crystalline silicon in alkaline solutions I. Orientation dependence and behavior of passivation layers. J. Electrochem. Soc. 1990, 137, 3612–3626. [Google Scholar] [CrossRef]
- Muto, S.; Matsui, T.; Tanabe, T. Non-destructive structural analysis of surface blistering by TEM and EELS in a reflection configuration. J. Nucl. Mater. 2001, 290–293, 131–134. [Google Scholar] [CrossRef]
- Zhou, N.; Lin, M.; Zhou, L.; Hu, Q.; Fang, H.; Wang, S. A modified cooling process in directional solidification of multicrystalline silicon. J. Cryst. Growth 2013, 381, 22–26. [Google Scholar] [CrossRef]
- Asuha, H.K.; Maida, O.; Takahashi, M.; Iwasa, H. Nitric acid oxidation of Si to form ultrathin silicon dioxide layers with a low leakage current density. J. Appl. Phys. 2003, 94, 7328–7335. [Google Scholar] [CrossRef]
- Schiavon, M.A.; Redondo, S.U.A.; Pina, S.R.O.; Yoshida, I.V.P. Investigation on kinetics of thermal decomposition in polysiloxane networks used as precursors of silicon oxycarbide glasses. J. Non-Cryst. Solids 2002, 304, 92–100. [Google Scholar] [CrossRef]
- Ootani, Y.; Xu, J.; Hatano, T.; Kubo, M. Contrasting Roles of Water at Sliding Interfaces between Silicon-Based Materials: First-Principles Molecular Dynamics Sliding Simulations. J. Phys. Chem. C 2018, 122, 10459–10467. [Google Scholar] [CrossRef]
- Deng, S.; Feng, N.; Kang, S.; Zhu, J.; Yu, B.; Chen, J.; Xie, X. Mechanochemical formation of chlorinated phenoxy radicals and their roles in the remediation of hexachlorobenzene contaminated soil. J. Hazard. Mater. 2018, 352, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Zolper, T.; Li, Z.; Chen, C.; Jungk, M.; Marks, T.; Chung, Y.-W.; Wang, Q. Lubrication Properties of Polyalphaolefin and Polysiloxane Lubricants: Molecular Structure–Tribology Relationships. Tribol. Lett. 2012, 48, 355–365. [Google Scholar] [CrossRef]
- Kobayashi, S. A model for oxygen precipitation in Czochralski silicon during crystal growth. J. Cryst. Growth 1997, 174, 163–169. [Google Scholar] [CrossRef]
- Tiller, W.A.; Hahn, S.; Ponce, F.A. Thermodynamic and kinetic considerations on the equilibrium shape for thermally induced microdefects in Czochralski silicon. J. Appl. Phys. 1986, 59, 3255–3266. [Google Scholar] [CrossRef]
- Bhushan, B.; Israelachvili, J.N.; Landman, U. Nanotribology: Friction, wear and lubrication at the atomic scale. Nature 1995, 374, 607–616. [Google Scholar] [CrossRef]
- Ramalho, A.; Miranda, J.C. The relationship between wear and dissipated energy in sliding systems. Wear 2006, 260, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Huq, M.Z.; Celis, J.P. Expressing wear rate in sliding contacts based on dissipated energy. Wear 2002, 252, 375–383. [Google Scholar] [CrossRef]
- Asay, D.B.; Kim, S.H. Effects of adsorbed water layer structure on adhesion force of silicon oxide nanoasperity contact in humid ambient. J. Chem. Phys. 2006, 124, 174712. [Google Scholar] [CrossRef] [PubMed]
- Barnette, A.L.; Kim, S.H. Attenuated Total Reflectance Infrared Spectroscopy Study of Hysteresis of Water and n-Alcohol Coadsorption on Silicon Oxide. Langmuir 2012, 28, 15529–15536. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, F. Single Asperity Tribochemical Wear of Silicon by Atomic Force Microscopy; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Wang, Y.G.; Zhang, L.C.; Biddut, A. Chemical effect on the material removal rate in the CMP of silicon wafers. Wear 2011, 270, 312–316. [Google Scholar] [CrossRef]
- Sinan Haliyo, D.; Régnier, S.; Bidaud, P. Manipulation of Micro-objects Using Adhesion Forces and Dynamical Effects. In Experimental Robotics VIII; Siciliano, B., Dario, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 5, pp. 382–391. [Google Scholar]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, J.; Xu, L.; Cheng, G.; Qian, L. An Investigation of the Wear on Silicon Surface at High Humidity. Materials 2018, 11, 1027. https://doi.org/10.3390/ma11061027
Wang X, Guo J, Xu L, Cheng G, Qian L. An Investigation of the Wear on Silicon Surface at High Humidity. Materials. 2018; 11(6):1027. https://doi.org/10.3390/ma11061027
Chicago/Turabian StyleWang, Xiaodong, Jian Guo, Lin Xu, Guanggui Cheng, and Linmao Qian. 2018. "An Investigation of the Wear on Silicon Surface at High Humidity" Materials 11, no. 6: 1027. https://doi.org/10.3390/ma11061027
APA StyleWang, X., Guo, J., Xu, L., Cheng, G., & Qian, L. (2018). An Investigation of the Wear on Silicon Surface at High Humidity. Materials, 11(6), 1027. https://doi.org/10.3390/ma11061027



