Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution
Abstract
:1. Introduction
2. Materials and Experimental
2.1. Materials
2.2. Electrochemical Measurements
2.3. Surface Analysis
2.4. Calculation Methods
3. Results and Discussion
3.1. EIS Analysis
3.2. Potentiodynamic Polarization Curves Analysis
3.3. Morphology Analysis
3.4. FT-IR Spectra
3.5. XPS Measurements
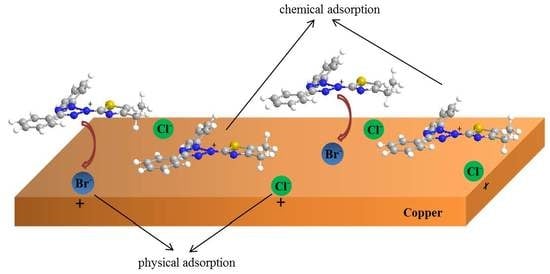
3.6. Adsorption Isotherm Analysis
3.7. Theoretical Calculation
4. Conclusions
- (1)
- The electrochemical tests, SEM, and AFM measurements demonstrate that the MTT as a mixed-type inhibitor can prevent copper corrosion effectively, and is efficiency increased with the addition of the MTT concentration.
- (2)
- The MTT molecules form metal complex film by N and S atoms to inhibit corrosion from FT-IR spectra and XPS spectra.
- (3)
- Adsorption isotherm studies demonstrated that adsorption for this work was a spontaneous mixed physical and chemical adsorption which obeyed Langmuir adsorption isotherm.
- (4)
- The theoretical calculations reflected that MTT molecules processed a stronger adsorption on copper surface by a parallel mode, occupying the active site to the greatest extent by hydrophobic film, and thus, showing excellent inhibition effect.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mousavi, M.; Baghgoli, T. Application of interaction energy in quantitative structure-inhibition relationship study of some benzenethiol derivatives on copper corrosion. Corros. Sci. 2016, 105, 170–176. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Zhang, D.; Wang, Y.; Yu, Y.; Gao, L.; Huang, M. Preparation of triazole compounds via click chemistry reaction and formation of the protective self-assembled membrane against copper corrosion. Colloids Surf. A 2018, 550, 145–154. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Han, J.; Chen, X.; Min, Y. A novel combination approach for the preparation of superhydrophobic surface on copper and the consequent corrosion resistance. Corros. Sci. 2016, 110, 105–113. [Google Scholar] [CrossRef]
- Gerengi, H.; Mielniczek, M.; Gece, G.; Solomon, M.M. Experimental and quantum chemical evaluation of 8-Hydroxyquinoline as a corrosion inhibitor for copper in 0.1 M HCl. Ind. Eng. Chem. Res. 2016, 55, 9614–9624. [Google Scholar] [CrossRef]
- Mo, S.; Luo, H.Q.; Li, N.B. Study on the influences of two thiazole flavor ingredients on Cu corrosion caused by chloride ion. J. Colloid Interface Sci. 2017, 505, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Tasić, Ž.Z.; Petrović Mihajlović, M.B.; Radovanović, M.B.; Simonović, A.T.; Antonijević, M.M. Cephradine as corrosion inhibitor for copper in 0.9% NaCl solution. J. Mol. Struct. 2018, 1159, 46–54. [Google Scholar] [CrossRef]
- Ghelichkhah, Z.; Sharifi-Asl, S.; Farhadi, K.; Banisaied, S.; Ahmadi, S.; Macdonald, D.D. l-cysteine/polydopamine nanoparticle-coatings for copper corrosion protection. Corros. Sci. 2015, 91, 129–139. [Google Scholar] [CrossRef]
- Hong, S.; Chen, W.; Zhang, Y.; Luo, H.Q.; Li, M.; Li, N.B. Investigation of the inhibition effect of trithiocyanuric acid on corrosion of copper in 3.0 wt.% NaCl. Corros. Sci. 2013, 66, 308–314. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, B.; Liang, Y.; Song, S.; Liu, C. Corrosion control of copper in 3.5 wt.% NaCl Solution by Domperidone: Experimental and Theoretical Study. Corros. Sci. 2014, 85, 77–86. [Google Scholar] [CrossRef]
- Finšgar, M.; Kek Merl, D. An electrochemical, long-term immersion, and XPS study of 2-mercaptobenzothiazole as a copper corrosion inhibitor in chloride solution. Corros. Sci. 2014, 83, 164–175. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Li, W.; Yin, L.; He, J.; Wu, J. Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros. Sci. 2014, 80, 383–392. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, S.; Zheng, D.; Wang, J.; Zhang, X.; Du, R.; Song, G.; Lin, C. Multifunctional inhibition based on layered double hydroxides to comprehensively control corrosion of carbon steel in concrete. Corros. Sci. 2017, 126, 166–179. [Google Scholar] [CrossRef]
- Chen, J.; Qin, Z.; Martino, T.; Shoesmith, D.W. Non-uniform film growth and micro/macro-galvanic corrosion of copper in aqueous sulphide solutions containing chloride. Corros. Sci. 2017, 114, 72–78. [Google Scholar] [CrossRef]
- Yu, H.J.; Li, C.X.; Yuan, B.Y.; Li, L.; Wang, C. The inhibitive effects of AC-treated mixed self-assembled monolayers on copper corrosion. Corros. Sci. 2017, 120, 231–238. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, L.; Zhao, Z.H.; Zheng, S.S.; Xu, Y.; Xiang, B.; Kaya, S. Anticorrosion potential of domperidone on copper in different concentration of hydrochloric acid solution. J. Adhes. Sci. Technol. 2018, 32, 1485–1502. [Google Scholar] [CrossRef]
- Ma, X.; Xu, L.; Wang, W.; Lin, Z.; Li, X. Synthesis and characterisation of composite nanoparticles of mesoporous silica loaded with inhibitor for corrosion protection of Cu-Zn alloy. Corros. Sci. 2017, 120, 139–147. [Google Scholar] [CrossRef]
- Nam, N.D.; Thang, V.Q.; Hoai, N.T.; Van Hien, P. Yttrium 3-(4-nitrophenyl)-2-propenoate used as inhibitor against copper alloy corrosion in 0.1 M NaCl solution. Corros. Sci. 2016, 112, 451–461. [Google Scholar] [CrossRef]
- Kovacevic, N.; Milosev, I.; Kokalj, A. How relevant is the adsorption bonding of imidazoles and triazoles for their corrosion inhibition of copper? Corros. Sci. 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Y.; Jing, C.; Huang, H.; Li, H.; Zhang, S.; Gao, F. Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: A new thought for the design of organic inhibitors. Corros. Sci. 2016, 113, 64–77. [Google Scholar] [CrossRef]
- Qafsaoui, W.; Kendig, M.W.; Joiret, S.; Perrot, H.; Takenouti, H. Ammonium pyrrolidine dithiocarbamate adsorption on copper surface in neutral chloride media. Corros. Sci. 2016, 106, 96–107. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Li, R.; Wang, D.; Li, T.; Yuan, M.; Wang, J. Simple preparation of aminothiourea-modified chitosan as corrosion inhibitor and heavy metal ion adsorbent. J. Colloid Interface Sci. 2014, 417, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Zhang, S.; Yan, S.; Zou, X.; Chen, S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Corros. Sci. 2017, 126, 295–304. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part I. Long-term immersion, 3D-profilometry, and electrochemistry. Corros. Sci. 2013, 72, 82–89. [Google Scholar] [CrossRef]
- Bokati, K.S.; Dehghanian, C.; Yari, S. Corrosion inhibition of copper, mild steel and galvanically coupled copper-mild steel in artificial sea water in presence of 1H-benzotriazole, sodium molybdate and sodium phosphate. Corros. Sci. 2017, 126, 272–285. [Google Scholar] [CrossRef]
- Lozano, I.; Mazario, E.; Olivares-Xometl, C.O.; Likhanova, N.V.; Herrasti, P. Corrosion behaviour of API 5LX52 steel in HCl and H2SO4 media in the presence of 1,3-dibencilimidazolio acetate and 1,3-dibencilimidazolio dodecanoate ionic liquids as inhibitors. Mater. Chem. Phys. 2014, 147, 191–197. [Google Scholar] [CrossRef]
- Ha, J.H.; Cho, J.-H.; Kim, J.H.; Cho, B.W.; Oh, S.H. 1-Butyl-1-methylpyrrolidinium chloride as an effective corrosion inhibitor for stainless steel current collectors in magnesium chloride complex electrolytes. J. Power Sources 2017, 355, 90–97. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Qiang, Y.; Xu, S.; Tan, B.; Chen, S. The synergistic corrosion inhibition study of different chain lengths ionic liquids as green inhibitors for X70 steel in acidic medium. Mater. Chem. Phys. 2018, 215, 229–241. [Google Scholar] [CrossRef]
- Kannan, P.; Rao, T.S.; Rajendran, N. Anti-corrosion behavior of benzimidazoliumtetra fluroborate ionic liquid in acid medium using electrochemical noise technique. J. Mol. Struct. 2016, 222, 586–595. [Google Scholar]
- Zheng, X.; Zhang, S.; Li, W.; Gong, M.; Yin, L. Experimental and theoretical studies of two imidazolium-based ionic liquids as inhibitors for mild steel in sulfuric acid solution. Corros. Sci. 2015, 95, 168–179. [Google Scholar] [CrossRef]
- Mehmeti, V.V.; Berisha, A.R. Corrosion study of mild steel in aqueous sulfuric acid solution using 4-methyl-4H-1,2,4-triazole-3-thiol and 2-mercaptonicotinic acid-An experimental and theoretical study. Front. Chem. 2017, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kaya, S.; Obot, I.B.; Zheng, X.; Qiang, Y. Toward understanding the anticorrosive mechanism of some thiourea derivatives for carbon steel corrosion: A combined DFT and molecular dynamics investigation. J. Colloid Interface Sci. 2017, 506, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.B.; Mouanga, M.; Druart, M.E.; Recloux, I.; Cossement, D.; Olivier, M.G. A SVET study of the inhibitive effects of benzotriazole and cerium chloride solely and combined on an aluminium/copper galvanic coupling model. Corros. Sci. 2016, 110, 143–156. [Google Scholar] [CrossRef]
- Sigircik, G.; Yildirim, D.; Tuken, T. Synthesis and inhibitory effect of N,N′-bis(1-phenylethanol) ethylenediamine against steel corrosion in HCl Media. Corros. Sci. 2017, 120, 184–193. [Google Scholar] [CrossRef]
- Hu, Z.; Meng, Y.; Ma, X.; Zhu, H.; Li, J.; Li, C.; Cao, D. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 2016, 112, 563–575. [Google Scholar] [CrossRef]
- Metikos-Hukovic, M.; Babic, R.; Paic, I. Copper corrosion at various pH values with and without the inhibitor. J. Appl. Electrochem. 2000, 30, 617–624. [Google Scholar] [CrossRef]
- Tian, H.W.; Li, W.H.; Hou, B.R.; Wang, D.P. Insights into corrosion inhibition behavior of multi-active compounds for X65 pipeline steel in acidic oilfield formation water. Corros. Sci. 2017, 117, 43–58. [Google Scholar] [CrossRef]
- Kannan, P.; Rao, T.S.; Rajendran, N. Improvement in the corrosion resistance of carbon steel in acidic condition using naphthalen-2-ylnaphthalene-2-carboxammide inhibitor. J. Colloid Interface Sci. 2018, 512, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Mashuga, M.; Olasunkanmi, L.; Adekunle, A.; Yesudass, S.; Kabanda, M.; Ebenso, E. Adsorption, thermodynamic and quantum chemical studies of 1-hexyl-3-methylimidazolium based ionic liquids as corrosion inhibitors for mild steel in HCl. Materials 2015, 8, 3607–3632. [Google Scholar] [CrossRef]
- Li, W.; Landon, J.; Irvin, B.; Zheng, L.F.; Ruh, K.; Kong, L.; Pelgen, J.; Link, D.; Figueroa, J.D.; Thompson, J.; et al. Use of Carbon Steel for Construction of Post-combustion CO2 Capture Facilities: A Pilot-Scale Corrosion Study. Ind. Eng. Chem. Res. 2017, 56, 4792–4803. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Xu, S.; Yin, L. The effect of 5-nitroindazole as an inhibitor for the corrosion of copper in a 3.0% NaCl solution. RSC Adv. 2015, 5, 63866–63873. [Google Scholar] [CrossRef]
- Song, P.; Guo, X.-Y.; Pan, Y.-C.; Shen, S.; Sun, Y.; Wen, Y.; Yang, H.-F. Insight in cysteamine adsorption behaviors on the copper surface by electrochemistry and Raman spectroscopy. Electrochim. Acta 2013, 89, 503–509. [Google Scholar] [CrossRef]
- Coelho, L.B.; Cossement, D.; Olivier, M.G. Benzotriazole and cerium chloride as corrosion inhibitors for AA2024-T3: An EIS investigation supported by SVET and ToF-SIMS analysis. Corros. Sci. 2018, 130, 177–189. [Google Scholar] [CrossRef]
- Stoian, D.; Bansode, A.; Medina, F.; Urakawa, A. Catalysis under microscope: Unraveling the mechanism of catalyst de- and re-activation in the continuous dimethyl carbonate synthesis from CO2 and methanol in the presence of a dehydrating agent. Catal. Today 2017, 283, 2–10. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Gong, Y.; Gao, F.; Luo, Z.; Zhang, S.; Li, H. Water soluble corrosion inhibitors for copper in 3.5 wt.% sodium chloride solution. Corros. Sci. 2017, 123, 339–350. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, F.; Peng, J.; Dong, Y.; Li, W.; Huang, Y. A robust bilayer nanofilm fabricated on copper foam for oil–water separation with improved performances. J. Mater. Chem. A 2016, 4, 10294–10303. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Han, G.-C.; Chen, S.-L.; Chen, Z. Inhibition of copper corrosion by the formation of Schiff base self-assembled monolayers. Appl. Surf. Sci. 2016, 389, 601–608. [Google Scholar] [CrossRef]
- Welbourn, R.J.L.; Truscott, C.L.; Skoda, M.W.A.; Zarbakhsh, A.; Clarke, S.M. Corrosion and inhibition of copper in hydrocarbon solution on a molecular level investigated using neutron reflectometry and XPS. Corros. Sci. 2017, 115, 68–77. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Huang, Y.; Qi, Y. The polymeric nanofilm of triazinedithiolsilane fabricated by self-assembled technique on copper surface. Part 2: Characterization of composition and morphology. Appl. Surf. Sci. 2015, 356, 191–202. [Google Scholar] [CrossRef]
- Appa Rao, B.V.; Narsihma Reddy, M. Formation, characterization and corrosion protection efficiency of self-assembled 1-octadecyl-1H-imidazole films on copper for corrosion protection. Arab. J. Chem. 2017, 10, S3270–S3283. [Google Scholar] [CrossRef]
- Yang, W.; Li, T.; Zhou, H.; Huang, Z.; Fu, C.; Chen, L.; Li, M.; Kuang, Y. Electrochemical and anti-corrosion properties of octadecanethiol and benzotriazole binary self-assembled monolayers on copper. Electrochim. Acta 2016, 220, 245–251. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Z.; Guo, X. Inhibition behavior of an imidazoline inhibitor for carbon steel in a supercritical CO2/H2O system. J. Electrochem. Soc. 2017, 164, C602–C609. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Li, J.; Zhang, D.; Gao, L. In situ click-assembling monolayers on copper surface with enhanced corrosion resistance. Corros. Sci. 2016, 113, 133–144. [Google Scholar] [CrossRef]
- Khalil, N. Quantum chemical approach of corrosion inhibition. Electrochim. Acta 2003, 48, 2635–2640. [Google Scholar] [CrossRef]
- Frau, J.; Glossman-Mitnik, D. Conceptual DFT descriptors of amino acids with potential corrosion inhibition properties calculated with the latest minnesota density functionals. Front. Chem. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Zhang, S.; Guo, L.; Zheng, X.; Xiang, B.; Chen, S. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- Guo, L.; Safi, Z.S.; Kaya, S.; Shi, W.; Tüzün, B.; Altunay, N.; Kaya, C. Anticorrosive effects of some thiophene derivatives against the corrosion of iron: A computational study. Front. Chem. 2018, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Zuriaga-Monroy, C.; Oviedo-Roa, R.; Montiel-Sanchez, L.E.; Vega-Paz, A.; Marin-Cruz, J.; Martinez-Magadan, J.M. Theoretical study of the aliphatic-chain length’s electronic effect on the corrosion inhibition activity of methylimidazole-based ionic liquids. Ind. Eng. Chem. Res. 2016, 55, 3506–3516. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, S.D.; Fu, H.; Xie, X.G. Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H3PO4 solution. Corros. Sci. 2014, 78, 29–42. [Google Scholar] [CrossRef]
- Guo, L.; Ou, Y.; Shen, X.; Kaya, S.; Shi, W.; Zhang, R.; Zheng, X.; Wang, J. Specific adsorption of halide ions on iron surface: A combined electrochemical and Monte Carlo simulation investigation. Int. J. Electrochem. Sci. 2017, 12, 7064–7074. [Google Scholar] [CrossRef]













| C (mM) | Rf (kΩ cm2) | Rct (kΩ cm2) | Rp (Ω cm2) | Q1 | Q2 | W × 10−3 (Ω cm2) | η (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| n1 | Cf (μF cm−2) | n2 | Cdl (μF cm−2) | |||||||
| Blank | 0 | 0.095 | 1.970 | 2.065 | 1 | 8.82 | 0.57 | 681.5 | 1.57 | – |
| MTT | 0.05 | 0.021 | 8.675 | 8.696 | 1 | 8.22 | 0.56 | 601.7 | – | 76.25 |
| 0.2 | 0.104 | 13.260 | 13.364 | 0.88 | 2.57 | 0.60 | 106.3 | – | 84.55 | |
| 0.5 | 0.133 | 17.310 | 17.443 | 0.94 | 0.89 | 0.57 | 116.8 | – | 88.16 | |
| 1 | 0.089 | 21.580 | 21.669 | 0.94 | 0.96 | 0.59 | 213.8 | – | 90.47 | |
| 5 | 0.382 | 47.990 | 48.372 | 0.99 | 0.38 | 0.52 | 123.8 | – | 95.73 | |
| C (mM) | Ecorr (mV) | Icorr (A cm−2) | βc (mV dec−1) | βa (mV dec−1) | η (%) | |
|---|---|---|---|---|---|---|
| Blank | 0 | −186 | 4.124 × 10−6 | −167.2 | 59.4 | – |
| MTT | 0.05 | −144 | 1.073 × 10−6 | −149.7 | 78.4 | 73.98 |
| 0.2 | −168 | 1.010 × 10−6 | −159.2 | 78.2 | 75.51 | |
| 0.5 | −176 | 5.877 × 10−7 | −138.1 | 128.7 | 85.75 | |
| 1 | −181 | 4.559 × 10−7 | −149.1 | 124.5 | 88.95 | |
| 5 | −186 | 3.184 × 10−7 | −117.7 | 118.4 | 92.28 |
| The Blank | Cu-MTT | |||||
|---|---|---|---|---|---|---|
| Chemical State | Binding Energy (ev) | FWHM | Chemical State | Binding Energy (ev) | FWHM | |
| C1s | C–C/C–H | 284.31 | 1.15 | C–C/C–H | 284.39 | 1.20 |
| C–O–C | 286.16 | 1.15 | C=N/C–S | 285.62 | 1.20 | |
| O–C=O | 287.70 | 1.15 | C–N | 286.61 | 1.20 | |
| O–C=O | 287.71 | 1.20 | ||||
| Cu2p | Cu(0)/Cu(I) | 931.90 | 1.13 | Cu(0)/Cu(I) | 931.95 | 1.7 |
| CuO | 933.80 | 1.7 | ||||
| O1s | CuO/Cu2O | 530.14 | 1.00 | CuO/Cu2O | 530.65 | 1.80 |
| O–C=O | 531.30 | 1.00 | O–C=O | 531.98 | 1.80 | |
| C–O–C | 532.10 | 1.00 | N=N | 398.45 | 1.00 | |
| N1s | N=N | 398.45 | 1.00 | |||
| N-N | 399.20 | 1.00 | ||||
| C=N | 400.10 | 1.00 | ||||
| C–N | 400.70 | 1.00 | ||||
| N:Cu | 401.77 | 1.00 | ||||
| S2p | S–C | 163.75 | 1.40 | |||
| S:Cu | 165.07 | 1.40 |
| Measurements | Kads (×103 L/mol) | ΔG0ads (KJ/mol) |
|---|---|---|
| Polarization | 29.41 | −35.44 |
| EIS | 29.07 | −35.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Zhang, S.; Qiang, Y.; Xu, Y.; Guo, L.; Madkour, L.H.; Chen, S. Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution. Materials 2018, 11, 1042. https://doi.org/10.3390/ma11061042
Feng L, Zhang S, Qiang Y, Xu Y, Guo L, Madkour LH, Chen S. Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution. Materials. 2018; 11(6):1042. https://doi.org/10.3390/ma11061042
Chicago/Turabian StyleFeng, Li, Shengtao Zhang, Yujie Qiang, Yue Xu, Lei Guo, Loutfy H. Madkour, and Shijin Chen. 2018. "Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution" Materials 11, no. 6: 1042. https://doi.org/10.3390/ma11061042
APA StyleFeng, L., Zhang, S., Qiang, Y., Xu, Y., Guo, L., Madkour, L. H., & Chen, S. (2018). Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution. Materials, 11(6), 1042. https://doi.org/10.3390/ma11061042