Nitrogen Oxides Mitigation Efficiency of Cementitious Materials Incorporated with TiO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial Titanium Dioxides
2.2. Cementitious Mixes Preparation
3. Results and Discussion
3.1. Mechanical Properties of Cement Pastes and Mortars
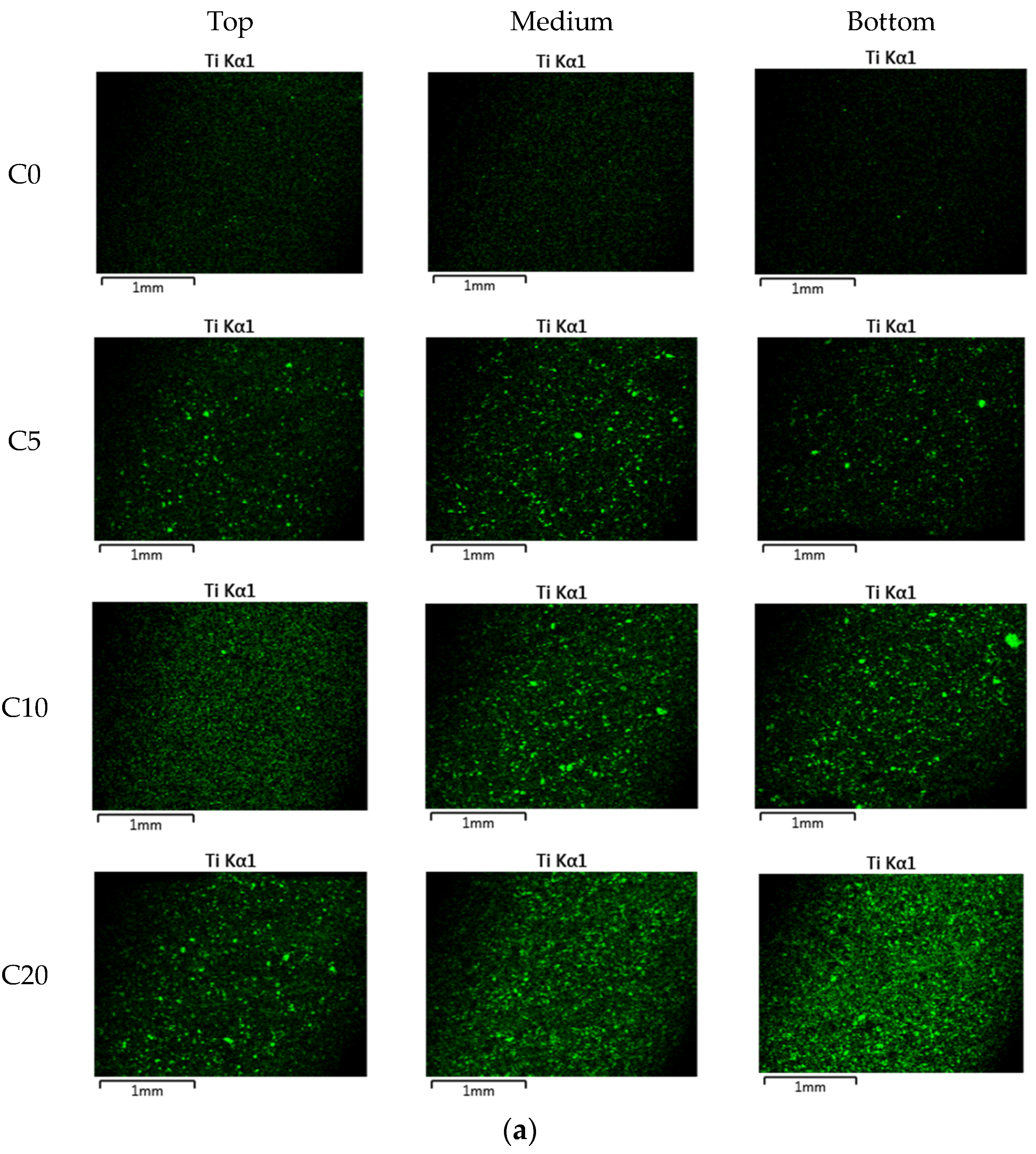
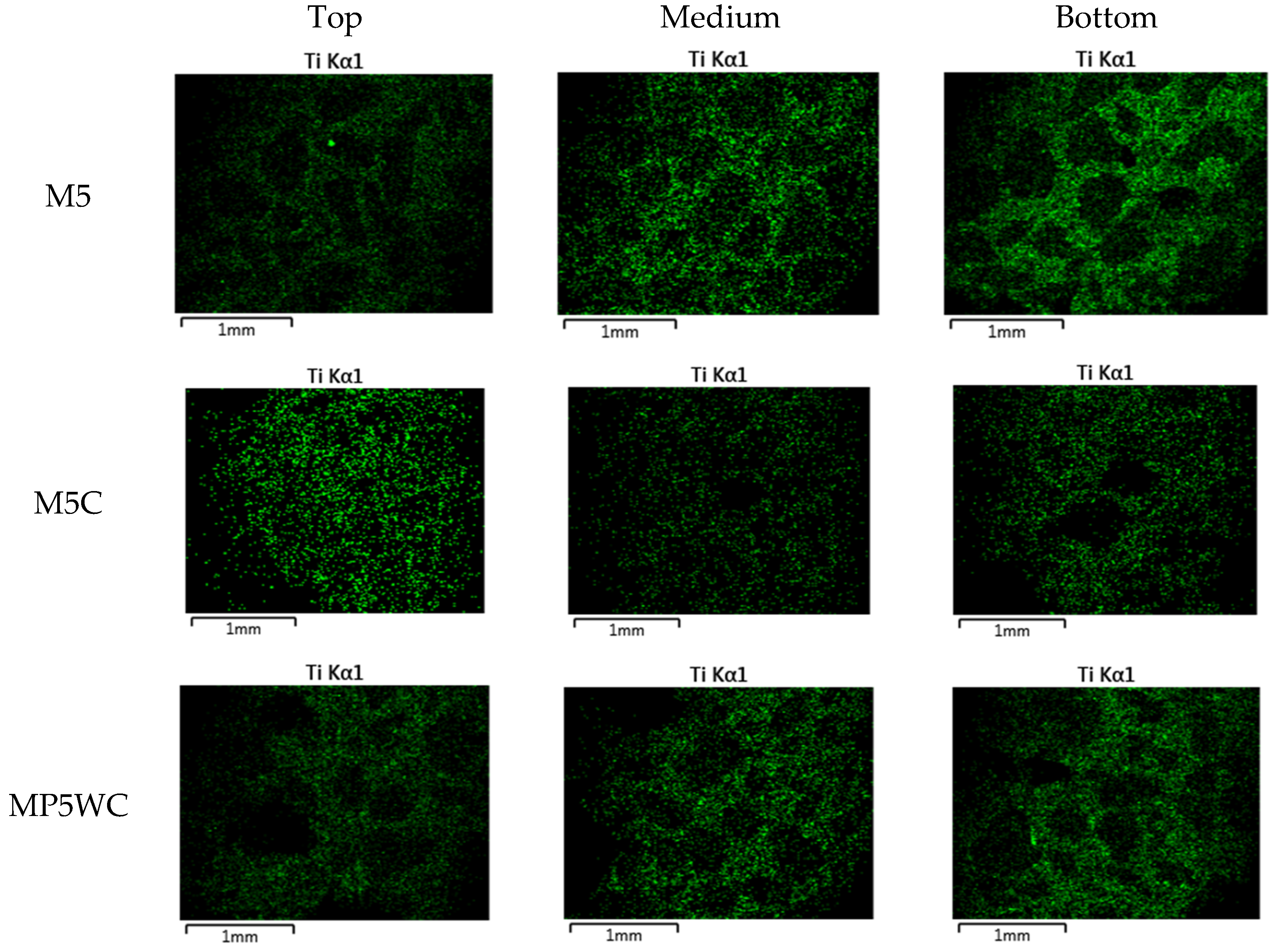
3.2. Nitrogen Oxide (NO) Removal by Cement Mixes Incorporating Titanium Dioxide
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aoa, C.H.; Lee, S.C.; Zoub, S.C.; Makc, C.L. Inhibition effect of SO2 on NO and VOCs during the photodegradation of synchronous indoor air pollutants at parts per billion (ppb) level by TiO2. Appl. Catal. B Environ. 2004, 49, 187–193. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Verran, J.; Stratton, J.; Maltby, J.; Bygott, C. Photocatalytic titania based surfaces: Environmental benefits. Polym. Degrad. Stab. 2008, 93, 1632–1646. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Chen, M.; Chu, J.-W. NO photocatalytic degradation on active concrete road surface—From experiment to real-scale application. J. Clean. Prod. 2011, 19, 1266–1272. [Google Scholar] [CrossRef]
- De Marco, T.; Fava, G.; Guerrini, G.L.; Manganelli, G.; Moriconi, G.; Riderelli, L. Use of photocatalytic products for sustainable construction development. In Proceedings of the Third International Conference on Sustainable Construction Materials and Technologies, Kyoto, Japan, 18–21 August 2013. [Google Scholar]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef]
- Ma, B.; Li, H.; Mei, J.; Li, X.; Chen, F. Effects of Nano-TiO2 on the Toughness and Durability of Cement-Based Material. Adv. Mater. Sci. Eng. 2015, 2015, 583106. [Google Scholar] [CrossRef]
- King, D. Surface and Microstructural Properties of Photocatalytic Cements for Pavement Applications. Master’s Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2016. [Google Scholar]
- Staub de Melo, J.V.; Triches, G. Study of the influence of nano-TiO2 on the properties of Portland cement concrete for application on road surfaces. Road Mater. Pavement Des. 2017, 19, 1011–1026. [Google Scholar] [CrossRef]
- Akhnoukh, A.K. Implementation of nanotechnology in improving the environmental compliance of construction projects in the United States. Part. Sci. Technol. 2018, 36, 357–361. [Google Scholar] [CrossRef]
- Ola, O.; Mercedes Maroro-Valer, M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.; Poon, C. Hydration and properties of nano-TiO2 blended cement composites. Cem. Concr. Compos. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Bouras, P.; Stathatos, E.; Lianos, P. Pure versus metal-ion-doped nanocrystalline titania for photocatalysis. Appl. Catal. B 2015, 73, 51–59. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kurtis, K.E. Influence of TiO2 nanoparticles on early C3S hydration. J. Am. Ceram. Soc. 2010, 93, 3399–3405. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The effects of TiO2 nanoparticles on properties of binary blended concrete. J. Compos. Mater. 2010, 45, 1181–1188. [Google Scholar] [CrossRef]
- Teixeira, K.P.; Rocha, I.P.; Carneiro, L.D.S.; Flores, J.; Dauer, E.A.; Ghahremaninezhad, A. The effect of curing temperature on the properties of cement pastes modified with TiO2 nanoparticles. Materials 2016, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of nano-TiO2 on the mechanical properties of cement mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Hasebe, M.; Edahiro, H. Experimental studies on basic properties of concrete using TIO2 as admixture. In Proceedings of the Thirteenth East Asia-Pacific Conference on Structural Engineering and Construction (EASEC-13), Sapporo, Japan, 11–13 September 2013; pp. 1–8. [Google Scholar]
- Martins, T.; Torgal, F.P.; Miraldo, S.; Aguiar, J.B.; Jesus, C. An experimental investigation on nano-TiO2 and fly ash based high performance concrete. Ind. Concr. J. 2016, 1, 23–31. [Google Scholar]
- Zhao, D.; Wang, F.; Liu, P.; Yang, L.; Hu, S.; Zhang, W. Preparation, physicochemical properties, and long-term performance of photocatalytic ceramsite sand in cementitious materials. Appl. Sci. 2017, 7, 828. [Google Scholar] [CrossRef]
- Lucas, S.S.; Ferreira, V.M.; Barroso de Aguiar, J.L. Incorporation of titanium dioxide nanoparticles in mortars—Influence of microstructure in the hardened state properties and photocatalytic activity. Cem. Concr. Res. 2013, 43, 112–120. [Google Scholar] [CrossRef]
- Folli, A. TiO2 Photocatalysis in Portland Cement System: Fundamentals of Self Cleaning Effect and Air Pollution Mitigation. Ph.D. Thesis, University of Aberdden, Aberdden, UK, 2010; pp. 91–149. [Google Scholar]
- Macphee, D.E.; Folli, A. Photocatalytic concretes—The interface between photocatalysis and cement chemistry. Cem. Concr. Res. 2016, 85, 48–54. [Google Scholar] [CrossRef]
- Qin, Z.B.; Zhang, W.T.; Qian, G.P.; Wu, X.L.; Li, Y. The effects of different ways of adding nano-TiO2 to concrete on the degradation performance of NO2. Mater. Res. Innov. 2015, 19, S10-148–S10-154. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOx pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Rhee, I.; Kim, J.H.; Kim, J.-H.; Roh, Y.S. Sensitivity of NO removal on recycled TiO2 in cement mortar. J. Korean Recycl. Constr. Res. 2016, 4, 388–395. (In Korean) [Google Scholar]
- Shon, H.K.; Vigneswarn, S.; Kim, I.S.; Cho, J.; Kim, G.J.; Kim, J.B.; Kim, J.H. Preparation of titanium dioxide (TiO2) from sludge produced by titanium tetrachloride (TiCl2) flocculation of wastewater. Environ. Sci. Technol. 2007, 41, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Okour, Y.; Shon, H.K.; El Saliby, I.J.; Naidu, R.; Kim, J.B.; Kim, J.H. Preparation and characterisation of titanium dioxide (TiO2) and thiourea-doped titanate nanotubes prepared from wastewater flocculated sludge. Bioresour. Technol. 2010, 101, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Chekli, L.; Kim, J.-B.; Shahid, M.; Shon, H.K.; Kim, P.S.; Lee, W.-S.; Lee, W.E.; Kim, J.-H. NO removal of mortar mixed with Titania produced from Ti-salt flocculated sludge. J. Ind. Eng. Chem. 2014, 20, 3851–3856. [Google Scholar] [CrossRef]
- Angelo, J.; Andrade, L.; Madeira, L.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide; ISO 22197-1:2016; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
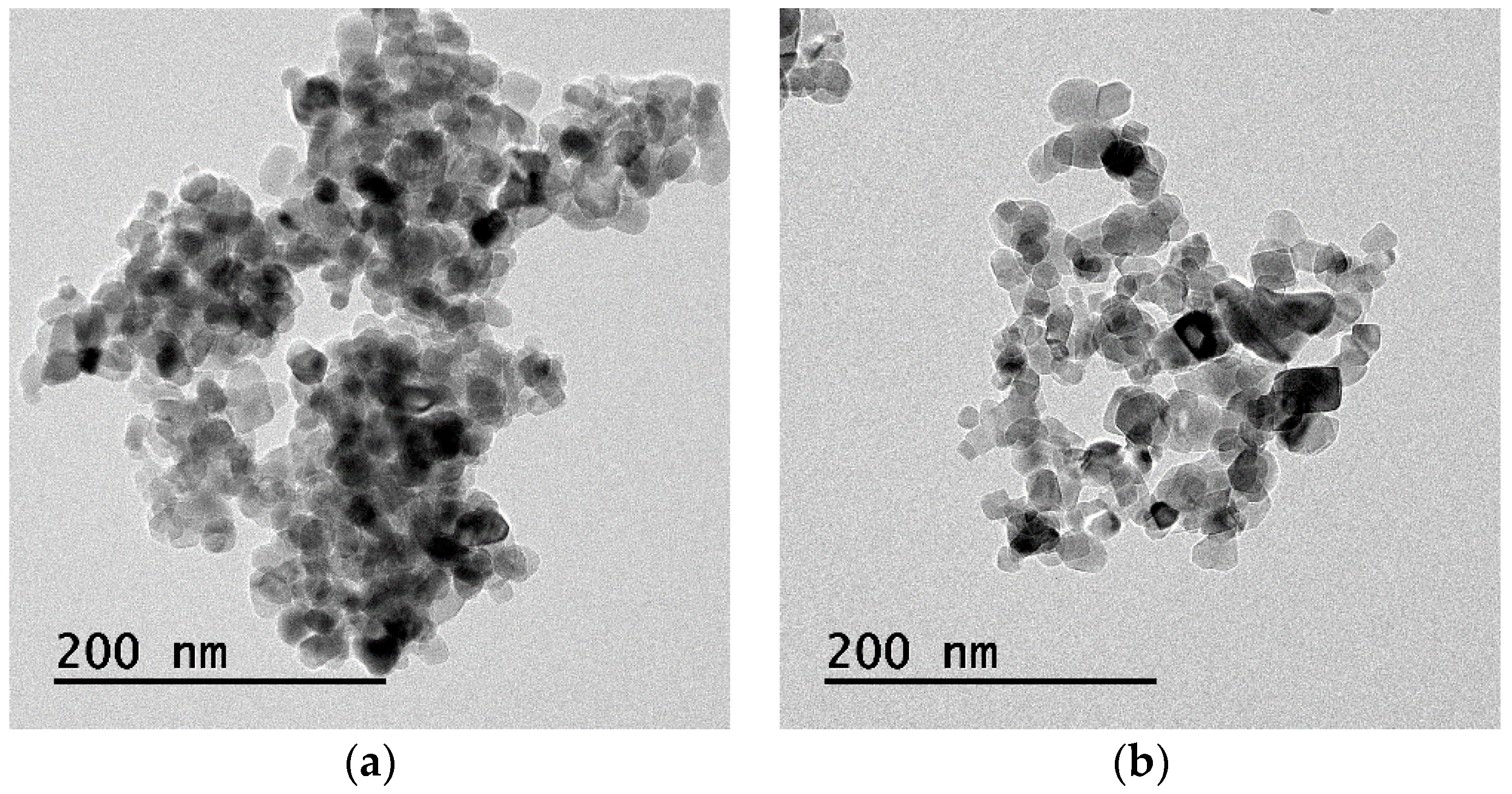






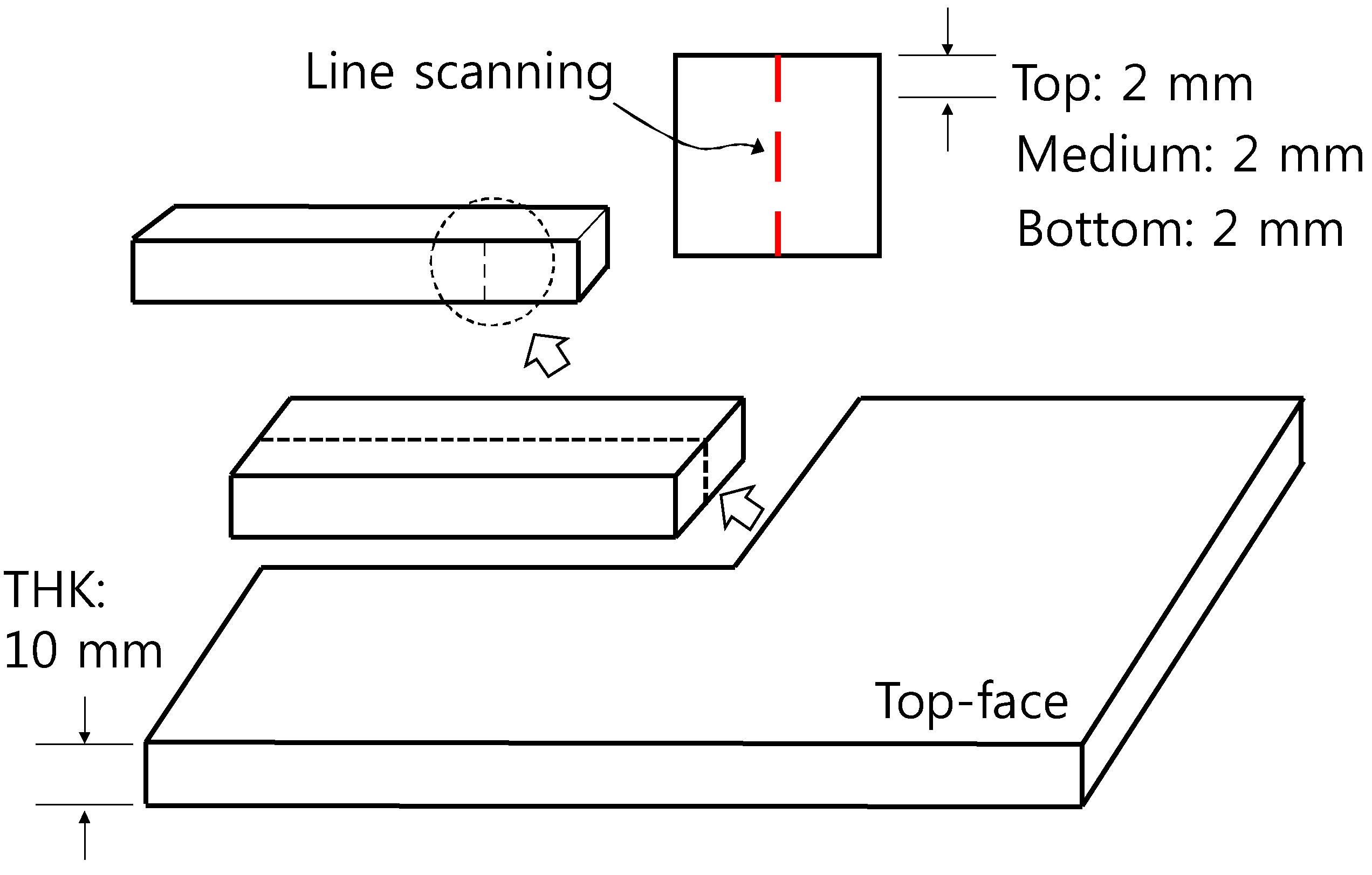





| Ingredients | Crystal Phase | Average Particle Size (nm) | Specific Surface Area (m2/g) | Apparent Density (g/mL) | True Density (g/mL) |
|---|---|---|---|---|---|
| NP-400 | Anatase | 26.6 (s = 8.52%) * | 66.5 | 0.45 | 3.70 |
| P-25 | Rutile/Anatase | 25.2 (s = 9.45%) * | 54.9 | 0.18 | 3.41 |
| Cement | Portland Type I | >44 μm | 0.33 | - | 3.15 |
| Sample Type | Label | Cement (g) | Sand (g) | Water (mL) | TiO2 (g) |
|---|---|---|---|---|---|
| Mortar (NP-400) | M0 | 120 | 240 | 60 | 0 |
| M5 | 234 | 6 | |||
| M10 | 228 | 12 | |||
| M20 | 216 | 24 | |||
| Mortar (P-25) | MP0 | 240 | 0 | ||
| MP5 | 234 | 6 | |||
| MP10 | 228 | 12 | |||
| MP15 | 222 | 18 | |||
| Cement paste (NP-400) | C0 | 280 | - | 140 | 0 |
| C5 | 266 | 14 | |||
| C10 | 252 | 28 | |||
| C20 | 224 | 56 | |||
| Cement paste (P-25) | CP0 | 280 | 0 | ||
| CP5 | 266 | 14 | |||
| CP10 | 252 | 28 | |||
| CP15 | 238 | 56 |
| Specimen Label | Face | NO Removal Rate (%) | NO Concentration Change, ΔC (μmol) | NO Initial Concentration, Ci (μmol) |
|---|---|---|---|---|
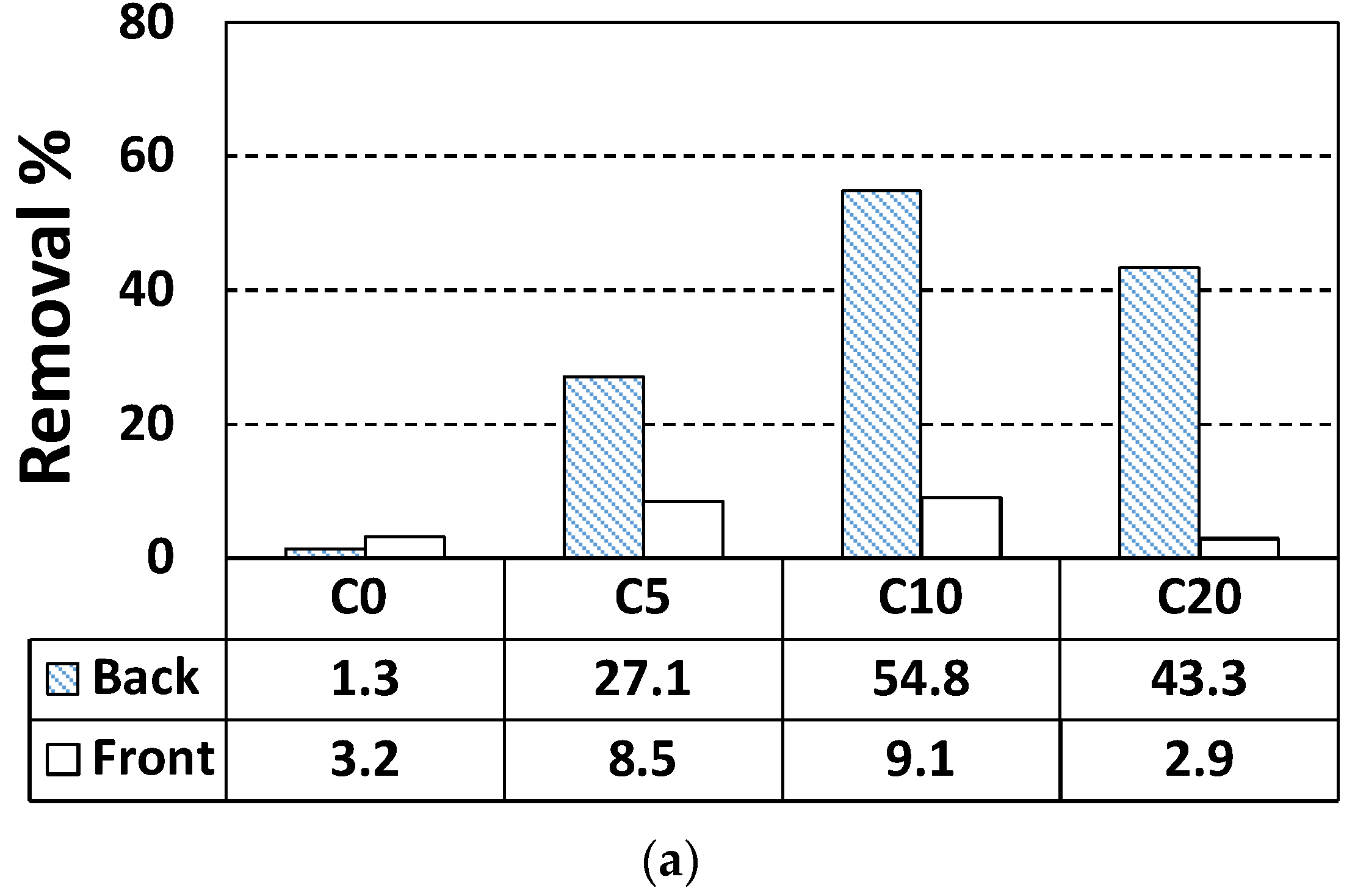
| C0 | Back | 1.3 | 0.50 | 38.38 |
| Front | 3.2 | 1.25 | 39.13 | |
| C5 | Back | 27.1 | 10.48 | 38.65 |
| Front | 8.5 | 3.25 | 38.21 | |
| C10 | Back | 43.3 | 16.47 | 38.04 |
| Front | 2.9 | 1.11 | 38.17 | |
| C20 | Back | 54.8 | 21.18 | 38.65 |
| Front | 9.0 | 3.49 | 38.81 | |
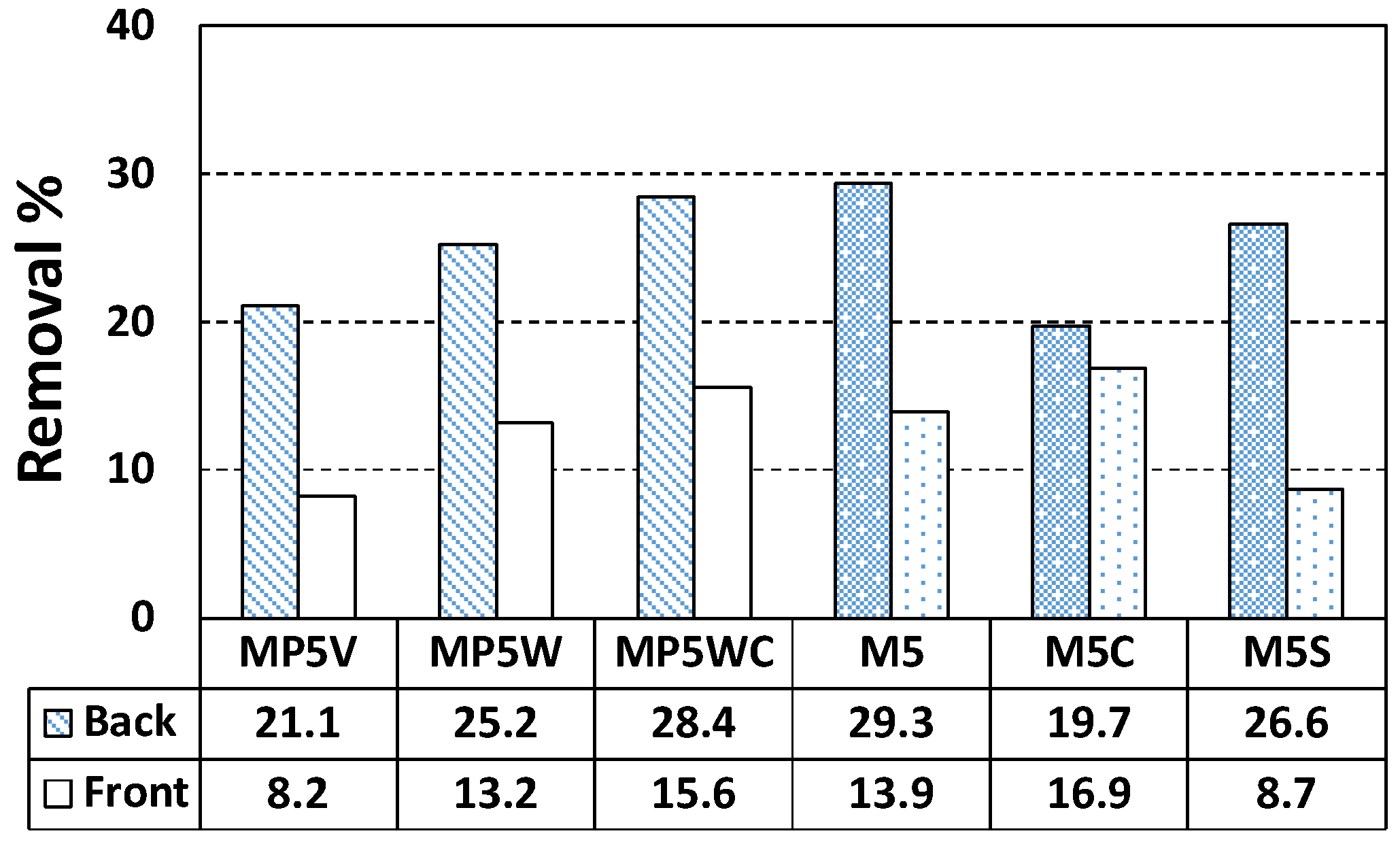
| M0 | Back | 2.2 | 0.87 | 39.46 |
| Front | 2.3 | 0.87 | 37.87 | |
| M5 | Back | 29.3 | 11.60 | 39.59 |
| Front | 13.9 | 5.55 | 39.94 | |
| M10 | Back | 29.5 | 11.50 | 38.97 |
| Front | 16.3 | 6.36 | 39.01 | |
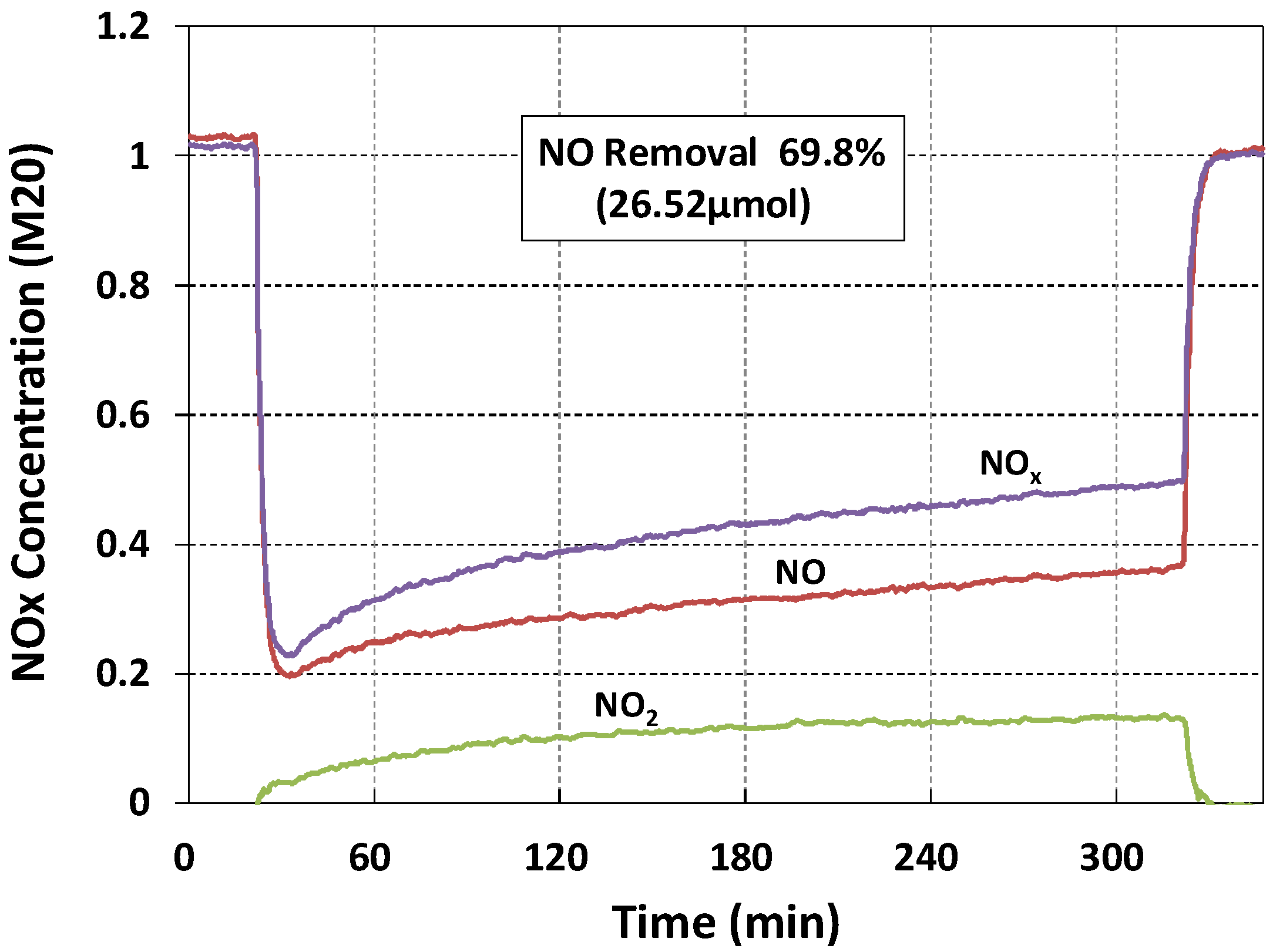
| M20 | Back | 69.8 | 26.52 | 38.00 |
| Front | 10.9 | 4.22 | 38.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhee, I.; Lee, J.-S.; Kim, J.B.; Kim, J.-H. Nitrogen Oxides Mitigation Efficiency of Cementitious Materials Incorporated with TiO2. Materials 2018, 11, 877. https://doi.org/10.3390/ma11060877
Rhee I, Lee J-S, Kim JB, Kim J-H. Nitrogen Oxides Mitigation Efficiency of Cementitious Materials Incorporated with TiO2. Materials. 2018; 11(6):877. https://doi.org/10.3390/ma11060877
Chicago/Turabian StyleRhee, Inkyu, Jun-Seok Lee, Jong Beom Kim, and Jong-Ho Kim. 2018. "Nitrogen Oxides Mitigation Efficiency of Cementitious Materials Incorporated with TiO2" Materials 11, no. 6: 877. https://doi.org/10.3390/ma11060877
APA StyleRhee, I., Lee, J.-S., Kim, J. B., & Kim, J.-H. (2018). Nitrogen Oxides Mitigation Efficiency of Cementitious Materials Incorporated with TiO2. Materials, 11(6), 877. https://doi.org/10.3390/ma11060877





