Experimental and Theoretical Studies on Corrosion Inhibition of Niobium and Tantalum Surfaces by Carboxylated Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Monte Carlo Simulations
2.3. Molecular Dynamic (MD) Simulations
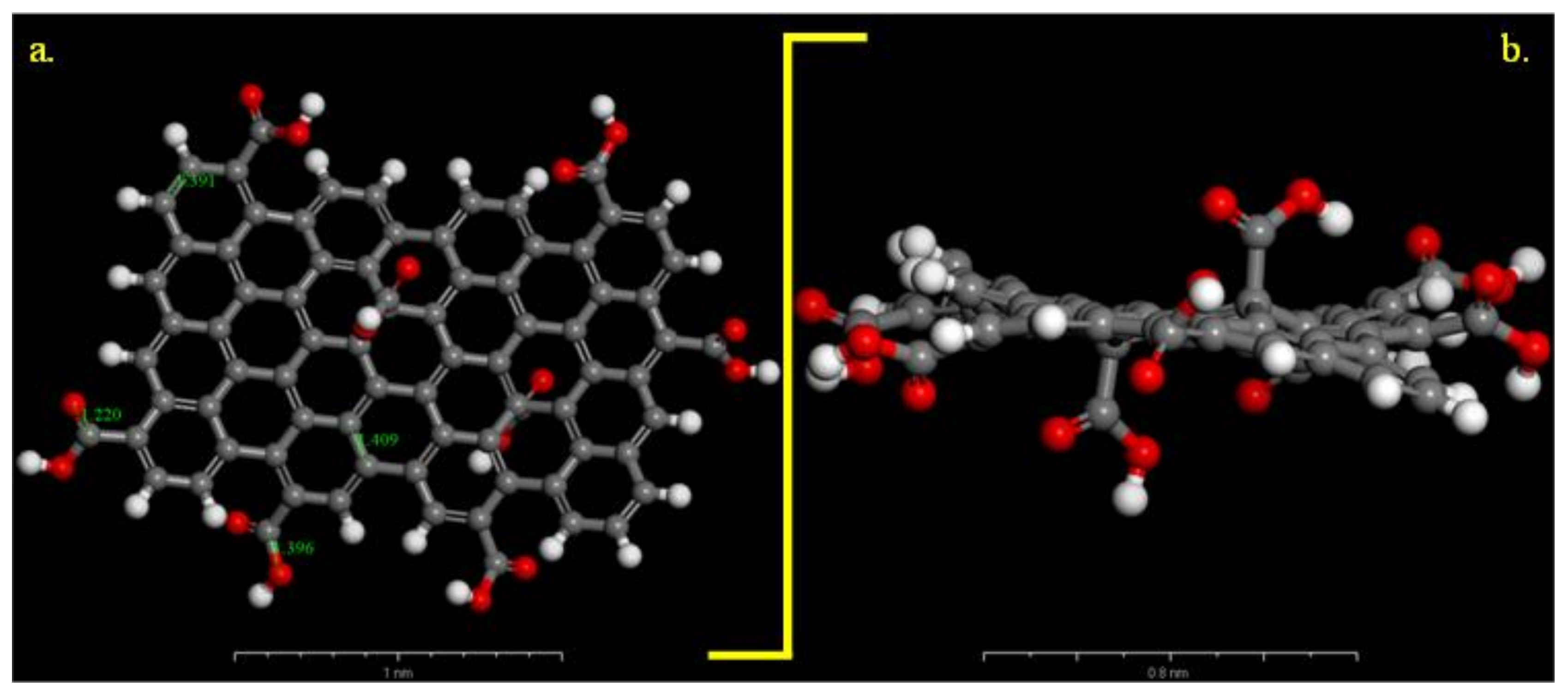
2.4. Adsorption Energy and Radial Distribution Function
3. Results and Discussion
3.1. Potentiodynamic Polarization
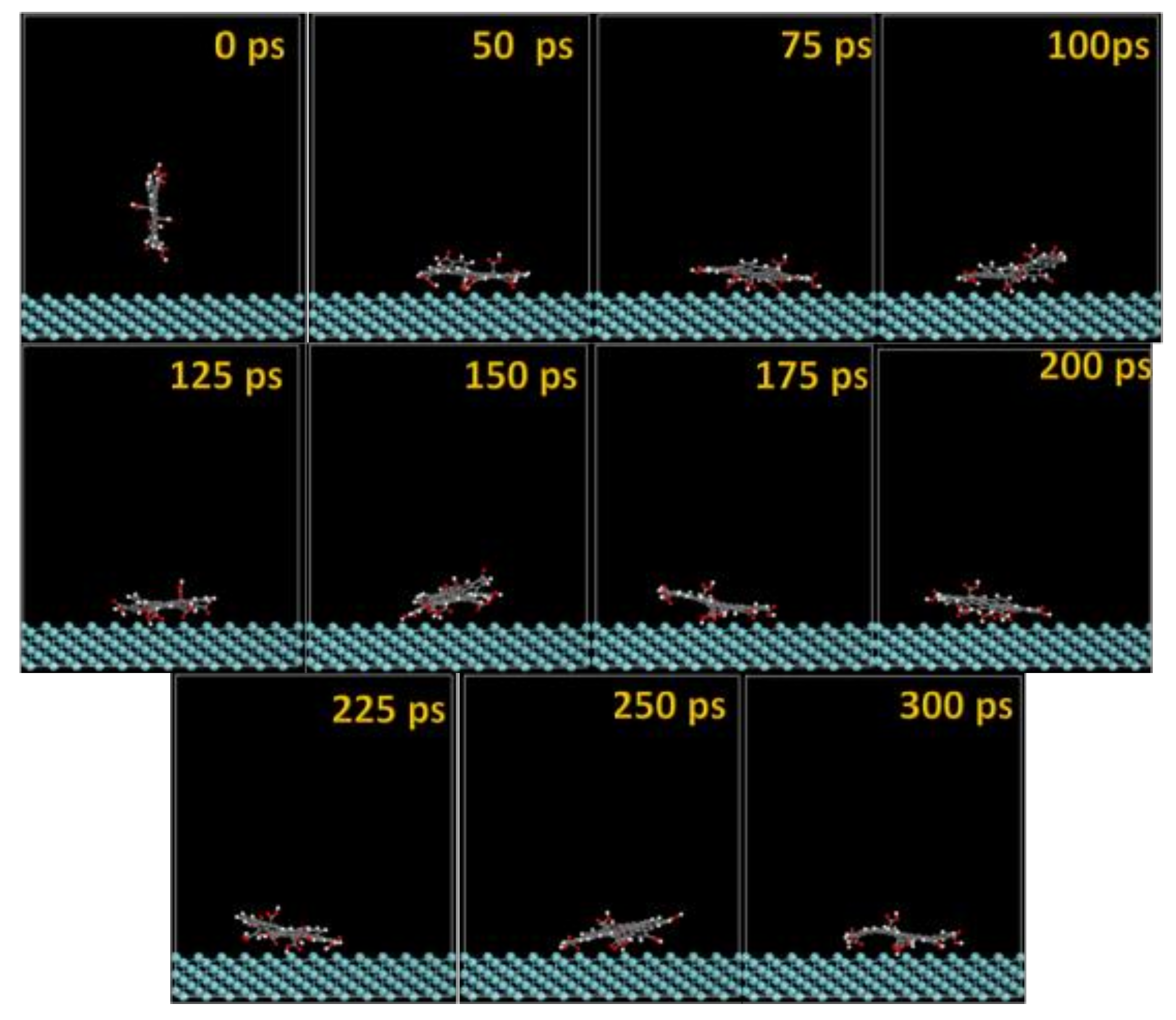
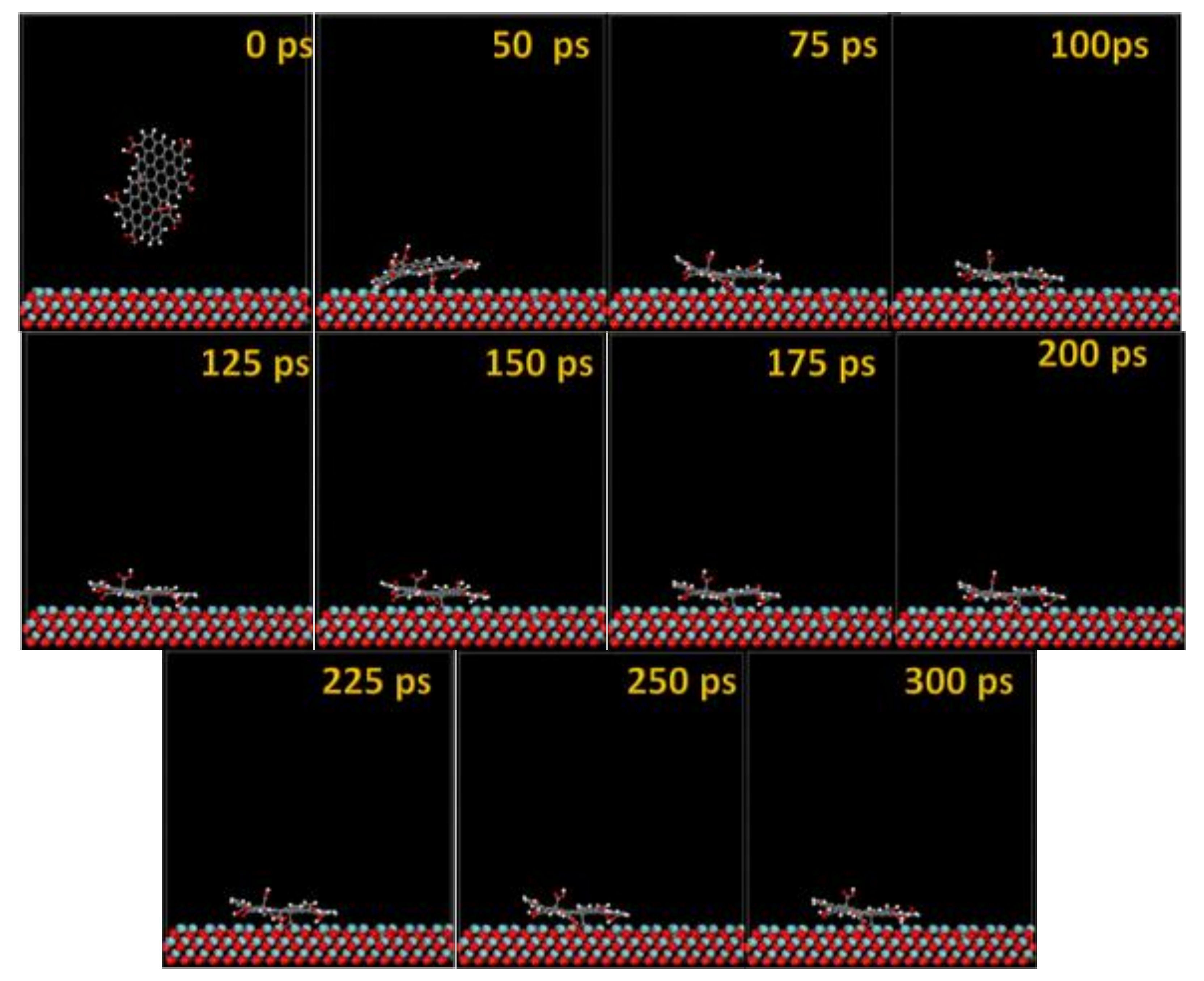
3.2. Molecular Dynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plankaert, R. Surface Coating in Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; VCH: Weinheim, Germany, 1994; p. 170. [Google Scholar]
- Lagrenée, M.; Mernari, B.; Bouanis, M.; Traisnel, M.; Bentiss, F. Study of the mechanism and inhibiting efficiency of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media. Corr. Sci. 2002, 44, 573–588. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Trabanelli, G. Inhibitors—An old remedy for a new challenge. Corrosion 1991, 47, 410–419. [Google Scholar] [CrossRef]
- Van Alsten, J.G. Self-assembled monolayers on engineering metals: structure, derivatization, and utility. Langmuir 1999, 15, 7605–76014. [Google Scholar] [CrossRef]
- Gunasekaran, G.; Natarajan, R.; Muralidharan, V.S.; Palaniswamy, N.; Appa Rao, B.V. Inhibition by phosphonic acids—An overview. Anti-Corrosion. Methods Mater. 1997, 44, 248–259. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials; World Scientific: Jurong East, Singapore, 2011; p. 232. ISBN 978-981-4322-50-8. [Google Scholar]
- Das, S.; Mitra, S.; Khurana, S.M.P.; Debnath, N. Nanomaterials for biomedical applications. Front. Life Sci. 2013, 7, 90–98. [Google Scholar] [CrossRef]
- Bai, Y.; Filippo, M.-S.; Angelis, D.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Ghiamati Yazdi, E.; Ghahfarokhi, Z.S.; Bagherzadeh, M. Protection of carbon steel corrosion in 3.5% NaCl medium by aryldiazonium grafted graphene coatings. New J. Chem. 2017, 41, 12470–12480. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Eom, S.H.; Chung, J.S.; Hur, S.H. Large-scale production of high-quality reduced graphene oxide. Chem. Eng. J. 2013, 233, 297–304. [Google Scholar] [CrossRef]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.-C.; Ruan, G.; et al. Coal as an abundant source of graphene quantum dots. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.; Likhanova, N.V.; Dominguez-Aguilar, M.A.; Martinez-Palou, R.; Vela, A.; Gazquez, J.L. Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles. J. Phys. Chem. B 2006, 110, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Khaled, K.F. Molecular simulation, quantum chemical calculations and electrochemical studies for inhibition of mild steel by triazoles. Electrochim. Acta 2008, 53, 3484–3492. [Google Scholar] [CrossRef]
- Khalil, N. Quantum chemical approach of corrosion inhibition. Electrochim. Acta 2003, 48, 2635–2640. [Google Scholar] [CrossRef]
- Khaled, K.F.; Amin, M.A. Computational and electrochemical investigation for corrosion inhibition of nickel in molar nitric acid by piperidines. J. Appl. Electrochem. 2008, 38, 1609–1629. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci. 2010, 52, 657–660. [Google Scholar] [CrossRef]
- Tang, Y.M.; Yang, W.Z.; Yin, X.S.; Liu, Y.; Wang, R.; Wang, J.T. Phenyl-substituted amino thiadiazoles as corrosion inhibitors for copper in 0.5 M H2SO4. Mater. Chem. Phys. 2009, 116, 479–483. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: Experimental and theoretical approach. Phys. Chem. Chem. Phys. 2006, 18, 17898–17911. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, H.U.; Olasunkanmi, L.O.; Ebenso, E.E. Experimental, quantum chemical and molecular dynamic simulations studies on the corrosion inhibition of mild steel by some carbazole derivatives. Sci. Rep. 2017, 7, 2436. [Google Scholar] [CrossRef] [PubMed]
- Jihong, W.; Ying, D.; Yan, W.; Qing, L.; Hongyan, W.; Qing, W. Adsorption of graphene on an Fe3O4 surface: A molecular dynamics simulation study. J. Adhes. 2018, 94, 238–253. [Google Scholar] [CrossRef]
- Mehmeti, V.V.; Berisha, A.R. Corrosion Study of Mild Steel in Aqueous Sulfuric Acid Solution Using 4-Methyl-4H-1,2,4-Triazole-3-Thiol and 2-Mercaptonicotinic Acid—An Experimental and Theoretical Study. Front. Chem. 2017, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Shen, X.; Chen, C.; Zhang, X. Molecular dynamics simulations of simple aromatic compounds adsorption on single-walled carbon nanotubes. RSC Adv. 2016, 6, 80972–80980. [Google Scholar] [CrossRef]
- Shtogun, Y.V.; Woods, L.M.; Dovbeshko, G.I. Adsorption of Adenine and Thymine and Their Radicals on Single-Wall Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 18174–18181. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: New York, NY, USA, 1966. [Google Scholar]
- Asselin, E.; Ahmed, T.M.; Alfantazi, A. Corrosion of niobium in sulphuric and hydrochloric acid solutions at 75 and 95 °C. Corrs. Sci. 2007, 49, 694–710. [Google Scholar] [CrossRef]
- De Souza, K.A.; Robin, A. Electrochemical behavior of Titanum-Tantalum alloys in sulfuric acid solutions. Mater. Corros. 2004, 55, 853–860. [Google Scholar] [CrossRef]
- Pan, T.J.; Chen, Y.; Zhang, B.; Hu, J.; Li, C. Corrosion behavior of Niobium coated 304 stainless steel in acid solution. Appl. Surf. Sci. 2016, 369, 320–325. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Ghahfarokhi, Z.S.; Yazdi, E.G. Electrochemical and surface evaluation of the anticorrosion properties of reduced graphene oxide. RSC Adv. 2016, 6, 22007–22015. [Google Scholar] [CrossRef]
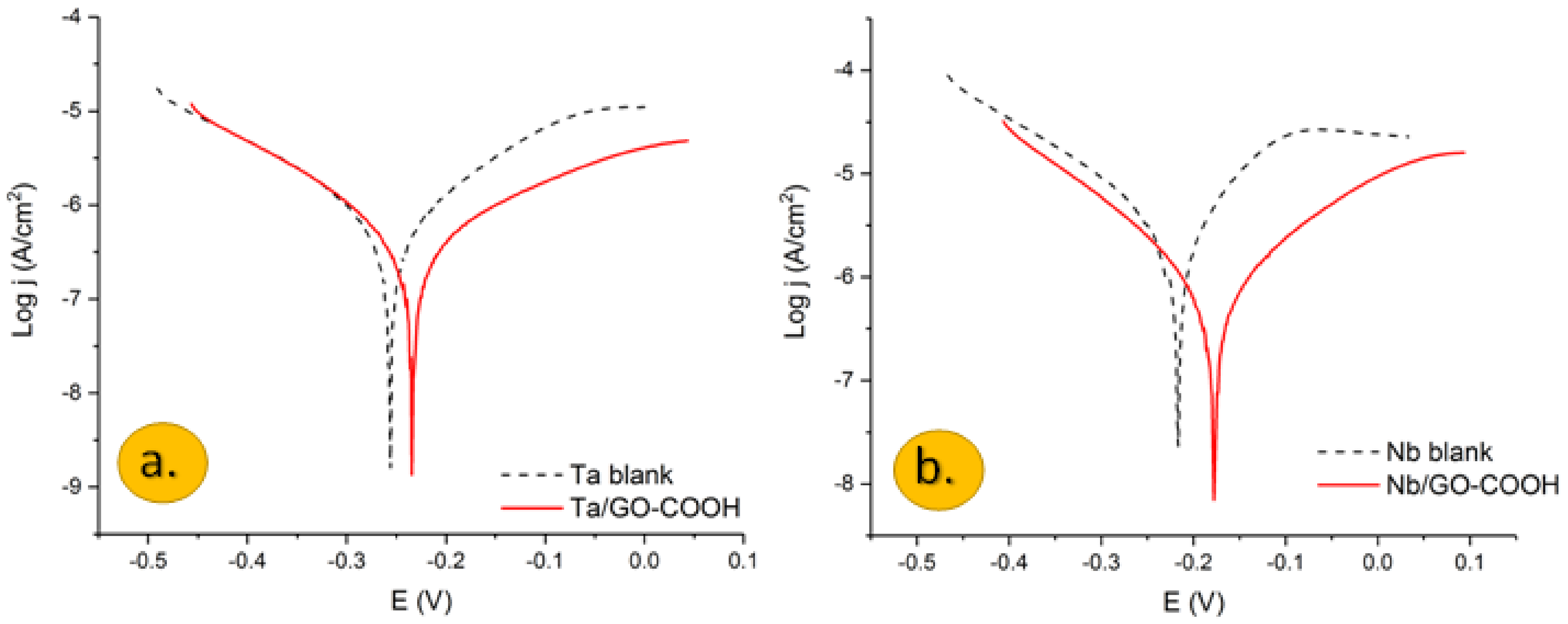










| Studied System (in 0.1 M H2SO4) | Bc(V dec−1) | Ba(V dec−1) | jcorr (Acm−2) | Ecorr (V/SCE) | IE (%) |
|---|---|---|---|---|---|
| Nb | 0.167 | 0.092 | 2.46 × 10−6 | −0.205 | - |
| Nb + GO-COOH | 0.139 | 0.149 | 7.33 × 10−7 | −0.175 | 70.22 |
| Ta | 0.165 | 0.131 | 5.71 × 10−7 | −0.247 | - |
| Ta + GO-COOH | 0.145 | 0.225 | 2.05 × 10−7 | −0.240 | 64.07 |
| Substrate (POS = Plane of Substrate) | dH(GO-COOH)-POS (Å) | dO(GO-COOH)-POS (Å) | dc(GO-COOH)-POS (Å) | dH(GO-COOH)-POS (50H2O) (Å) | dO(GO-COOH)-POS (50H2O) (Å) | dcentroid(GO-COOH)-POS (50H2O) (Å) | Ads. Geometry |
|---|---|---|---|---|---|---|---|
| Nb(gas) 111 | 1.850 | 1.534 | 2.259 | - | - | 4.216 | Planar |
| Nb(H2O) 111 | 5.722 | 6.697 | 6.947 | 1.705 | 2.262 | 8.727 | Planar |
| NbO(gas) 111 | 2.132 | 1.716 | 1.419 | - | - | 4.345 | Planar |
| NbO(H2O) 111 | 2.131 | 1.810 | 2.653 | 1.279 | 1.182 | 4.897 | Planar |
| Ta(gas) 111 | 1.818 | 1.533 | 2.285 | - | - | 4.342 | Planar |
| Ta(H2O) 111 | 6.419 | 5.689 | 6.621 | 2.419 | 2.651 | 10.607 | Side |
| TaO(gas) 111 | 0.841 | 0.758 | 1.261 | - | - | 3.305 | Side |
| TaO(H2O) 111 | 2.122 | 2.156 | 2.981 | 2.244 | 2.648 | 7.988 | Side |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmeti, V.; Podvorica, F.I. Experimental and Theoretical Studies on Corrosion Inhibition of Niobium and Tantalum Surfaces by Carboxylated Graphene Oxide. Materials 2018, 11, 893. https://doi.org/10.3390/ma11060893
Mehmeti V, Podvorica FI. Experimental and Theoretical Studies on Corrosion Inhibition of Niobium and Tantalum Surfaces by Carboxylated Graphene Oxide. Materials. 2018; 11(6):893. https://doi.org/10.3390/ma11060893
Chicago/Turabian StyleMehmeti, Valbonë, and Fetah I. Podvorica. 2018. "Experimental and Theoretical Studies on Corrosion Inhibition of Niobium and Tantalum Surfaces by Carboxylated Graphene Oxide" Materials 11, no. 6: 893. https://doi.org/10.3390/ma11060893





