Semi-Continuous Reverse Membrane Bioreactor in Two-Stage Anaerobic Digestion of Citrus Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum
2.2. Substrate
2.3. Membrane Sachet Preparation and Cell Encasement
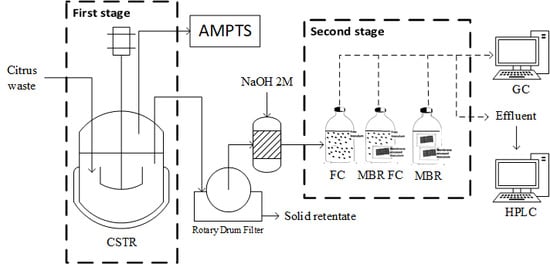
2.4. Bioreactor Set-Up
2.5. Analysis
3. Results and Discussion
3.1. Methane Yield and Biogas Composition from Anaerobic Digestion of Citrus Waste
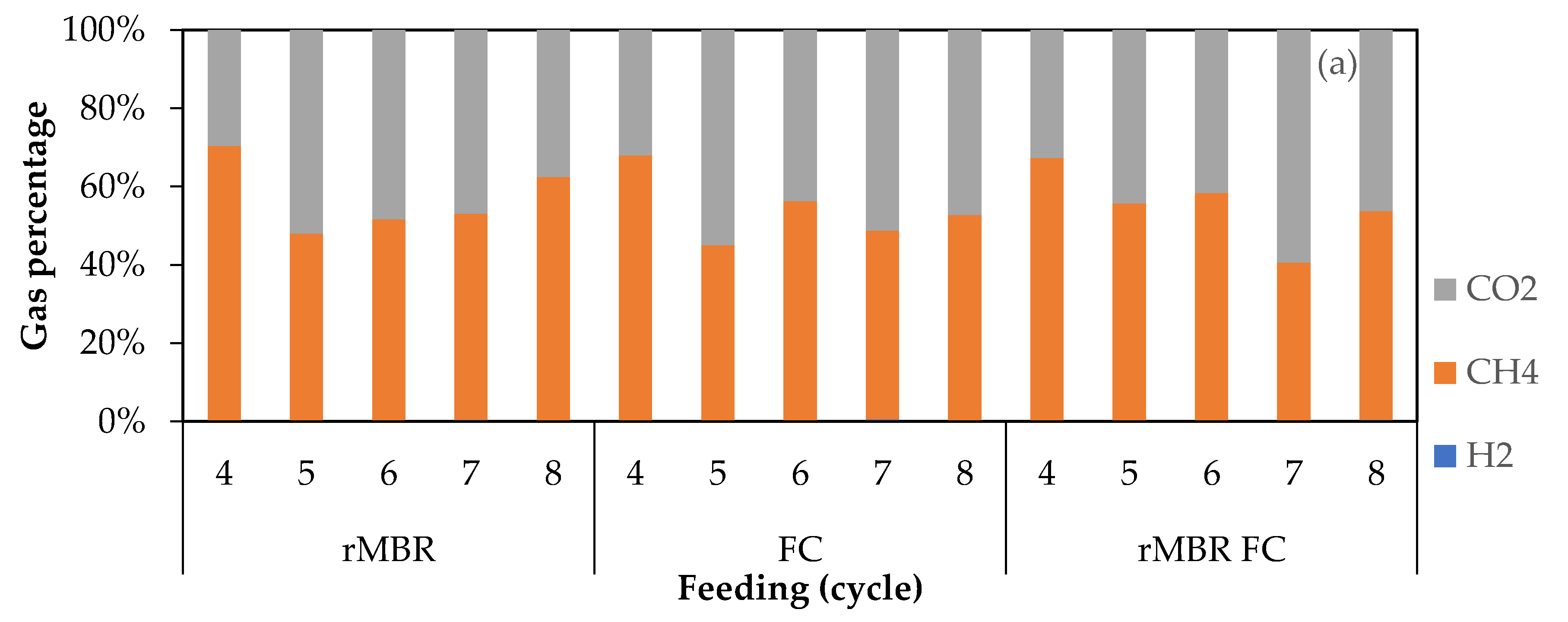
3.2. Biogas Composition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. Available online: http://www.faostat.fao.org (accessed on 10 January 2018).
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K.; Cameron, R.G. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour. Technol. 2007, 98, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Mizuki, E.; Akao, T.; Saruwatari, T. Inhibitory effect of Citrus unshu peel on anaerobic digestion. Biol. Wastes 1990, 33, 161–168. [Google Scholar] [CrossRef]
- Ferry, J.G. Methanogenesis: Ecology, Physiology, Biochemistry & Genetics; Springer: New York, NY, USA, 2012. [Google Scholar]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Snape, J.B.; Dunn, I.J.; Ingham, J.; Prenosil, J.E. Dynamics of Environmental Bioprocesses: Modelling & Simulation; VCH Verlagsgesellschaft mbH.: Weinheim, Germany, 1995. [Google Scholar]
- Youngsukkasem, S.; Rakshit, S.K.; Taherzadeh, M.J. Biogas Production by Encapsulated Methane-Producing Bacteria. Bioresources 2011, 7, 56–65. [Google Scholar]
- Gioannis, G.D.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Energy recovery from one- and two-stage anaerobic digestion of food waste. Waste Manag. 2017, 68, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Youngsukkasem, S.; Akinbomi, J.; Rakshit, S.K.; Taherzadeh, M.J. Biogas production by encased bacteria in synthetic membranes: Protective effects in toxic media and high loading rates. Environ. Technol. 2013, 34, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J. Biogas Production from Citrus Waste by Membrane Bioreactor. Membranes 2014, 4, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Youngsukkasem, S.; Millati, R.; Taherzadeh, M.J. Performance of semi-continuous membrane bioreactor in biogas production from toxic feedstock containing d-Limonene. Bioresour. Technol. 2014, 170, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Pourbafrani, M.; Forgács, G.; Horváth, I.S.; Niklasson, C.; Taherzadeh, M.J. Production of biofuels, limonene and pectin from citrus wastes. Bioresour. Technol. 2010, 101, 4246–4250. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. Laboratory Analytical Procedure (LAP); NREL: Golden, CO, USA, 2008.
- Sant’ana, T.C.P.D.; Blank, A.F.; Vieira, S.D.; Arrigoni-Blank, M.D.F.; Jesus, H.C.R.D.; Alves, P.B. Influência do armazenamento de folhas secas no óleo essencial de patchouli (Pogostemon cablin Benth.). Quím Nova 2010, 33, 1263–1265. [Google Scholar] [Green Version]
- Dohany, J.E. Fluorine-Containing Polymers, Poly (Vinylidene Fluoride); John Wiley & Sons Inc.: New Delhi, India, 2000. [Google Scholar]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Grumezescu, A.M. Emulsions: Nanotechnology in the Agri-Food Industry; Academic Press: London, UK, 2016. [Google Scholar]
- Izaxon, C.; Pagels, J.; Wiertzbicka, A.; Eriksson, A.; Gudmundsson, A.; Nielsen, J.; Dierschke, K.; Assarsson, E.; Andersson, U.; Kleno, J.; et al. Generation of Nano-Size Particles from Limonene/Ozone Reactions, for Controlled Human Exposures in a Chamber. In Proceedings of the European Aerosol Conference 2009, Karlsruhe, Germany, 6–11 September 2009; Lund University: Karlsruhe, Germany, 2009. [Google Scholar]
- Millati, R.; Permanasari, E.D.; Sari, K.W.; Cahyanto, M.N.; Niklasson, C.; Taherzadeh, M.J. Anaerobic digestion of citrus waste using two-stage membrane bioreactor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012063. [Google Scholar] [CrossRef] [Green Version]






| VFA Content (g/L) | ||
|---|---|---|
| VFA | Substrate Cycles 1–7 | Substrate Cycle 8 |
| acetate | 4.12 | 7.89 |
| propionate | 1.88 | 0.84 |
| isobutyrate | 0.56 | 0.08 |
| butyrate | 1.39 | 0.14 |
| isovalerate | 0.00 | 0.00 |
| caproate | 0.00 | 0.29 |
| valerate | 0.06 | 0.18 |
| total VFA | 8.01 | 9.42 |
| OLR | Time Required to Reach Steady Methane Production (Day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rMBR | FC | rMBR–FC | ||||||||
| Feeding (cycle) | 0.6 | 1.2 | 3.6 | 0.6 | 1.2 | 3.6 | 0.6 | 1.2 | 3.6 | |
| 1 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | |
| 2 | 9 | 8 | 8 | 7 | 8 | 8 | 8 | 8 | 9 | |
| 3 | 4 | 7 | 4 | 4 | 7 | 4 | 4 | 6 | 4 | |
| 5 | 4 | 3 | 3 | 4 | 3 | 3 | 5 | 3 | ||
| 5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| 6 | 3 | 4 | 5 | 3 | 4 | 4 | 3 | 5 | 5 | |
| 7 | 4 | 5 | 5 | 4 | 2 | 4 | 4 | 3 | 5 | |
| 8 | 5 | 5 | 4 | 4 | 3 | 4 | 3 | 4 | 4 | |
| Configuration | OLR (g COD/(L Cycle)) | Methane Percentage Range (%) |
|---|---|---|
| rMBR | 0.6 | 42.7–70.4 |
| 1.2 | 51.1–71.1 | |
| 3.6 | 53.8–73.8 | |
| rMBR–FC | 0.6 | 40.5–71.6 |
| 1.2 | 41.1–66.9 | |
| 3.6 | 50.2–68.1 | |
| FC | 0.6 | 45.0–67.9 |
| 1.2 | 40.0–66.3 | |
| 3.6 | 51.7–66.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, T.; Lukitawesa; Hanifah, I.; Wikandari, R.; Millati, R.; Taherzadeh, M.J.; Niklasson, C. Semi-Continuous Reverse Membrane Bioreactor in Two-Stage Anaerobic Digestion of Citrus Waste. Materials 2018, 11, 1341. https://doi.org/10.3390/ma11081341
Kurniawan T, Lukitawesa, Hanifah I, Wikandari R, Millati R, Taherzadeh MJ, Niklasson C. Semi-Continuous Reverse Membrane Bioreactor in Two-Stage Anaerobic Digestion of Citrus Waste. Materials. 2018; 11(8):1341. https://doi.org/10.3390/ma11081341
Chicago/Turabian StyleKurniawan, Tonny, Lukitawesa, Ilma Hanifah, Rachma Wikandari, Ria Millati, Mohammad J. Taherzadeh, and Claes Niklasson. 2018. "Semi-Continuous Reverse Membrane Bioreactor in Two-Stage Anaerobic Digestion of Citrus Waste" Materials 11, no. 8: 1341. https://doi.org/10.3390/ma11081341