Biofilm Removal and Bacterial Re-Colonization Inhibition of a Novel Erythritol/Chlorhexidine Air-Polishing Powder on Titanium Disks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Profilometry
2.3. Strains and Growth Conditions
2.4. Preventive Anti-Adhesion Activity
2.5. Plaque Removal
2.6. Morphological Analysis
2.7. Statistical Analysis of Data
3. Results
3.1. Surface Analysis by Profilometry
3.2. Preventive Anti-Bacteria Adhesion Activity Evaluation
3.3. Plaque Removal Evaluation
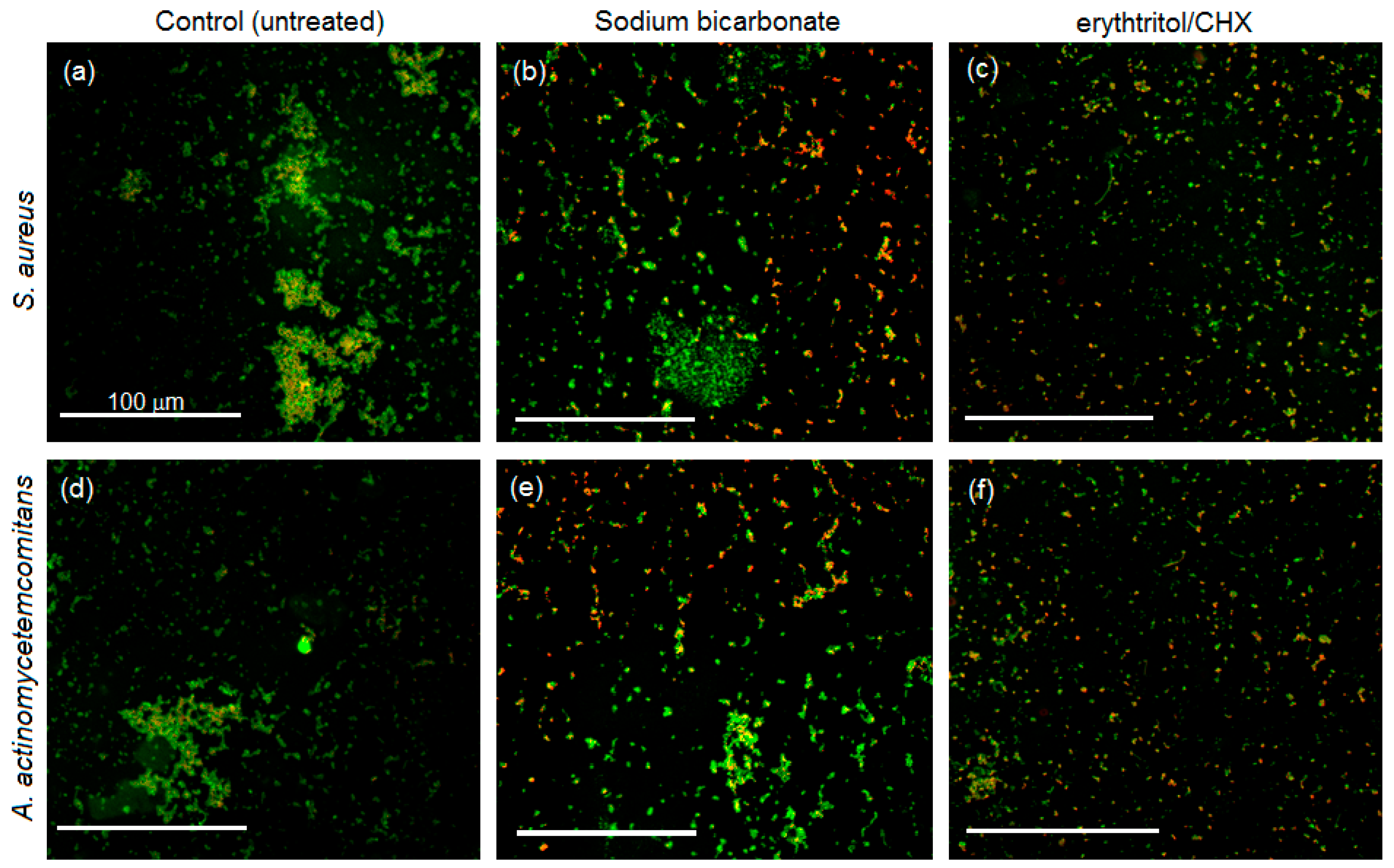
3.4. Biofilm Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Listgarten, M. The Structure of Dental Plaque. Periodontology 2000 1994, 5, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial Colonization Immediately after Installation on Oral Titanium Implants. Clin. Oral Implants Res. 2007, 184, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N. Microbiological and Immuno-Pathological Aspects of Peri-Implant Diseases. Arch. Oral Biol. 2014, 591, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Vogels, R.; Peeters, W.; Van Steenberghe, D.; Naert, I.; Haffajee, A. Dynamics of Initial Subgingival Colonization of “Pristine” Peri-Implant Pockets. Clin. Oral Implants Res. 2006, 171, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Heuer, W.; Stiesch, M.; Abraham, W.R. Microbial Diversity of Supra- and Subgingival Biofilms on Freshly Colonized Titanium Implant Abutments in the Human Mouth. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 302, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The Microbiota Associated with Sucessful or Failing Osseointegrated Titanium Implants. Oral Microbiol. Immunol. 1987, 24, 145–151. [Google Scholar] [CrossRef]
- Hultin, M.; Gustafsson, A.; Hallström, H.; Johansson, L.Å.; Ekfeldt, A.; Klinge, B. Microbiological Findings and Host Response in Patients with Peri-Implantitis. Clin. Oral Implants Res. 2002, 134, 349–358. [Google Scholar] [CrossRef]
- Grusovin, M.G.; Coulthard, P.; Worthington, H.V.; George, p.; Esposito, M. Interventions for Replacing Missing Teeth: Maintaining and Recovering Soft Tissue Health around Dental Implants. Cochrane Database Syst. Rev. 2010, 8, CD003069. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacingmissing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; van der Weijden, F. Influence of Mechanical Instruments on the Biocompatibility of Titanium Dental Implants Surfaces: A Systematic Review. Clin. Oral Implants Res. 2015, 2510, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.E.; Auschill, T.M.; Heumann, C.; Frankenberger, R.; Eick, S.; Sculean, A.; Arweiler, N.B. Influence of Different Instrumentation Modalities on the Surface Characteristics and Biofilm Formation on Dental Implant Neck, in Vitro. Clin. Oral Implants Res. 2017, 28, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Garcia, F.; Tanomaru-Filho, M.; Chávez-Andrade, G.M.; Bosso-Martelo, R.; Basso-Bernardi, M.I.; Guerreiro-Tanomaru, J.M. Effect of Silver Nanoparticles on Physicochemical and Antibacterial Properties of Calcium Silicate Cements. Braz. Dent. J. 2016, 27, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Hao, L.; Zhang, L.; Wang, K.; Zheng, W.; Wang, X.; Zhou, X.; Li, W.; Zhang, L. Tea Polyphenols Functionalized and Reduced Graphene Oxide-ZnO Composites for Selective Pb(2+) Removal and Enhanced Antibacterial Activity. J. Biomed. Nanotechnol. 2018, 14, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ghaemy, M.; Ghadiri, A.A.; Mohseni, M. Photocurable, Antimicrobial Quaternary Ammonium-modified Nanosilica. J. Dent. Res. 2015, 94, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Koidou, V.P.; Argyris, P.P.; Skoe, E.P.; Mota Siqueira, J.; Chen, X.; Zhang, L.; Hinrichs, J.E.; Costalonga, M.; Aparicio, C. Peptide coatings enhance keratinocyte attachment towards improving the peri-implant mucosal seal. Biomater. Sci. 2018, 6, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F.A. Titanium Surface Alterations Following the Use of Different Mechanical Instruments: A Systematic Review. Clin. Oral Implants Res. 2012, 236, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Becker, K.; Renvert, S. Efficacy of Air Polishing for the Non-Surgical Treatment of Peri-Implant Diseases: A Systematic Review. J. Clin. Periodontol. 2015, 4210, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Moëne, R.; Cancela, J.A.; Mombelli, A. Subgingival Air-Polishing with Erythritol during Periodontal Maintenance: Randomized Clinical Trial of Twelve Months. J. Clin. Periodontol. 2014, 419, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Hägi, T.T.; Hofmänner, P.; Eick, S.; Donnet, M.; Salvi, G.E.; Sculean, A.; Ramseier, C.A. The Effects of Erythritol Air-Polishing Powder on Microbiologic and Clinical Outcomes during Supportive Periodontal Therapy: Six-Month Results of a Randomized Controlled Clinical Trial. Quintessence Int. 2015, 461, 31–41. [Google Scholar] [CrossRef]
- Schwarz, F.; Ferrari, D.; Popovski, K.; Hartig, B.; Becker, J. Influence of Different Air-Abrasive Powders on Cell Viability at Biologically Contaminated Titanium Dental Implants Surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 881, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Nuesry, E.; Bieling, K.; Herten, M.; Becker, J. Influence of an Erbium, Chromium-Doped Yttrium, Scandium, Gallium, and Garnet (Er,Cr:YSGG) Laser on the Reestablishment of the Biocompatibility of Contaminated Titanium Implant Surfaces. J. Periodontol. 2006, 7711, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sculean, A.; Romanos, G.; Herten, M.; Horn, N.; Scherbaum, W.; Becker, J. Influence of Different Treatment Approaches on the Removal of Early Plaque Biofilms and the Viability of SAOS2 Osteoblasts Grown on Titanium Implants. Clin. Oral Investig. 2005, 92, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ronay, V.; Merlini, A.; Attin, T.; Schmidlin, P.R.; Sahrmann, P. In Vitro Cleaning Potential of Three Implant Debridement Methods. Simulation of the Non-Surgical Approach. Clin. Oral Implants Res. 2017, 28, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Papanicolau, P.; Rothamel, D.; Beck, B.; Herten, M.; Becker, J. Influence of Plaque Biofilm Removal on Reestablishment of the Biocompatibility of Contaminated Titanium Surfaces. J. Biomed. Mater. Res. 2006, 77A, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.M.; Covey, D.; Watanabe, H.; Simetich, B.; Schulte, J.R.; Chen, H. An in Vitro Comparison of the Effects of Various Air Polishing Powders on Enamel and Selected Esthetic Restorative Materials. J. Clin. Dent. 2014, 254, 76–87. [Google Scholar]
- Salerno, M.; Giacomelli, L.; Derchi, G.; Patra, N.; Diaspro, A. Atomic Force Microscopy in Vitro Study of Surface Roughness and Fractal Character of a Dental Restoration Composite after Air-Polishing. Biomed. Eng. Online 2010, 91, 59. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Fini, M.; Carrassi, A.; Migliario, M.; Visai, L.; Rimondini, L. Effect of Air Polishing with Glycine Powder on Titanium Abutment Surfaces. Clin. Oral Implants Res. 2013, 248, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Del Fabbro, M.; Bortolin, M.; Vassena, C.; De Vecchi, E.; Taschieri, S. Biofilm Removal and Antimicrobial Activity of Two Different Air-Polishing Powders: An in Vitro Study. J. Periodontol. 2014, 8511, e363–e369. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Bernt, W.O.; Borzelleca, J.F.; Flamm, G.; Lynch, B.S.; Kennepohl, E.; Bär, E.A.; Modderman, J. Erythritol: An Interpretive Summary of Biochemical, Metabolic, Toxicological and Clinical Data. Food Chem. Toxicol. 1998, 3612, 1139–1174. [Google Scholar] [CrossRef]
- Godswill, A.C. Sugar Alcohols: Chemistry, Production, Health Concerns and Nutritional Importance of Mannitol, Sorbitol, Xylitol, and Erythritol. Int. J. Adv. Acad. Res. 2017, 3, 31–66. [Google Scholar]
- Bradshaw, D.J.; Marsh, P.D. Effect of Sugar Alcohols on the Composition and Metabolism of a Mixed Culture of Oral Bacteria Grown in a Chemostat. Caries Res. 1994, 284, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.K.; Saag, M.; Isotupa, K.P.; Olak, J.; Nõmmela, R.; Söderling, E.; Mäkinen, P.L. Similarity of the Effects of Erythritol and Xylitol on Some Risk Factors of Dental Caries. Caries Res. 2005, 393, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol Alters Microstructure and Metabolomic Profiles of Biofilm Composed of Streptococcus Gordonii and Porphyromonas Gingivalis. Mol. Oral Microbiol. 2013, 286, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Söderling, E.M.; Hietala-Lenkkeri, A.M. Xylitol and Erythritol Decrease Adherence of Polysaccharide-Producing Oral Streptococci. Curr. Microbiol. 2010, 601, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Bortolin, M.; Taschieri, S.; De Vecchi, E.; Agrappi, S.; Del Fabbro, M.; Francetti, L.; Mattina, R. Erythritol/chlorhexidine Combination Reduces Microbial Biofilm and Prevents Its Formation on Titanium Surfaces in Vitro. J. Oral Pathol. Med. 2017, 468, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Azzimonti, B.; Cochis, A.; El Beyrouthy, M.; Iriti, M.; Uberti, F.; Sorrentino, R.; Landini, M.M.; Rimondini, L.; Varoni, E.M. Essential Oil from Berries of Lebanese Juniperus excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells. Molecules 2015, 20, 9344–9357. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Truffa Giachet, F.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C 2017, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, R.; Tonelli, M.; Carnevale, G.; Mombelli, A.; Nyman, S.; Lang, N. Experimentally Induced Periimplant mucositis. A Clinical Study in Humans. Clin. Oral Implants Res. 1994, 54, 254–259. [Google Scholar] [CrossRef]
- Brambilla, E.; Ionescu, A.; Gagliani, M.; Cochis, A.; Arciola, C.R.; Rimondini, L. Biofilm formation on composite resins for dental restorations: An in situ study on the effect of chlorhexidine mouthrinses. Int. J. Artif. Organs 2012, 35, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Quintas, V.; Prada-López, I.; Donos, N.; Suárez-Quintanilla, D.; Tomás, I. In Situ Neutralisation of the Antibacterial Effect of 0.2% Chlorhexidine on Salivary Microbiota: Quantification of Substantivity. Arch. Oral Biol. 2015, 60, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Honkala, S.; Runnel, R.; Olak, J.; Nõmmela, R.; Russak, S.; Saag, M.; Mäkinen, P.L.; Mäkinen, K.; Vahlberg, T.; et al. Long-Term Effect of Erythritol on Dental Caries Development during Childhood: A Posttreatment Survival Analysis. Caries Res. 2016, 50, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Van Der Mei, H.C.; Bollen, C.M.; Schotte, A.; Marechal, M.; Doornbusch, G.I.; Naert, I.; Busscher, H.J.; Van Steenberghe, D. An in Vivo Study of the Influence of the Surface Roughness of Implants on the Microbiology of Supra- and Subgingival Plaque. J. Dent. Res. 1993, 729, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Borsari, V.; Fini, M.; Giavaresi, G.; Tschon, M.; Chiesa, R.; Chiusoli, L.; Salito, A.; Rimondini, L.; Giardino, R. Comparative in vivo evaluation of porous and dense duplex titanium and hydroxyapatite coating with high roughnesses in different implantation environments. J. Biomed. Mater. Res. A 2009, 89, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Venturello, A.; Miola, M.; Cochis, A.; Rimondini, L.; Spriano, S. Antibacterial and bioactive nanostructured titanium surfaces for bone integration. Appl. Surf. Sci. 2014, 311, 279–291. [Google Scholar] [CrossRef]
- Monje, A.; Aranda, L.; Diaz, K.T.; Alarcón, M.A.; Bagramian, R.A.; Wang, H.L.; Catena, A. Impact of Maintenance Therapy for the Prevention of Peri-Implant Diseases. J. Dent. Res. 2016, 954, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Dmytryk, J.J.; Fox, S.C.; Moriarty, J.D. The Effects of Scaling Titanium Implant Surfaces with Metal and Plastic Instruments on Cell Attachment. J. Periodontol. 1990, 618, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Petersilka, G.; Faggion, C.M.; Stratmann, U.; Gerss, J.; Ehmke, B.; Haeberlein, I.; Flemmig, T.F. Effect of Glycine Powder Air-Polishing on the Gingiva. J. Clin. Periodontol. 2008, 354, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Petersilka, G.; Heckel, R.; Koch, R.; Ehmke, B.; Arweiler, N. Evaluation of an ex vivo porcine model to investigate the effect of low abrasive airpolishing. Clin. Oral Investig. 2018, 22, 2669–2673. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensi, M.; Cochis, A.; Sordillo, A.; Uberti, F.; Rimondini, L. Biofilm Removal and Bacterial Re-Colonization Inhibition of a Novel Erythritol/Chlorhexidine Air-Polishing Powder on Titanium Disks. Materials 2018, 11, 1510. https://doi.org/10.3390/ma11091510
Mensi M, Cochis A, Sordillo A, Uberti F, Rimondini L. Biofilm Removal and Bacterial Re-Colonization Inhibition of a Novel Erythritol/Chlorhexidine Air-Polishing Powder on Titanium Disks. Materials. 2018; 11(9):1510. https://doi.org/10.3390/ma11091510
Chicago/Turabian StyleMensi, Magda, Andrea Cochis, Annamaria Sordillo, Francesca Uberti, and Lia Rimondini. 2018. "Biofilm Removal and Bacterial Re-Colonization Inhibition of a Novel Erythritol/Chlorhexidine Air-Polishing Powder on Titanium Disks" Materials 11, no. 9: 1510. https://doi.org/10.3390/ma11091510
APA StyleMensi, M., Cochis, A., Sordillo, A., Uberti, F., & Rimondini, L. (2018). Biofilm Removal and Bacterial Re-Colonization Inhibition of a Novel Erythritol/Chlorhexidine Air-Polishing Powder on Titanium Disks. Materials, 11(9), 1510. https://doi.org/10.3390/ma11091510








