In Vitro Osteogenesis Stimulation via Nano-Hydroxyapatite/Carbon Nanotube Thin Films on Biomedical Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
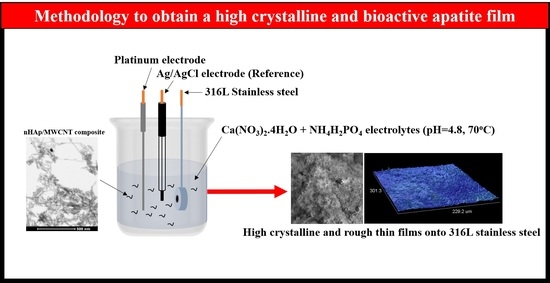
2.1. Electrophoretic Deposition
2.2. Characterization of nHAp/MWCNT Thin Films
2.3. Cytotoxicity Test
2.4. Gene Expression Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walczak, J.; Shahgaldi, F.; Heatley, F. In vivo corrosion of 316l stainless-steel hip implants: Morphology and elemental compositions of corrosion products. Biomaterials 1998, 19, 229–237. [Google Scholar] [CrossRef]
- Grosgogeat, B.; Reclaru, L.; Lissac, M.; Dalard, F. Measurement and evaluation of galvanic corrosion between titanium/Ti6Al4V implants and dental alloys by electrochemical techniques and auger spectrometry. Biomaterials 1999, 20, 933–941. [Google Scholar] [CrossRef]
- Caicedo, M.S.; Pennekamp, P.H.; McAllister, K.; Jacobs, J.J.; Hallab, N.J. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J. Biomed. Mater. Res. Part A 2010, 93A, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Corrosion of metal orthopaedic implants. JBJS 1998, 80, 268–282. [Google Scholar] [CrossRef]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef]
- Groessner-Schreiber, B.; Tuan, R.S. Enhanced extracellular matrix production and mineralization by osteoblasts cultured on titanium surfaces in vitro. J. Cell Sci. 1992, 101, 209–217. [Google Scholar] [PubMed]
- Lobo, A.O.; Marciano, F.R.; Corat, E.J.; Trava-Airoldi, V.J. Processo para produção de nanocompósitos de nanoapatitas e os ditos nanocompósitos; Instituto Nacional da Propriedade Industrial-INPI: Rio de Janeiro, Barzil, 2013. [Google Scholar]
- Rodrigues, B.V.M.; Leite, N.C.; Cavalcanti, B.D.N.; da Silva, N.S.; Marciano, F.R.; Corat, E.J.; Webster, T.J.; Lobo, A.O. Graphene oxide/multi-walled carbon nanotubes as nanofeatured scaffolds for the assisted deposition of nanohydroxyapatite: Characterization and biological evaluation. Int. J. Nanomed. 2016, 11, 2569–2585. [Google Scholar]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Gopi, D.; Shinyjoy, E.; Sekar, M.; Surendiran, M.; Kavitha, L.; Sampath Kumar, T.S. Development of carbon nanotubes reinforced hydroxyapatite composite coatings on titanium by electrodeposition method. Corros. Sci. 2013, 73, 321–330. [Google Scholar] [CrossRef]
- Prodana, M.; Duta, M.; Ionita, D.; Bojin, D.; Stan, M.S.; Dinischiotu, A.; Demetrescu, I. A new complex ceramic coating with carbon nanotubes, hydroxyapatite and TiO2 nanotubes on ti surface for biomedical applications. Ceram. Int. 2015, 41, 6318–6325. [Google Scholar] [CrossRef]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Ustundag, C.B.; Avciata, O.; Kaya, F.; Kaya, C. Hydrothermally mixed hydroxyapatite–multiwall carbon nanotubes composite coatings on biomedical alloys by electrophoretic deposition. J. of Phys. Chem. B 2013, 117, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zeng, Y.; He, R.; Li, Z.; Tian, L.; Wang, J.; Wan, Q.; Li, X.; Bao, H. Single-walled carbon nanotubes/hydroxyapatite coatings on titanium obtained by electrochemical deposition. Appl. Surf. Sci. 2014, 295, 71–80. [Google Scholar] [CrossRef]
- Lobo, A.O.; Zanin, H.; Siqueira, I.A.W.B.; Leite, N.C.S.; Marciano, F.R.; Corat, E.J. Effect of ultrasound irradiation on the production of nHAp/MWCNT nanocomposites. Mater. Sci. Eng. C 2013, 33, 4305–4312. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Antunes, E.F.; de Resende, V.G.; Mengui, U.A.; Cunha, J.B.M.; Corat, E.J.; Massi, M. Analyses of residual iron in carbon nanotubes produced by camphor/ferrocene pyrolysis and purified by high temperature annealing. Appl. Surf. Sci. 2011, 257, 8038–8043. [Google Scholar] [CrossRef]
- Zou, Z.; Lin, K.; Chen, L.; Chang, J. Ultrafast synthesis and characterization of carbonated hydroxyapatite nanopowders via sonochemistry-assisted microwave process. Ultrason. Sonochem. 2012, 19, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Liao, S.; Xu, G.; Wang, W.; Watari, F.; Cui, F.; Ramakrishna, S.; Chan, C.K. Self-assembly of nano-hydroxyapatite on multi-walled carbon nanotubes. Acta Biomater. 2007, 3, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Gopi, D.; Indira, J.; Prakash, V.C.A.; Kavitha, L. Spectroscopic characterization of porous nanohydroxyapatite synthesized by a novel amino acid soft solution freezing method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Seesala, V.S.; Sen, M.; Sengupta, S.; Dhara, S.; Saha, P.; Das, K.; Das, S. Mwcnt reinforced bone like calcium phosphate—Hydroxyapatite composite coating developed through pulsed electrodeposition with varying amount of apatite phase and crystallinity to promote superior osteoconduction, cytocompatibility and corrosion protection performance compared to bare metallic implant surface. Surf. Coat. Technol. 2017, 325, 496–514. [Google Scholar]
- Lee, M.; Ku, S.H.; Ryu, J.; Park, C.B. Mussel-inspired functionalization of carbon nanotubes for hydroxyapatite mineralization. J. Mater. Chem. 2010, 20, 8848–8853. [Google Scholar] [CrossRef]
- Rodrigues, B.V.; Silva, A.S.; Melo, G.F.; Vasconscellos, L.M.; Marciano, F.R.; Lobo, A.O. Influence of low contents of superhydrophilic mwcnt on the properties and cell viability of electrospun poly (butylene adipate-co-terephthalate) fibers. Mater. Sci. Eng. C 2016, 59, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Gao, J.; Wang, M.; Fu, J.; Wang, D.; Zhu, Y. Ultra-trace silver-doped hydroxyapatite with non-cytotoxicity and effective antibacterial activity. Mater. Sci. Eng. C 2015, 55, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Zancanela, D.C.; Sper Simão, A.M.; Matsubara, E.Y.; Rosolen, J.M.; Ciancaglini, P. Defective multilayer carbon nanotubes increase alkaline phosphatase activity and bone-like nodules in osteoblast cultures. J. Nanosci. Nanotechnol. 2016, 16, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Khalili, A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Prado, R.F.d.; de Oliveira, F.S.; Nascimento, R.D.; de Vasconcellos, L.M.R.; Carvalho, Y.R.; Cairo, C.A.A. Osteoblast response to porous titanium and biomimetic surface: In vitro analysis. Mater. Sci. Eng. C 2015, 52, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Lu, L.; Runge, M.B.; Zeng, H.; Yaszemski, M.J.; Dadsetan, M. Nanocomposite bone scaffolds based on biodegradable polymers and hydroxyapatite. J. Biomed. Mater. Res. Part A 2015, 103, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Bellows, C.G.; Aubin, J.E.; Heersche, J.N.M. Differential effects of fluoride during initiation and progression of mineralization of osteoid nodules formed in vitro. J. Bone Miner. Res. 1993, 8, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Zhao, W.; Qiao, B.; Cui, H.; Fan, J.; Li, H.; Tu, X.; Jiang, D. In vitro and in vivo biocompatibility and osteogenesis of graphene-reinforced nanohydroxyapatite polyamide66 ternary biocomposite as orthopedic implant material. Int. J. Nanomed. 2016, 11, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Lincks, J.; Boyan, B.D.; Blanchard, C.R.; Lohmann, C.H.; Liu, Y.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials 1998, 19, 2219–2232. [Google Scholar] [CrossRef]
- Bagherifard, S.; Hickey, D.J.; de Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316l stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R.; Zerler, B.; Moran, E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Sawase, T.; Jimbo, R.; Baba, K.; Shibata, Y.; Ikeda, T.; Atsuta, M. Photo-induced hydrophilicity enhances initial cell behavior and early bone apposition. Clin. Oral Implants Res. 2008, 19, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Vedakumari, W.S.; Priya, V.M.; Sastry, T.P. Deposition of superparamagnetic nanohydroxyapatite on iron–fibrin substrates: Preparation, characterization, cytocompatibility and bioactivity studies. Colloids Surf. B Biointerfaces 2014, 120, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L.; Niziolek, P.J.; Haberstroh, K.M.; Nauman, E.A.; Webster, T.J. Decreased fibroblast and increased osteoblast adhesion on nanostructured NaOH-etched PLGA scaffolds. Int. J. Nanomed. 2007, 2, 383–388. [Google Scholar]
- Annunziata, M.; Oliva, A.; Buosciolo, A.; Giordano, M.; Guida, A.; Guida, L. Bone marrow mesenchymal stem cell response to nano-structured oxidized and turned titanium surfaces. Clin. Oral Implants Res. 2012, 23, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Oliva, A.; Basile, M.A.; Giordano, M.; Nastri, L.; Annunziata, M. Human gingival fibroblast functions are stimulated by oxidized nano-structured titanium surfaces. J. Dent. 2013, 41, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Calandrelli, L.; Annunziata, M.; Della Ragione, F.; Laurienzo, P.; Malinconico, M.; Oliva, A. Development and performance analysis of PCL/silica nanocomposites for bone regeneration. J. Mater. Sci. Mater. Med. 2010, 21, 2923–2936. [Google Scholar] [CrossRef] [PubMed]
- Thakur, T.; Xavier, J.R.; Cross, L.; Jaiswal, M.K.; Mondragon, E.; Kaunas, R.; Gaharwar, A.K. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J. Biomed. Mater. Res. Part A 2016, 104, 879–888. [Google Scholar] [CrossRef] [PubMed]



| Gene Symbol/(Access Number) | Gene Name | Primer Sequences | Function |
|---|---|---|---|
| β-actin/ACTB (NM_001101) | Actin Beta | 5′-ACCAACTGGGAC GACATGGAGAAA-3′ 5′-TAGCACAGCCTG GATAGCAACGTA-3′ | Related to cell motility, structure, and integrity |
| ALPL (NM_000478.4) | Alkaline phosphatase | 5′-CCGTGGCAACT CTATCTTTGG-3′ 5′-GCCATACAGGA TGGCAGTGA-3′ | Evolved in bone mineralization |
| OPN/SPP1 (NM_1251830) | Secreted phosphoprotein 1/Osteopontin | 5′-AGACACATAT GATGGCCGAG-3′ 5′-GGCCTTGTATG CACCATTCAA-3′ | Specific to cell osteoclast attachment and mineralization of the bone matrix |
| OC/BGLAP (NM_199173) | Osteocalcin/Bone gamma-carboxyglutamate protein | 5′-AAGAGACCCA GGCGCTACCT-3′ 5′-AACTCGTCACA GTCCCGGATTG-3′ | Directly secreted by osteoblasts during bone remodeling |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, N.M.; Ribeiro, M.J.G.; Ricci, R.; Marques, M.A.; Lobo, A.O.; Marciano, F.R. In Vitro Osteogenesis Stimulation via Nano-Hydroxyapatite/Carbon Nanotube Thin Films on Biomedical Stainless Steel. Materials 2018, 11, 1555. https://doi.org/10.3390/ma11091555
Martinelli NM, Ribeiro MJG, Ricci R, Marques MA, Lobo AO, Marciano FR. In Vitro Osteogenesis Stimulation via Nano-Hydroxyapatite/Carbon Nanotube Thin Films on Biomedical Stainless Steel. Materials. 2018; 11(9):1555. https://doi.org/10.3390/ma11091555
Chicago/Turabian StyleMartinelli, Natalia M., Maria Julia G. Ribeiro, Ritchelli Ricci, Miller A. Marques, Anderson Oliveira Lobo, and Fernanda Roberta Marciano. 2018. "In Vitro Osteogenesis Stimulation via Nano-Hydroxyapatite/Carbon Nanotube Thin Films on Biomedical Stainless Steel" Materials 11, no. 9: 1555. https://doi.org/10.3390/ma11091555
APA StyleMartinelli, N. M., Ribeiro, M. J. G., Ricci, R., Marques, M. A., Lobo, A. O., & Marciano, F. R. (2018). In Vitro Osteogenesis Stimulation via Nano-Hydroxyapatite/Carbon Nanotube Thin Films on Biomedical Stainless Steel. Materials, 11(9), 1555. https://doi.org/10.3390/ma11091555