Surface Modification of Calcium Silicate via Mussel-Inspired Polydopamine and Effective Adsorption of Extracellular Matrix to Promote Osteogenesis Differentiation for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PDA-Coated CS
2.2. Setting Time and Injectability
2.3. Physicochemical Properties
2.4. Immersion Behavior
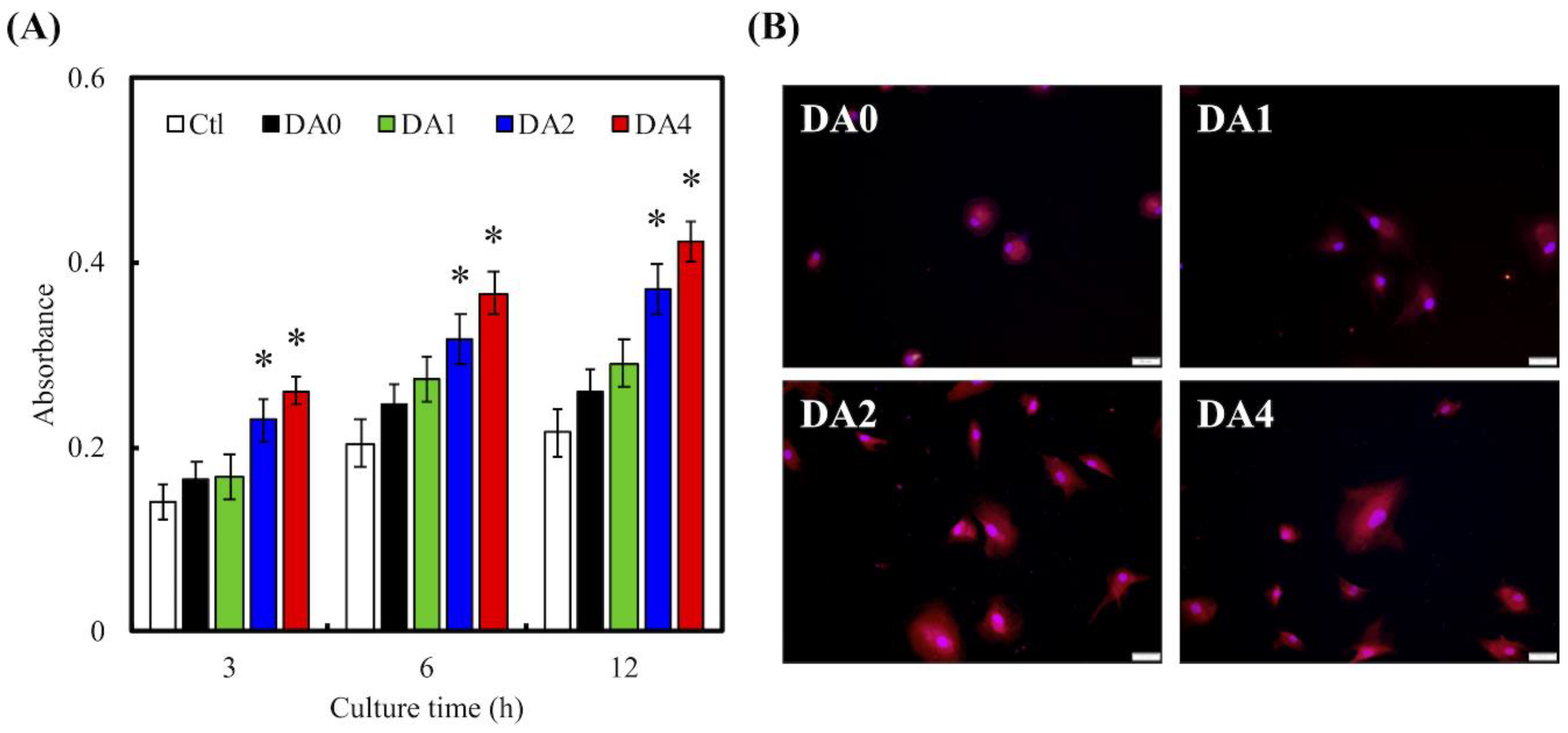
2.5. Cell Adhesion and Proliferation
2.6. Cell Morphology
2.7. Ion Released and ECM Secretion
2.8. Cell Adhesion-Related Protein
2.9. Osteogenesis-Related Genes and Protein Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PDA-Coated CS
3.2. Morphology and Strength of the Cements after Immersion in DMEM
3.3. Cell Adhesion
3.4. Ion Release and ECM Secretion
3.5. Cell Adhered-Related Protein
3.6. Cell Proliferation
3.7. Osteogenic Differentiation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiao, H.; Tang, T. Engineering 3D approaches to model the dynamic microenvironments of cancer bone metastasis. Bone Res. 2018, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Chiang, W.H.; Chen, I.W.P.; Liu, W.Y.; Chen, Y.W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate-graphene composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Hsu, T.T.; Huang, T.H.; Lin, C.Y.; Shie, M.Y. Fabrication and characterization of polycaprolactone and tricalcium phosphate composites for tissue engineering applications. J. Dent. Sci. 2017, 12, 33–43. [Google Scholar] [CrossRef]
- Schamel, M.; Bernhardt, A.; Quade, M.; Würkner, C.; Gbureck, U.; Moseke, C.; Gelinsky, M.; Lode, A. Cu2+, Co2+ and Cr3+ doping of a calcium phosphate cement influences materials properties and response of human mesenchymal stromal cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chiu, Y.C.; Shen, Y.F.; Wu, Y.H.; Shie, M.Y. Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 11. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Y.; Chen, Y.W.; Kao, C.T.; Hsu, T.T.; Huang, T.H.; Shie, M.Y. Human dental pulp cells responses to apatite precipitation from dicalcium silicates. Materials 2015, 8, 4491–4504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Yeh, C.H.; Shie, M.Y. Stimulatory effects of the fast setting and degradable Ca–Si–Mg cement on both cementogenesis and angiogenesis differentiation of human periodontal ligament cells. J. Mater. Chem. B 2015, 3, 7099–7108. [Google Scholar] [CrossRef]
- Huang, M.H.; Shen, Y.F.; Hsu, T.T.; Huang, T.H.; Shie, M.Y. Physical characteristics, antimicrobial and odontogenesis potentials of calcium silicate cement containing hinokitiol. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.F.; Ho, C.C.; Shie, M.Y.; Wang, K.; Fang, H.Y. Hinokitiol-loaded mesoporous calcium silicate nanoparticle induce apoptotic cell death through regulation of the function of MDR1 in lung adenocarcinoma cells. Materials 2016, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Y.; Kao, C.T.; Hung, C.J.; Huang, T.H.; Shie, M.Y. An evaluation of the inflammatory response of lipopolysaccharide-treated primary dental pulp cells with regard to calcium silicate-based cements. Int. J. Oral Sci. 2014, 6, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, M.G.; Chen, Y.W.; Shie, M.Y. Macrophage-mediated osteogenesis activation in co-culture with osteoblast on calcium silicate cement. J. Mater. Sci. Mater. Med. 2015, 26, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Landish, B.; Zhang, C.; Cui, N.; Du, J.; Lu, S.; Lin, X. 3D printing hydrogel with graphene oxide is functional in cartilage protection by influencing the signal pathway of Rank/Rankl/OPG. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 244–252. [Google Scholar]
- Zhai, D.; Xu, M.; Liu, L.; Chang, J.; Wu, C. Silicate-based bioceramics regulating osteoblast differentiation through a BMP2 signalling pathway. J. Mater. Chem. B 2017, 5, 7297–7306. [Google Scholar] [CrossRef]
- Huang, K.H.; Lin, Y.H.; Shie, M.Y.; Lin, C.P. Effects of bone morphogenic protein-2 loaded on the 3D-printed MesoCS scaffolds. J. Formosan Med. Assoc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Chen, Y.W.; Wang, K.; Shie, M.Y. Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(ε-caprolactone) scaffolds by stereolithography. J. Mater. Chem. B 2016, 4, 6307–6315. [Google Scholar] [CrossRef]
- Xu, M.; Zhai, D.; Xia, L.; Li, H.; Chen, S.; Fang, B.; Chang, J.; Wu, C. Hierarchical bioceramic scaffolds with 3D-plotted macropores and mussel-inspired surface nanolayers for stimulating osteogenesis. Nanoscale 2016, 8, 13790–13803. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.J.; Chen, Y.W.; Shieh, D.E.; Fang, H.Y.; Shie, M.Y. The effects of injectable calcium silicate-based composites with the Chinese herb on an osteogenic accelerator in vitro. Biomed. Mater. 2015, 10, 055004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Cheng, L.; Zhao, J.; Jin, R.; Sun, B.; Shi, Y.; Zhang, L.; Zhang, Y.; Cui, W. bFGF-grafted electrospun fibrous scaffolds via poly(dopamine) for skin wound healing. J. Mater. Chem. B 2014, 2, 3636–3645. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Wu, C.; Han, P.; Liu, X.; Xu, M.; Tian, T.; Chang, J.; Xiao, Y. Mussel-inspired bioceramics with self-assembled Ca-P/polydopamine composite nanolayer: Preparation, formation mechanism, improved cellular bioactivity and osteogenic differentiation of bone marrow stromal cells. Acta Biomater. 2014, 10, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Ball, V.; Del Frari, D.; Toniazzo, V.; Ruch, D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: Insights in the polydopamine deposition mechanism. J. Colloid Interface Sci. 2012, 386, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, M.; Chen, M.; Chen, M.; Ran, Z.; Zhu, C.; Liao, H. The reinforcing effect of polydopamine functionalized graphene nanoplatelets on the mechanical properties of epoxy resins at cryogenic temperature. Polym. Test. 2017, 58, 262–269. [Google Scholar] [CrossRef]
- Sharma, S.; Kothiyal, N.C. Influence of graphene oxide as dispersed phase in cement mortar matrix in defining the crystal patterns of cement hydrates and its effect on mechanical, microstructural and crystallization properties. RSC Adv. 2015, 5, 52642–52657. [Google Scholar] [CrossRef]
- Ma, T.; Gao, H.L.; Cong, H.P.; Yao, H.B.; Wu, L.; Yu, Z.Y.; Chen, S.M.; Yu, S.H. A bioinspired interface design for improving the strength and electrical conductivity of graphene-based fibers. Adv. Mater. 2018, 30, 1706435. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Moghaddam, E.; Shirazi, S.F.S.; Baradaran, S.; Mehrali, M.; Latibari, S.T.; Metselaar, H.S.C.; Kadri, N.A.; Zandi, K.; Osman, N.A.A. Synthesis, mechanical properties, and in vitro biocompatibility with osteoblasts of calcium silicate-reduced graphene oxide composites. ACS Appl. Mater. Interfaces 2014, 6, 3947–3962. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Huang, T.H.; Hung, C.J.; Lai, W.Y.; Kao, C.T.; Shie, M.Y. The synergistic effects of fibroblast growth factor-2 and mineral trioxide aggregate on an osteogenic accelerator in vitro. Int. Endod. J. 2014, 47, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Zancanela, D.C.; de Faria, A.N.; Simão, A.M.S.; Gonçalves, R.R.; Ramos, A.P.; Ciancaglini, P. Multi and single walled carbon nanotubes: Effects on cell responses and biomineralization of osteoblasts cultures. J. Mater. Sci. Mater. Med. 2016, 27, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Ho, C.C.; Huang, T.H.; Hsu, T.T.; Shie, M.Y. The ionic products from mineral trioxide aggregate–induced odontogenic differentiation of dental pulp cells via activation of the Wnt/β-catenin signaling pathway. J. Endod. 2016, 42, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chaudhuri, O. Regulation of breast cancer progression by extracellular matrix mechanics: Insights from 3D culture models. ACS Biomater. Sci. Eng. 2017, 4, 302–313. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J. Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.J.; Hsu, H.I.; Lin, C.C.; Huang, T.H.; Wu, B.C.; Kao, C.T.; Shie, M.Y. The role of integrin αv in proliferation and differentiation of human dental pulp cell response to calcium silicate cement. J. Endod. 2014, 40, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Chang, H.C.; Ding, S.J. Effects of altering the Si/Ca molar ratio of a calcium silicate cement on in vitro cell attachment. Int. Endod. J. 2012, 45, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Valerio, P.; Pereira, M.M.; Goes, A.M.; Leite, M.F. The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials 2004, 25, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chen, Y.W.; Shie, M.Y.; Fang, H.Y. Poly(dopamine)-assisted immobilization of Xu Duan on 3D printed poly(lactic acid) scaffolds to up-regulate osteogenic and angiogenic markers of bone marrow stem cells. Materials 2015, 8, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Makkar, P.; Paul, K.; Lee, B. Development of BMP-2 immobilized polydopamine mediated multichannelled biphasic calcium phosphate granules for improved bone regeneration. Mater. Lett. 2017, 208, 122–125. [Google Scholar] [CrossRef]
- Tu, M.G.; Ho, C.C.; Hsu, T.T.; Huang, T.H.; Lin, M.J.; Shie, M.Y. Mineral Trioxide Aggregate with mussel-inspired surface nanolayers for stimulating odontogenic differentiation of dental pulp cells. J. Endod. 2018, 44, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Zhou, Y.Z.; Fan, W.; Xiao, Y. A comparative study of mesoporous glass/silk and non-mesoporous glass/silk scaffolds: Physiochemistry and in vivo osteogenesis. Acta Biomater. 2011, 7, 2229–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Qu, S.; Zheng, X.; Xiong, X.; Fu, R.; Tang, K.; Zhong, Z.; Weng, J. Effect of polydopamine on the biomimetic mineralization of mussel-inspired calcium phosphate cement in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Niu, H.; Zhang, W.; Ma, Y.; Yuan, Y.; Liu, C. Microporous density-mediated response of MSCs on 3D trimodal macro/micro/nano-porous scaffolds via fibronectin/integrin and FAK/MAPK signaling pathways. J. Mater. Chem. B 2017, 5, 3586–3599. [Google Scholar] [CrossRef]
- Parsons, J.T. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003, 116, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.C.; Kao, C.T.; Huang, T.H.; Hung, C.J.; Shie, M.Y.; Chung, H.Y. Effect of verapamil, a calcium channel blocker, on the odontogenic activity of human dental pulp cells cultured with silicate-based materials. J. Endod. 2014, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, Y.T.; Zou, R.; Daniel, R.; Ko, C.C. Polydopamine-laced biomimetic material stimulation of bone marrow derived mesenchymal stem cells to promote osteogenic effects. Sci. Rep. 2017, 7, 12984. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Fu, S.J. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.Y.; Tsai, W.B. Poly(dopamine)-assisted immobilization of Arg-Gly-Asp peptides, hydroxyapatite, and bone morphogenic protein-2 on titanium to improve the osteogenesis of bone marrow stem cells. ACS Appl. Mater. Interfaces 2013, 5, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.Y.; Liu, T.Y.; Kuo, W.H.; Wang, M.J.; Tsai, W.B. Dopamine-assisted immobilization of hydroxyapatite nanoparticles and RGD peptides to improve the osteoconductivity of titanium. J. Biomed. Mater. Res. Part A 2013, 101, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Rim, N.G.; Kim, S.J.; Shin, Y.M.; Jun, I.; Lim, D.W.; Park, J.H.; Shin, H. Mussel-inspired surface modification of poly(L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surf. B 2012, 91, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, L.; Ostrovidov, S.; Shu, Y.; Khademhosseini, A.; Wu, H. Stretchable and micropatterned membrane for osteogenic differentation of stem cells. ACS Appl. Mater. Interfaces 2014, 6, 11915–11923. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Vohra, P.K.; Bhattacharya, R.; Dutta, S.; Sinha, S.; Mukhopadhyay, D. Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2. J. Cell Sci. 2009, 122, 3385–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Runx2 | 5′-TCAGGCATGTCCCTCGGTAT-3′ | 5′-TGGCAGGTAGGTATGGTAGTGG-3′ |
| ALP | 5′-TCAGAAGCTAACACCAACG-3′ | 5′-TTGTACGTCTTGGAGAGGGC-3′ |
| Col I | 5′-CTGCCCAGAAGAATATGTATCACC-3′ | 5′-GAAGCAAAGTTTCCTCCAAGACC-3′ |
| OC | 5′-GCGCTCTGTCTCTCTCTGACCT-3′ | 5′-TTTGTAGGCGGTCTTCAAGC-3′ |
| GAPDH | 5′-CTCACTCAAGATTGTCAGCA-3′ | 5′-GTCATCATACTTGGCAGGTT-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-T.; Chen, Y.-J.; Ng, H.-Y.; Lee, A.K.-X.; Huang, T.-H.; Lin, T.-F.; Hsu, T.-T. Surface Modification of Calcium Silicate via Mussel-Inspired Polydopamine and Effective Adsorption of Extracellular Matrix to Promote Osteogenesis Differentiation for Bone Tissue Engineering. Materials 2018, 11, 1664. https://doi.org/10.3390/ma11091664
Kao C-T, Chen Y-J, Ng H-Y, Lee AK-X, Huang T-H, Lin T-F, Hsu T-T. Surface Modification of Calcium Silicate via Mussel-Inspired Polydopamine and Effective Adsorption of Extracellular Matrix to Promote Osteogenesis Differentiation for Bone Tissue Engineering. Materials. 2018; 11(9):1664. https://doi.org/10.3390/ma11091664
Chicago/Turabian StyleKao, Chia-Tze, Yen-Jen Chen, Hooi-Yee Ng, Alvin Kai-Xing Lee, Tsui-Hsien Huang, Tz-Feng Lin, and Tuan-Ti Hsu. 2018. "Surface Modification of Calcium Silicate via Mussel-Inspired Polydopamine and Effective Adsorption of Extracellular Matrix to Promote Osteogenesis Differentiation for Bone Tissue Engineering" Materials 11, no. 9: 1664. https://doi.org/10.3390/ma11091664





