Effects of Heat-Treatment Temperature on the Microstructure and Mechanical Properties of Steel by MgO Nanoparticle Additions
Abstract
:1. Introduction
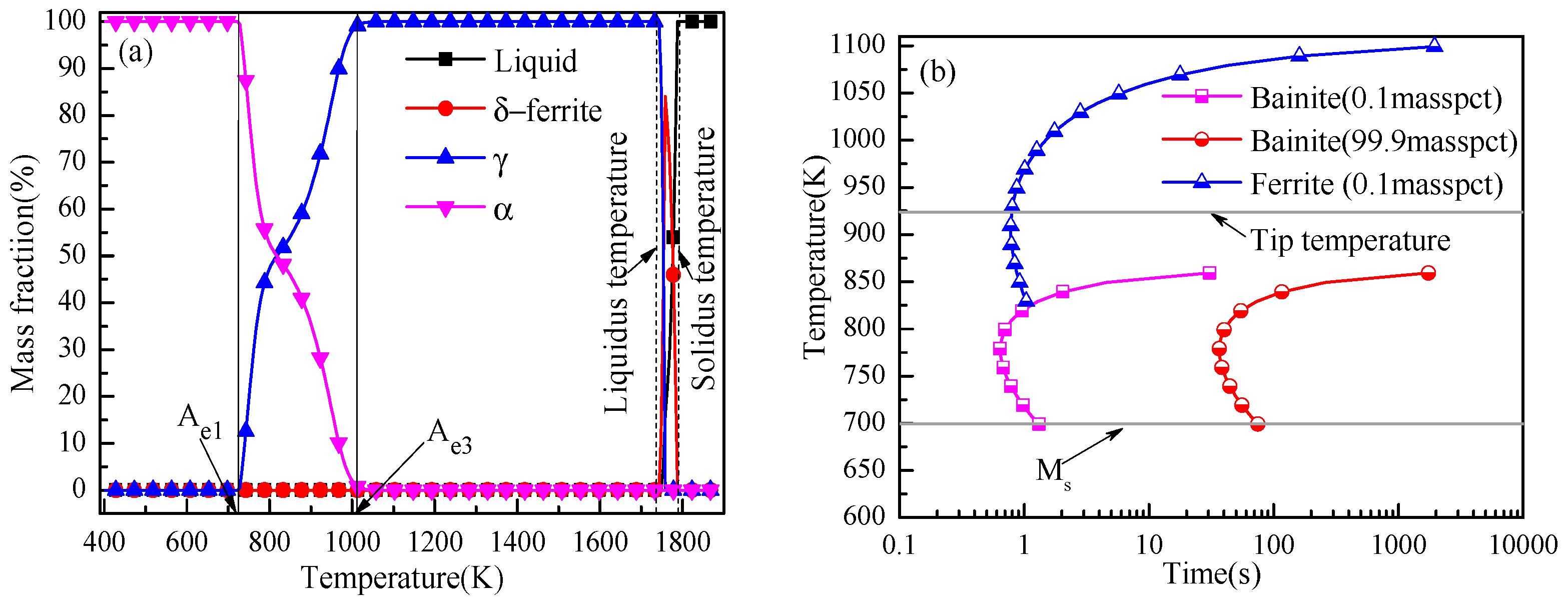
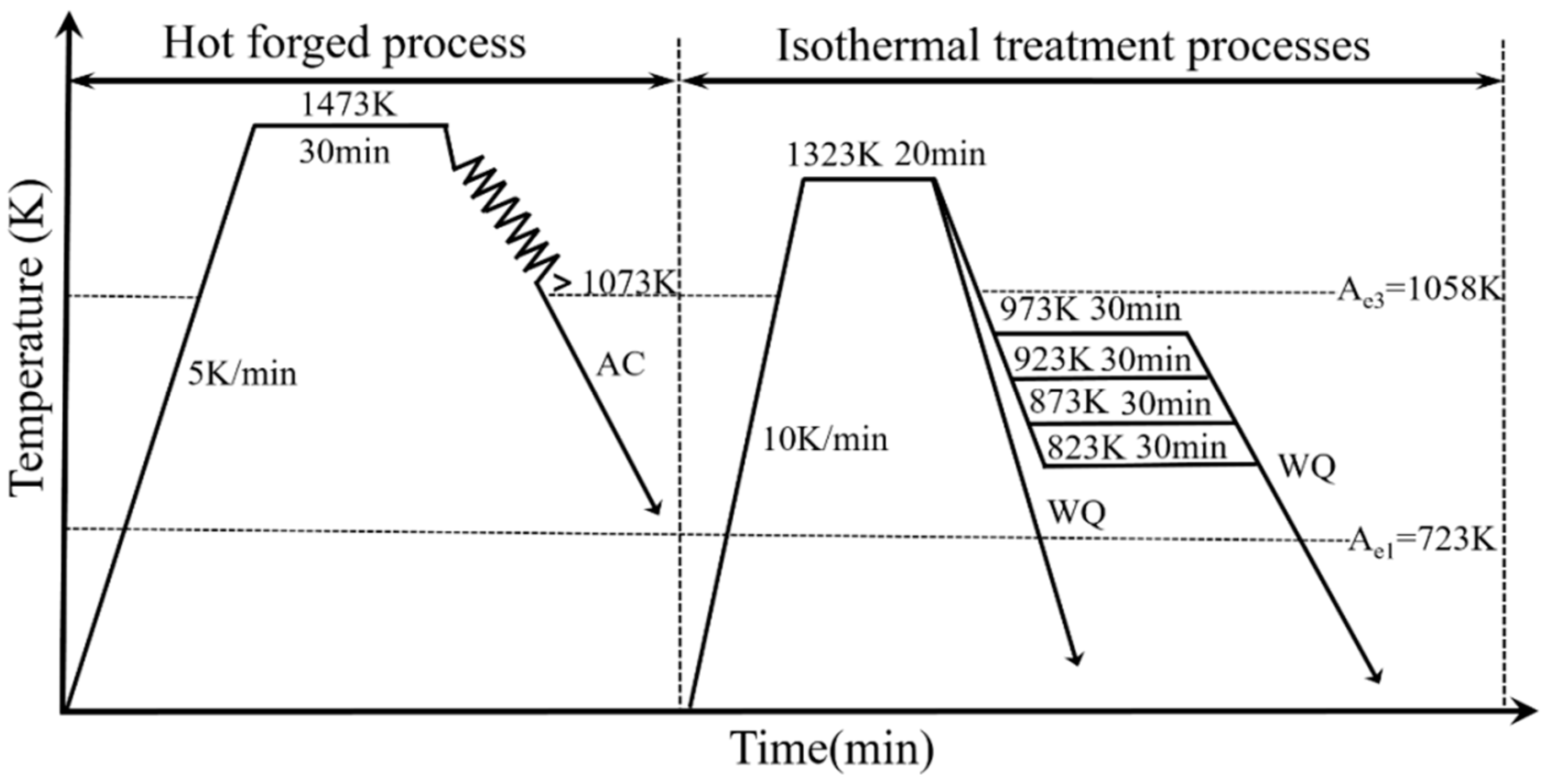
2. Experimental
2.1. Experimental Procedure
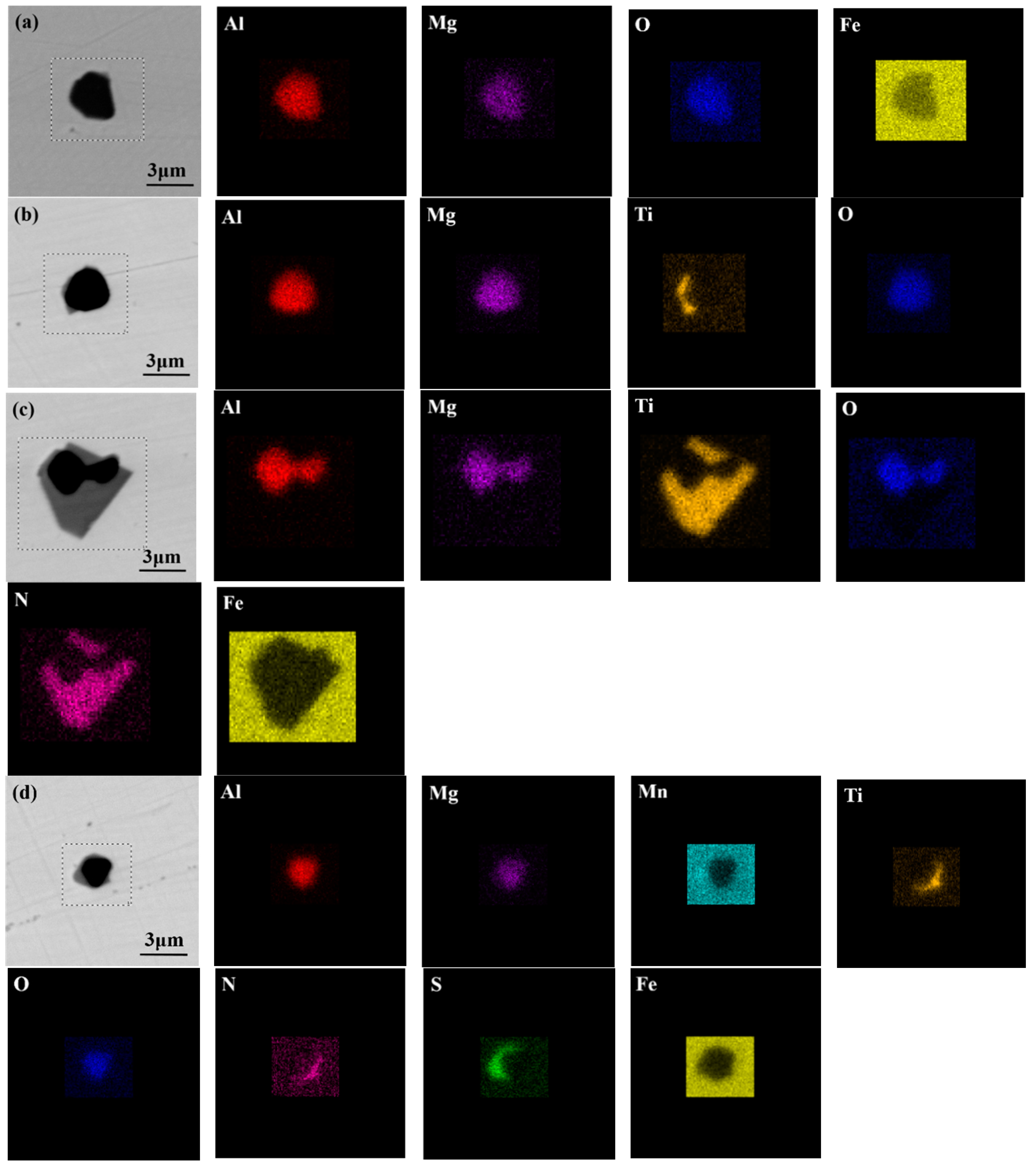
2.2. Characterization of Inclusions
2.3. Mechanical Properties and Microstructural Characteristics
3. Experimental Results
3.1. Characteristics of Inclusions
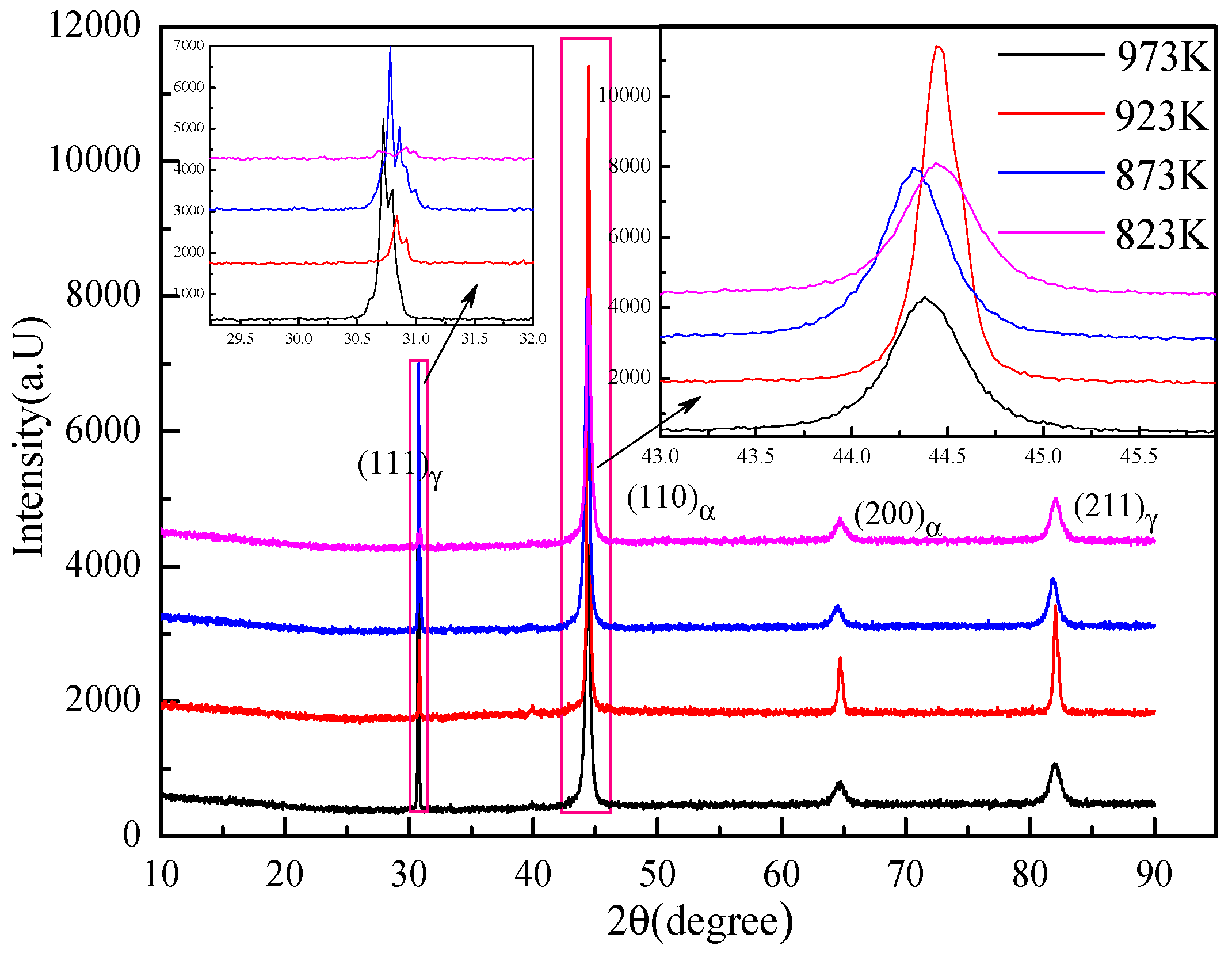
3.2. Microstructural Characteristics
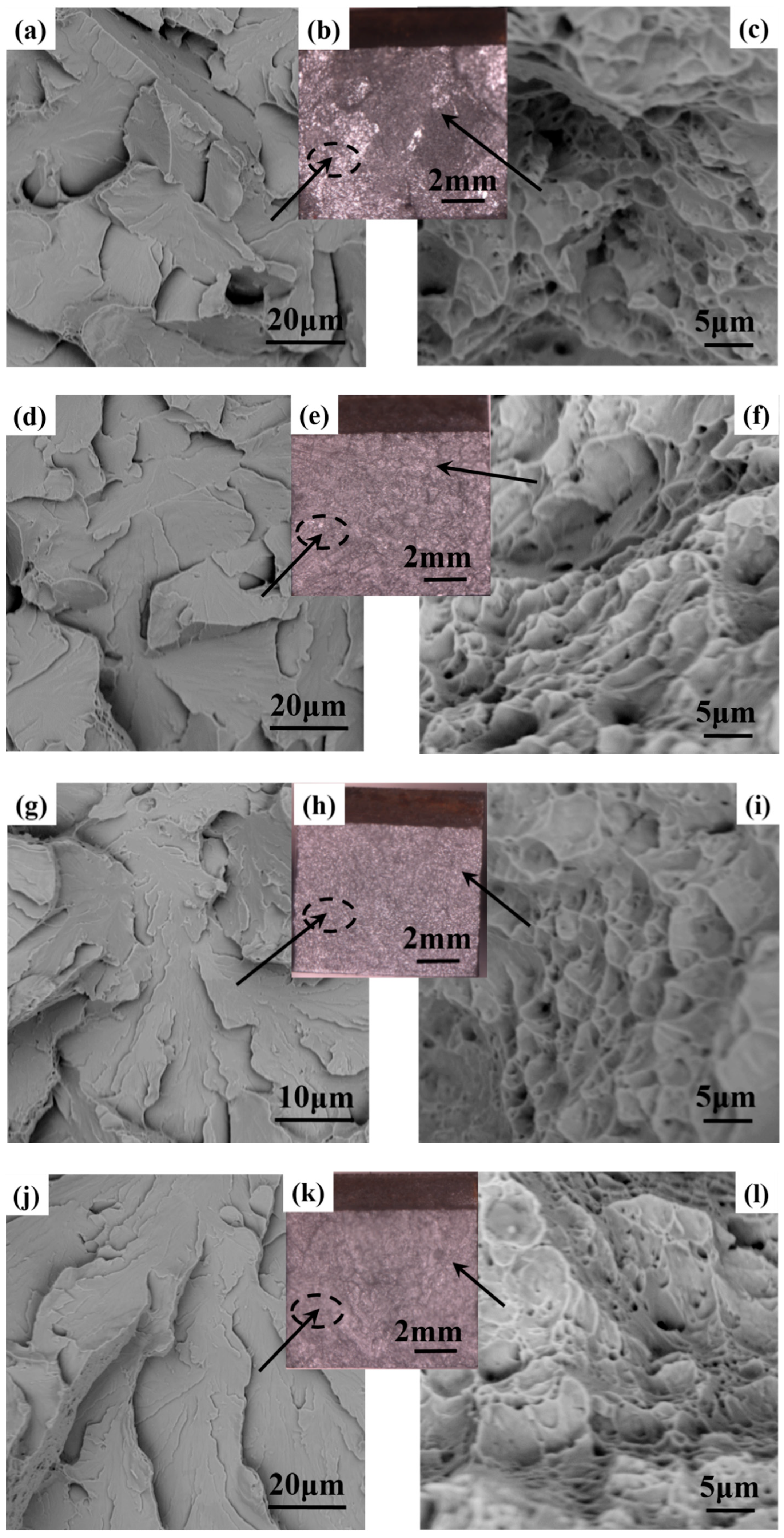
3.3. Mechanical Properties
4. Discussion
4.1. Effect of Inclusions on the Mechanism of IAF at Different Isothermal Heat-Treatment Temperatures
4.2. Refined Microstructure and Improved Mechanical Properties of Steel
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaushik, P.; Lehmann, J.; Nadif, M. State of the art in control of inclusions, their characterization, and future requirements. Metall. Mater. Trans. B 2012, 43, 710–725. [Google Scholar] [CrossRef]
- Wang, L.Z.; Yang, S.F.; Li, J.S.; Zhang, S.; Ju, J.T. Effect of Mg addition on the refinement and homogenized distribution of inclusions in steel with different Al contents. Metall. Mater. Trans. B 2017, 48, 805–818. [Google Scholar] [CrossRef]
- Beretta, S.; Murakami, Y. Largest-extreme-value distribution analysis of multiple inclusion types in determining steel cleanliness. Metall. Mater. Trans. B 2001, 32, 517–523. [Google Scholar] [CrossRef]
- Yang, S.F.; Wang, Q.Q.; Zhang, L.F.; Li, J.S.; Peaslee, K. Formation and modification of MgO·Al2O3-based inclusions in alloy steels. Metall. Mater. Trans. B 2012, 43, 731–750. [Google Scholar] [CrossRef]
- Yang, G.W.; Wang, X.H. Inclusion evolution after calcium addition in low carbon Al-killedsteel with ultra low sulfur content. ISIJ Int. 2015, 55, 126–133. [Google Scholar] [CrossRef]
- Takamura, J.I.; Mizoguchi, S. Roles of oxides in steels performance. In Proceedings of the Sixth International Iron and Steel Congress, Nagoya, Japan, 21–26 October 1990. [Google Scholar]
- Mizoguchi, S. Control of oxides as inoculants. In Proceedings of the Sixth International Iron and Steel Congress, Nagoya, Japan, 21–26 October 1990. [Google Scholar]
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the role of non-metallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Lu, Z.P.; Jiang, S.H.; He, J.Y.; Zhou, J.; Song, W.L.; Wu, Y.; Wang, H.; Liu, X.J. Second phase strengthening in advanced metal materials. Acta Metall. Sin. 2016, 52, 1183–1198. [Google Scholar]
- Xu, L.Y.; Yang, J.; Wang, R.Z.; Wang, Y.N.; Wang, W.L. Effect of Mg content on the microstructure and toughness of heat-affected zone of steel plate after high heat input welding. Metall. Mater. Trans. A 2016, 47, 3354–3364. [Google Scholar] [CrossRef]
- Kim, H.S.; Chang, C.H.; Lee, H.G. Evolution of inclusions and resultant microstructural change with Mg addition in Mn/Si/Ti deoxidized steels. Scr. Mater. 2005, 53, 1253–1258. [Google Scholar] [CrossRef]
- Kang, Y.; Jang, J.; Park, J.H.; Lee, C. Influence of Ti on non-metallic inclusion formation and acicular ferrite nucleation in high-strength low-alloy steel weld metals. Met. Mater. Int. 2014, 20, 119–127. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.H.; Jiang, M.; Hu, Z.Y.; Yang, C.W. Effects of Ti–Al complex deoxidization inclusions on nucleation of intragranular acicular ferrite in C–Mn steel. Steel Res. Int. 2016, 87, 445–455. [Google Scholar] [CrossRef]
- Guo, Z.; Kimura, N.; Tagashira, S.; Furuhara, T.; Maki, T. Kinetics and crystallography of intragranular pearlite transformation nucleated at (MnS + VC) complex precipitates in hypereutectoid Fe-Mn-C alloys. ISIJ Int. 2002, 42, 1033–1041. [Google Scholar] [CrossRef]
- Deng, X.X.; Jiang, M.; Wang, X.H. Mechanisms of inclusion evolution and intra-granular acicular ferrite formation in steels containing rare earth elements. Acta Metall. Sin. Engl. Lett. 2012, 25, 241–248. [Google Scholar]
- Li, X.B.; Min, Y.; Liu, C.J.; Jiang, M.F. Effect of Mg addition on the characterization of γ-α phase transformation during continuous cooling in low carbon steel. Steel Res. Int. 2016, 86, 1530–1540. [Google Scholar] [CrossRef]
- Zhou, B.W.; Li, G.Q.; Wan, X.L.; Li, Y.; Wu, K.M. In-situ observation of grain refinement in the simulated heat-affected zone of high-strength low-alloy steel by Zr-Ti combined deoxidation. Met. Mater. Int. 2016, 22, 267–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.B.; Ma, H. Enhancement of heat-affected zone toughness of a low carbon steel by TiN particle. Metall. Mater. Trans. B 2016, 47, 2148–2156. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, J.K.; Choo, W.Y. Effect of Ti addition on the potency of MnS for ferrite nucleation in C-Mn-V steels. ISIJ Int. 2007, 40, 1253–1259. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takeshita, K. Strengthening of steel by the method of spraying oxide particles into molten steel stream. Metall. Trans. B 1978, 9, 383–388. [Google Scholar] [CrossRef]
- Kiviö, M.; Holappa, L.; Iung, T. Addition of dispersoid titanium oxide inclusions in steel and their influence on grain refinement. Metall. Mater. Trans. B 2010, 41, 1194–1204. [Google Scholar] [CrossRef]
- Mu, W.Z.; Shibata, H.; Hedström, P.; Jönsson, P.G.; Nakajima, K. Ferrite formation dynamics and microstructure due to inclusion engineering in low-alloy steels by Ti2O3, and TiN addition. Metall. Mater. Trans. B 2016, 47, 1–15. [Google Scholar] [CrossRef]
- Xuan, C.J.; Mu, W.Z.; Olano, Z.I.; Jönsson, P.G.; Nakajima, K. Effect of the Ti, Al contents on the inclusion characteristics in steels with TiO2 and TiN particle additions. Steel Res. Int. 2016, 87, 911–920. [Google Scholar] [CrossRef]
- Kiviö, M.; Holappa, L. Addition of titanium oxide inclusions into liquid steel to control nonmetallic inclusions. Metall. Mater. Trans. B 2012, 43, 233–240. [Google Scholar] [CrossRef]
- Mu, W.Z.; Jönsson, P.G.; Nakajima, K. Prediction of intragranular ferrite nucleation from TiO, TiN, and VN inclusions. J. Mater. Sci. 2016, 51, 1–13. [Google Scholar] [CrossRef]
- Gao, X.Z.; Yang, S.F.; Li, J.S.; Yang, Y.D.; Chattopadhyay, K.; Mclean, A. Addition of MgO nanoparticles to carbon structural steel and the effect on inclusion characteristics and microstructure. Metall. Mater. Trans. B 2016, 47, 1124–1136. [Google Scholar] [CrossRef]
- Gao, X.Z.; Yang, S.F.; Li, J.S.; Yang, Y.D.; Chattopadhyay, K.; Mclean, A. Effects of MgO nanoparticle additions on the structure and mechanical properties of continuously cast steel billets. Metall. Mater. Trans. A 2016, 47, 461–470. [Google Scholar] [CrossRef]
- Gao, X.Z.; Yang, S.F.; Li, J.S.; Ma, A.; Chattopadhyay, K. Improvement of utilization ratio of nanoparticles in steel and its influence on acicular ferrite formation. Steel Res. Int. 2017, 88, 1–13. [Google Scholar] [CrossRef]
- Mu, W.Z.; Mao, H.H.; Jönsson, P.G.; Nakajima, K. Effect of carbon content on the potency of the intragranular ferrite formation. Steel Res. Int. 2016, 87, 311–319. [Google Scholar] [CrossRef]
- Kang, Y.; Han, K.; Park, J.H.; Lee, C. Mn-depleted zone formation in rapidly cooled high-strength low-alloy steel welds. Metall. Mater. Trans. A 2014, 45, 4753–4757. [Google Scholar] [CrossRef]
- Lee, T.Y.; Kim, H.J.; Kang, B.Y.; Hwang, S.K. Effect of inclusion size on the nucleation of acicular ferrite in welds. ISIJ Int. 2000, 40, 1260–1268. [Google Scholar] [CrossRef]
- Wan, X.L.; Wu, K.M.; Cheng, L.; Wei, R. In-situ observations of acicular ferrite growth behavior in the simulated coarse-grained heat-affected zone of high-strength low-alloy steels. ISIJ Int. 2015, 55, 679–685. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zheng, W.; Li, G.Q.; Matsuura, H.; Tsukihashi, F. Effect of inclusions’ behavior on the microstructure in Al-Ti deoxidized and magnesium-treated steel with different aluminum contents. Metall. Mater. Trans. B 2015, 46, 1226–1241. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K.; Mayerhofer, A.; Bernhard, C. On the capability of nonmetallic inclusions to act as nuclei for acicular ferrite in different steel grades. Metall. Mater. Trans. B 2017, 48, 1–15. [Google Scholar] [CrossRef]
- Zhang, D.; Terasaki, H.; Komizo, Y.I. In situ observation of the formation of intragranular acicular ferrite at non-metallic inclusions in C–Mn steel. Acta Mater. 2010, 58, 1369–1378. [Google Scholar] [CrossRef]
- Song, M.M.; Song, B.; Zhang, S.H.; Xue, Z.L.; Yang, Z.B.; Xu, R.S. Role of lanthanum addition on acicular ferrite transformation in C–Mn steel. ISIJ Int. 2017, 57, 1261–1267. [Google Scholar] [CrossRef]
- Seo, J.Y.; Park, S.K.; Kwon, H.; Cho, K.S. Influence of carbide modifications on the mechanical properties of ultra-high-strength stainless steels. Metall. Mater. Trans. A 2017, 48, 4477–4485. [Google Scholar] [CrossRef]
- Isasti, N.; Jorge-Badiola, D.; Taheri, M.L.; Uranga, P. Microstructural features controlling mechanical properties in Nb-Mo microalloyed steels. Part II: Impact toughness. Metall. Mater. Trans. A 2014, 45, 4972–4982. [Google Scholar] [CrossRef]
- Moon, J.; Lee, C. Behavior of (Ti, Nb)(C, N) complex particle during thermomechanical cycling in the weld CGHAZ of a microalloyed steel. Acta Mater. 2009, 57, 2311–2320. [Google Scholar] [CrossRef]
- Zhang, D.; Shintaku, Y.; Suzuki, S.; Komizo, Y. In situ observation of phase transformation in low-carbon, boron-treated steels. Metall. Mater. Trans. A 2012, 43, 447–458. [Google Scholar] [CrossRef]
- Karasev, A.V.; Suito, H. Effect of particle size distribution on austenite grain growth in Fe–0.05mass%C alloy deoxidized with Mn–Si, Ti, Mg, Zr and Ce. ISIJ Int. 2006, 46, 718–727. [Google Scholar] [CrossRef]
- Wang, C.; Nuhfer, N.T.; Sridhar, S. Transient behavior of inclusion chemistry, shape, and structure in Fe-Al-Ti-O melts: Effect of titanium source and laboratory deoxidation simulation. Metall. Mater. Trans. B 2009, 40, 1005–1021. [Google Scholar] [CrossRef]
- Shim, J.H.; Cho, Y.W.; Chung, S.H.; Shim, J.D.; Lee, D.N. Nucleation of intragranular ferrite at Ti2O3 particle in low carbon steel. Acta Mater. 1999, 47, 2751–2760. [Google Scholar] [CrossRef]
- Shim, J.H.; Oh, Y.J.; Suh, J.Y.; Cho, Y.W.; Shim, J.D.; Byun, J.S.; Lee, D.N. Ferrite nucleation potency of non-metallic inclusions in medium carbon steels. Acta Mater. 2001, 49, 2115–2122. [Google Scholar] [CrossRef]
- Byun, J.S.; Shim, J.H.; Cho, Y.W.; Lee, D.N. Non-metallic inclusion and intragranular nucleation of ferrite in Ti-killed C–Mn steel. Acta Mater. 2003, 51, 1593–1606. [Google Scholar] [CrossRef]
- Furuhara, T.; Shinyoshi, T.; Miyamoto, G.; Yamaguchi, J.; Sugita, N.; Kimura, N.; Takemura, N.; Maki, T. Multiphase crystallography in the nucleation of intragranular ferrite on MnS + V(C, N) complex precipitate in austenite. ISIJ Int. 2003, 43, 2028–2037. [Google Scholar] [CrossRef]
- Enomoto, M.; Lange, W.F.; Aaronson, H.I. The kinetics of ferrite nucleation at austenite grain edges in Fe-C and Fe-C-X alloys. Metall. Trans. A 1986, 17, 1399–1407. [Google Scholar] [CrossRef]
- Zheng, X.F.; Hayes, P.C.; Lee, H.G. Particle removal from liquid phase using fine gas bubbles. ISIJ Int. 1997, 37, 1091–1097. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhang, C.; Pan, T. The mechanism of intragranular ferrite nucleation on inclusion in steel. Mater. Sci. Forum 2005, 475, 113–116. [Google Scholar] [CrossRef]
- Nam, K.S.; Lee, H.J.; Lee, S.H.; Lee, G.H.; Song, Y.S.; Lee, D.Y. The effect of an atmospheric pressure plasma treated MgO layer on the discharge performance of an ac plasma display panel. Surf. Coat. Technol. 2006, 201, 2567–2572. [Google Scholar] [CrossRef]
- Gao, E.; Zou, G.; Wang, W.L.; Ma, F.J.; Luo, X.C. Undercooling and wettability behavior of interstitial-free steel on TiN, Al2O3, and MgAl2O4 under controlled oxygen partial pressure. Metall. Mater. Trans. B 2016, 48, 1–10. [Google Scholar] [CrossRef]
- Yin, J.Q.; Hillert, M.; Borgenstam, A. Morphology of proeutectoid ferrite. Metall. Mater. Trans. A 2017, 48, 1425–1443. [Google Scholar] [CrossRef]
- Wang, W.; Fu, L.M. Effect of the inclusion/precipitate size on the intragranular ferrite nucleation. Acta Metall. Sin. 2008, 44, 723–728. [Google Scholar]













| C | Si | Mn | Nb | Ti | Ni | Al | S | N |
|---|---|---|---|---|---|---|---|---|
| 0.09 | 0.38 | 1.58 | 0.04 | 0.01 | 0.25 | 0.025 | 0.008 | 0.003 |
| Specimen | Yield Strength (MPa) | Tensile Strength (Mpa) | Elongation (%) |
|---|---|---|---|
| M1 | 535 | 769 | 9.6 |
| M2 | 529 | 763 | 10.8 |
| M3 | 531 | 803 | 13.6 |
| M4 | 540 | 775 | 8.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, S.; Li, J.; Liu, W.; Dong, A. Effects of Heat-Treatment Temperature on the Microstructure and Mechanical Properties of Steel by MgO Nanoparticle Additions. Materials 2018, 11, 1707. https://doi.org/10.3390/ma11091707
Zhou Y, Yang S, Li J, Liu W, Dong A. Effects of Heat-Treatment Temperature on the Microstructure and Mechanical Properties of Steel by MgO Nanoparticle Additions. Materials. 2018; 11(9):1707. https://doi.org/10.3390/ma11091707
Chicago/Turabian StyleZhou, Yutao, Shufeng Yang, Jingshe Li, Wei Liu, and Anping Dong. 2018. "Effects of Heat-Treatment Temperature on the Microstructure and Mechanical Properties of Steel by MgO Nanoparticle Additions" Materials 11, no. 9: 1707. https://doi.org/10.3390/ma11091707





