The Effect of Gradient Bias Design on Electrochemistry and Tribology Behaviors of PVD CrN Film in a Simulative Marine Environment
Abstract
:1. Introduction
2. Experimental
2.1. Deposition
2.2. Characterization
3. Results and Discussion
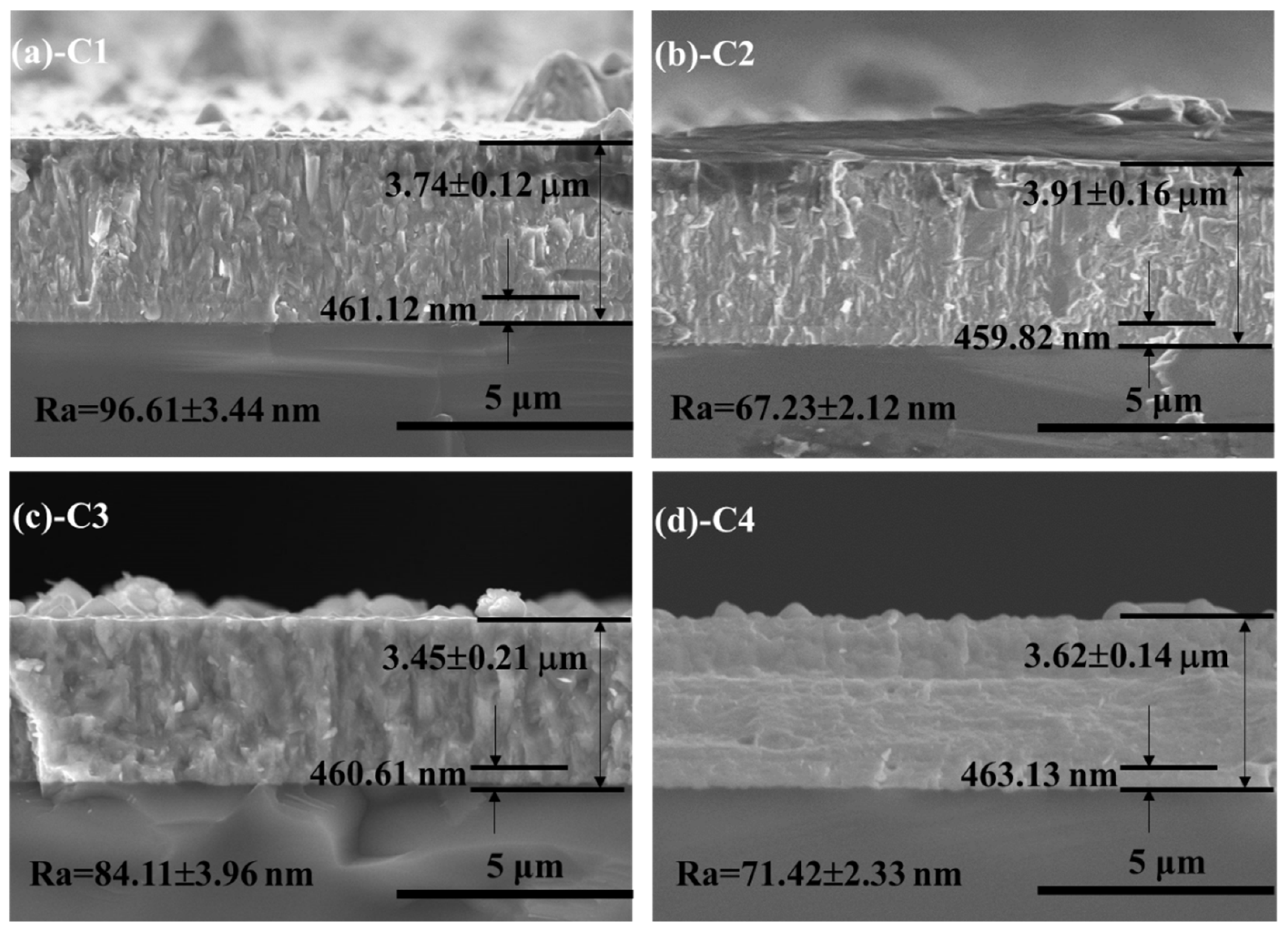
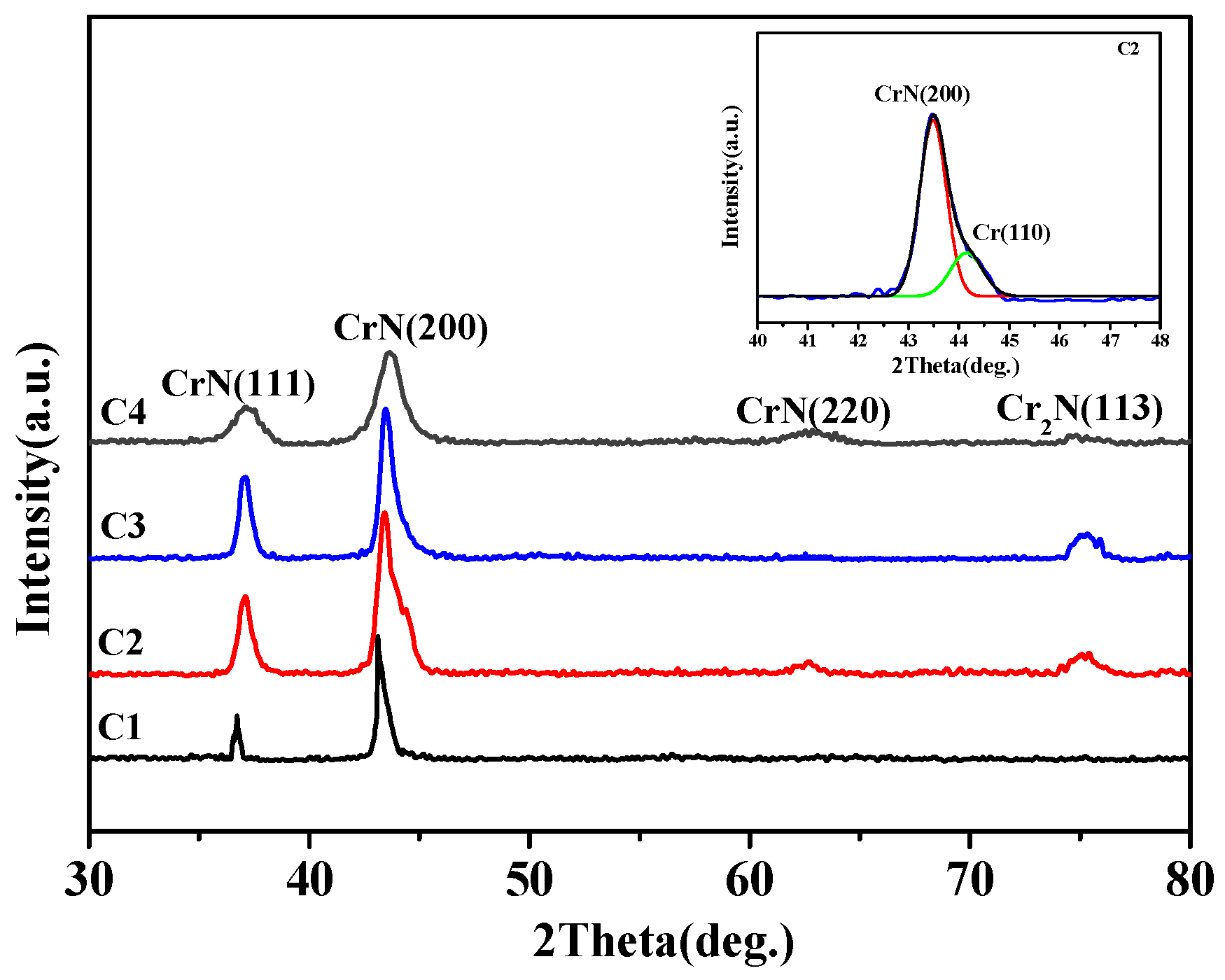
3.1. Microstructures
3.2. Mechanical Performances
3.3. Corrosion Performances
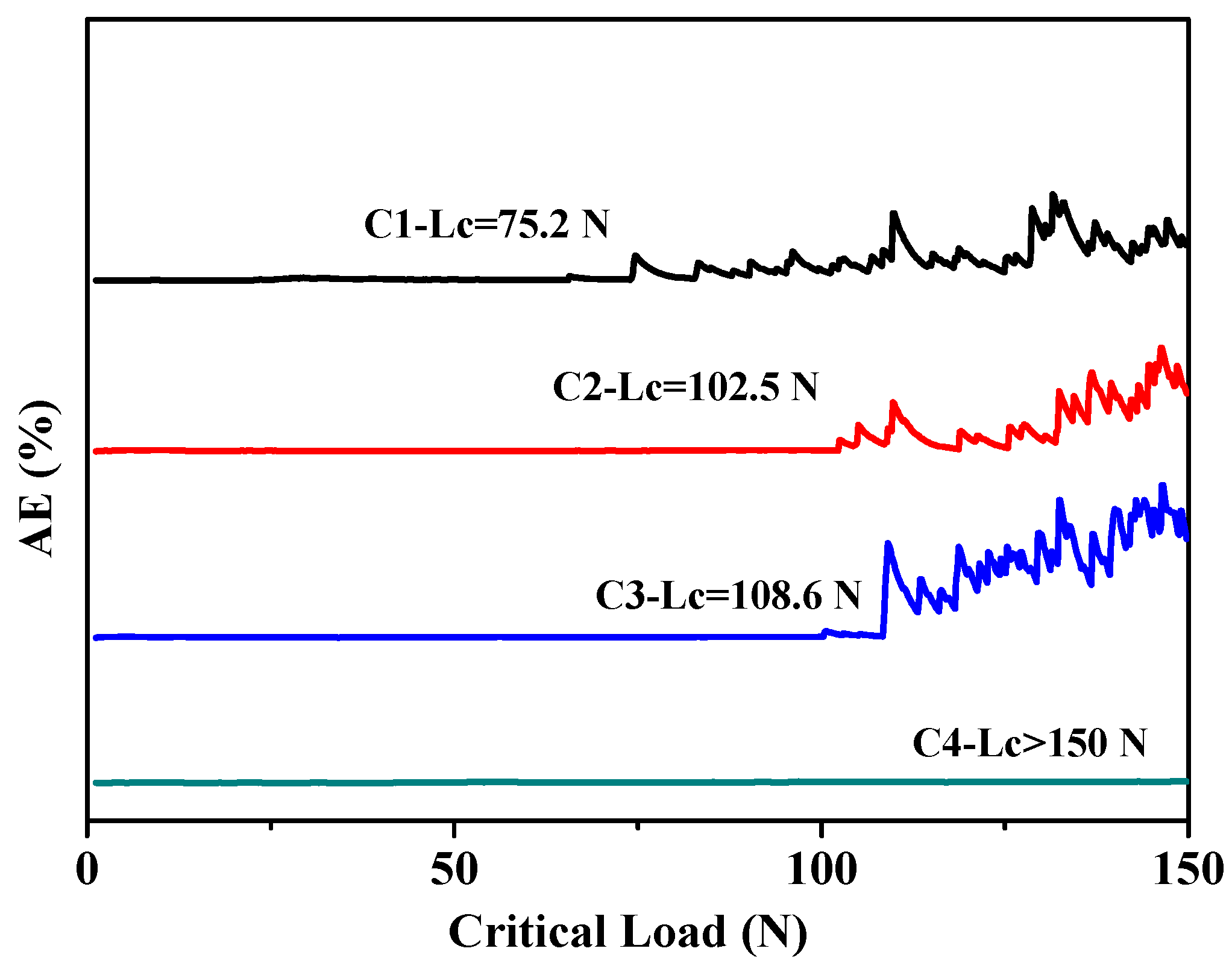
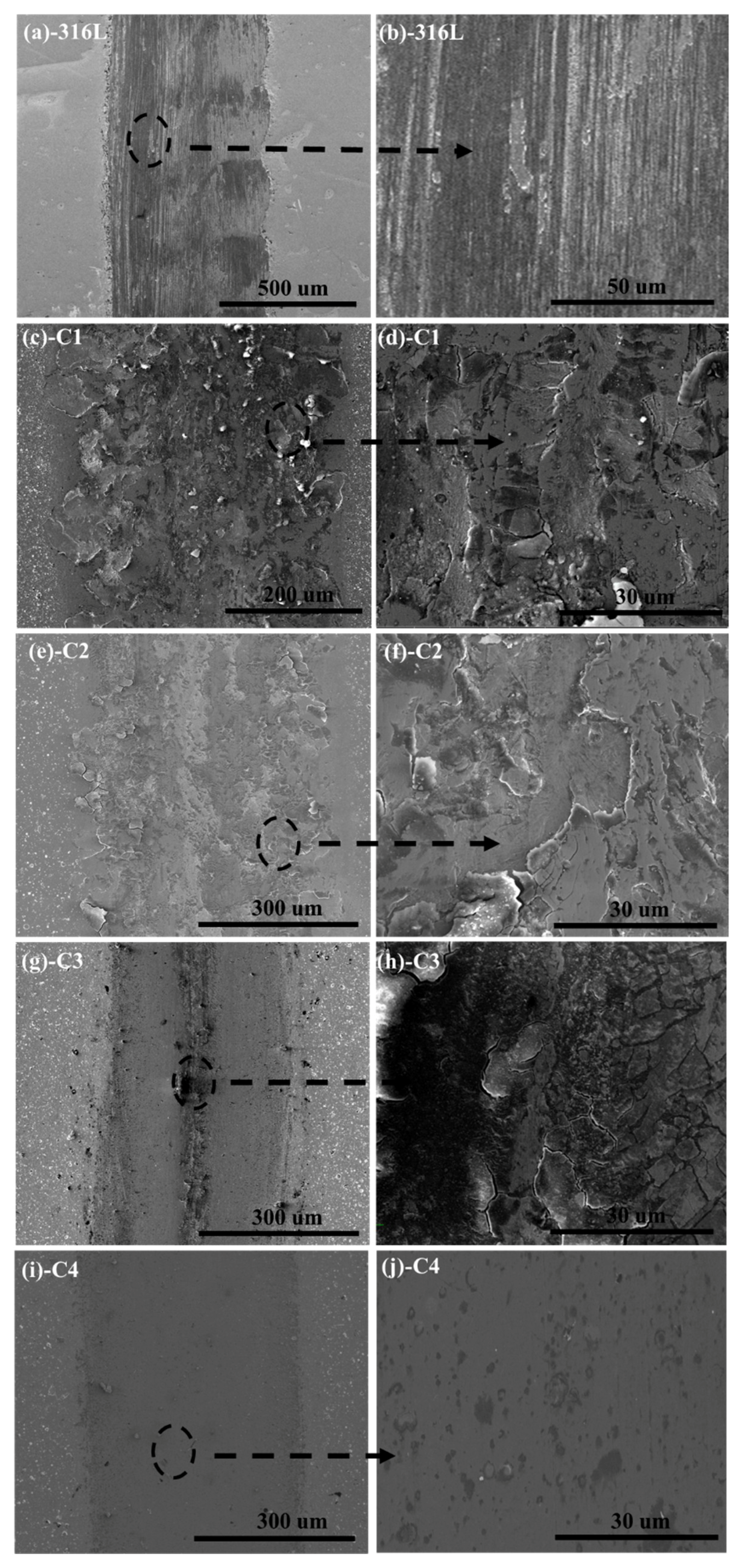
3.4. Tribological Performances
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shan, L.; Wang, Y.; Li, J.; Li, H.; Lu, X.; Chen, J. Structure and mechanical properties of thick Cr/Cr2N/CrN multilayer coating deposited by multi-arc ion plating. Trans. Nonferr. Met. Soc. China 2015, 25, 1135–1143. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, Y.; Wang, Y.; Zhang, Q.; Xue, Q. Tribocorrosion behaviors of PVD CrN coated stainless steel in seawater. Wear 2016, 362–363, 97–104. [Google Scholar] [CrossRef]
- Wang, J.; Pu, J.; Zhang, G.; Wang, L. Architecture of super thick diamond-like carbon films with excellent high temperature wear resistance. Tribol. Int. 2015, 81, 129–138. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Wang, C.; Li, L.; Yao, Y. Doping carbon to improve the tribological performance of CrN coatings in seawater. Tribol. Int. 2015, 90, 362–371. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Chen, H.; Li, J.; Zhou, S.; Xue, Q. Influences of bias voltage on the microstructures and tribological performances of Cr-C-N coatings in seawater. Surf. Coat. Technol. 2015, 270, 305–313. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Zhang, G.; Jiang, X.; Wang, L.; Xue, Q. A novel duplex PDMS/CrN coating with superior corrosion resistance for marine applications. RSC Adv. 2016, 6, 87003–87012. [Google Scholar] [CrossRef]
- Major, L.; Janusz, M.; Kot, M.; Lackner, J.; Major, B. Development and complex characterization of bio-tribological Cr/CrN + a-C:H (doped Cr) nano-multilayer protective coatings for carbon–fiber-composite materials. RSC Adv. 2015, 5, 9405–9415. [Google Scholar] [CrossRef]
- Hui, Z.; Tang, X.; Wei, R.; Hu, L.; Yang, J.; Luo, H.; Dai, J.; Song, W.; Liu, X.; Zhu, X.; et al. Facile chemical solution deposition of nanocrystalline CrN thin films with low magneto resistance. RSC Adv. 2014, 4, 12568–12571. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Ding, X.; Zhou, F.; Wang, C.; Zhang, W.; Lid, L.; Lee, S. Microstructure and water-lubricated friction and wear properties of CrN(C) coatings with different carbon contents. Appl. Surf. Sci. 2013, 268, 579–587. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, Y.; Wang, Y.; Li, J.; Jiang, X.; Chen, J. Corrosion and wear behaviors of PVD CrN and CrSiN coatings in seawater. Trans. Nonferr. Met. Soc. China 2016, 26, 175–184. [Google Scholar] [CrossRef]
- Xu, J.; Umehara, H.; Kojima, I. Effect of deposition parameters on composition, structures, density and topography of CrN films deposited by r.f. magnetron sputtering. Appl. Surf. Sci. 2002, 201, 208–218. [Google Scholar] [CrossRef]
- Shan, L.; Wang, Y.; Li, J.; Chen, J. Effect of N2 flow rate on microstructure and mechanical properties of PVD CrNx coatings for tribological application in seawater. Surf. Coat. Technol. 2014, 242, 74–82. [Google Scholar] [CrossRef]
- Shan, L.; Wang, Y.; Li, J.; Jiang, X.; Chen, J. Architecture of multilayer Cr/CrN coatings for wear protection in seawater: Cr/CrN ratio and total thickness. Tribol. Trans. 2015, 58, 914–923. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS comparison on corrosion performance of PVD TiN and CrN coated mild steel in 0.5 N NaCl aqueous solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
- Sproul, D. Multilayer, multicomponent, and multiphase physical vapor deposition coatings for enhanced performance. J. Vac. Sci. Technol. A 1994, 12, 1595–1601. [Google Scholar] [CrossRef]
- Hauert, R. Patscheider, from alloying to nanocomposites-improved performance of hard coatings. J. Adv. Eng. Mater. 2000, 2, 247–259. [Google Scholar] [CrossRef]
- Kok, Y.N.; Hovsepian, P.E.; Luo, Q.; Lewis, D.B.; Wen, J.G.; Petrov, I. Influence of the bias voltage on the structure and the tribological performance of nanoscale multilayer C/Cr PVD coatings. Thin Solid Films 2005, 475, 219–226. [Google Scholar] [CrossRef]
- Warcholinski, B.; Gilewicz, A. Effect of substrate bias voltage on the properties of CrCN and CrN coatings deposited by cathodic arc evaporation. Vacuum 2013, 90, 145–150. [Google Scholar] [CrossRef]
- Dai, W.; Ke, P.; Wang, A. Influence of bias voltage on microstructure and properties of Al-containing diamond-like carbon films deposited by a hybrid ion beam system. Surf. Coat. Technol. 2013, 229, 217–221. [Google Scholar] [CrossRef]
- Shaha, K.P.; Pei, Y.T.; Martinez-Martinez, D.; Sanchez-Lopez, J.C.; Hosson, J.T.M.D. Effect of process parameters on mechanical and tribological performance of pulsed-DC sputtered TiC/aC: H nanocomposite films. Surf. Coat. Technol. 2010, 205, 2633–2642. [Google Scholar] [CrossRef]
- Almer, J.; Oden, M.; Hakansson, G. Microstructure, stress and mechanical properties of arc-evaporated Cr–C–N coatings. Thin Solid Films 2001, 385, 190–197. [Google Scholar] [CrossRef]
- Bunshah, R.; Raghuram, A. Activated reactive evaporation process for high rate deposition of compounds. J. Vac. Sci. Technol. 1972, 9, 1385–1388. [Google Scholar] [CrossRef]
- Wang, D.; Oki, T. The morphology and orientation of Cr-N films deposited by reactive ion plating. Thin Solid Films 1990, 185, 219–230. [Google Scholar] [CrossRef]
- Bertrand, G.; Mahdjoub, H.; Meunier, C. A study of the corrosion behaviour and protective quality of sputtered chromium nitride coatings. Surf. Coat. Technol. 2000, 126, 199–209. [Google Scholar] [CrossRef]
- Zhi, X.; Liu, J.; Xing, J.; Ma, S. Effect of cerium modification on microstructure and properties of hypereutectic high chromium cast iron. Mater. Sci. Eng. A 2014, 603, 98–103. [Google Scholar] [CrossRef]
- Ahn, S.; Choi, Y.; Kim, J.; Han, J. A study on corrosion resistance characteristics of PVD Cr-N coated steels by electrochemical method. Surf. Coat. Technol. 2002, 150, 319–326. [Google Scholar] [CrossRef]
- Bull, S.; Rickerby, D. Compositional, microstructural and morphological effects on the mechanical and tribological properties of chromium nitrogen films. Metall. Coat. Thin Films 1990, 43–44, 732–744. [Google Scholar]
- Cunha, L.; Andritschky, M.; Pischow, K.; Wang, Z. Microstructure of CrN coatings produced by PVD techniques. Thin Solid Films 1999, 355, 465–471. [Google Scholar] [CrossRef]
- Patscheider, J.; Zehnder, T.; Diserens, M. Structure-performance relations in nanocomposite coatings. Surf. Coat. Technol. 2001, 146, 201–208. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, W.; Zhou, Y. Investigation of the relationship between elastic modulus and hardness based on depth-sensing indentation measurements. Acta. Mater. 2004, 52, 5397–5404. [Google Scholar] [CrossRef]
- Musil, Y.; Jirout, M. Toughness of hard nanostructured ceramic thin films. Surf. Coat. Technol. 2007, 201, 5148–5152. [Google Scholar] [CrossRef]
- Heinke, W.; Leyland, A.; Matthews, A.; Berg, G.; Friedrich, C.; Broszeit, E. Evaluation of PVD nitride coatings, using impact, scratch and Rockwell-C adhesion tests. Thin Solid Films 1995, 270, 431–438. [Google Scholar] [CrossRef]
- Lawn, B.; Evans, A.; Marshall, D. Elastic-plastic indentation damage in ceramics the median-radial crack system. J. Am. Ceram. Soc. 1980, 63, 574–581. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Meng, X. Structure, hardness and corrosion behavior of a gradient CrN x thick coating applied to turbine blades. Appl. Surf. Sci. 2009, 255, 7408–7413. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Kim, K.; Pan, F. Structural and mechanical properties of compositionally gradient CrNx coatings prepared by arc ion plating. Appl. Surf. Sci. 2009, 255, 9200–9205. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Meng, F.; Li, J. Evaluation of fatigue resistance of a gradient CrNx coating applied to turbine blades. Mater. Sci. Eng. A 2010, 527, 1436–1443. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Yan, F. Friction and wear behaviors of several polymers sliding against GCr15 and 316 steel under the lubrication of sea water. Tribol. Lett. 2011, 42, 17–25. [Google Scholar] [CrossRef]
- Wang, J.; Yan, F.; Xue, Q. Friction and wear behavior of ultra-high molecular weight polyethylene sliding against GCr15 steel and electroless Ni-P alloy coating under the lubrication of seawater. Tribol. Lett. 2009, 35, 85–95. [Google Scholar] [CrossRef]
- Polcar, T.; Kubart, T.; Novak, R.; Kopecky, L.; Siroky, P. Comparison of tribological behaviour of TiN, TiCN and CrN at elevated temperatures. Surf. Coat. Technol. 2005, 193, 192–199. [Google Scholar] [CrossRef]








| Solution | NaCl | Na2SO4 | MgCl2 | CaCl2 | SrCl2 | KCl | NaHCO3 | KBr | H3BO3 | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 24.53 | 4.09 | 5.20 | 1.16 | 0.025 | 0.695 | 0.201 | 0.101 | 0.027 | 0.003 |
| Film | I(200)/I(111) | Average Grain Size (nm) |
|---|---|---|
| C1 | 3.27 | 198 ± 18 |
| C2 | 2.05 | 132 ± 11 |
| C3 | 1.83 | 142 ± 16 |
| C4 | 2.38 | 79 ± 9 |
| Sample | H (GPa) | H/E | H3/E2 (GPa) |
|---|---|---|---|
| 316L | 3.6 ± 0.7 | 0.020 | 0.001 |
| C1 | 18.2 ± 1.5 | 0.049 | 0.044 |
| C2 | 19.7 ± 1.6 | 0.057 | 0.064 |
| C3 | 22.4 ± 1.1 | 0.064 | 0.092 |
| C4 | 21.5 ± 1.2 | 0.063 | 0.085 |
| Sample | βa (V dec−1) | βc (V dec−1) | icorr (A/cm2) | Ecorr (V vs. SCE) |
|---|---|---|---|---|
| 316L | 0.36 | −0.28 | 5.11 × 10−6 | −1.24 |
| C1 | 0.42 | −0.32 | 9.96 × 10−7 | −0.93 |
| C2 | 0.46 | −0.39 | 7.42 × 10−7 | −0.72 |
| C3 | 0.53 | −0.45 | 6.18 × 10−7 | −0.31 |
| C4 | 0.83 | −0.67 | 3.82 × 10−7 | −0.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, S.; Lv, X.; Xu, B.; Xu, Y. The Effect of Gradient Bias Design on Electrochemistry and Tribology Behaviors of PVD CrN Film in a Simulative Marine Environment. Materials 2018, 11, 1753. https://doi.org/10.3390/ma11091753
Cen S, Lv X, Xu B, Xu Y. The Effect of Gradient Bias Design on Electrochemistry and Tribology Behaviors of PVD CrN Film in a Simulative Marine Environment. Materials. 2018; 11(9):1753. https://doi.org/10.3390/ma11091753
Chicago/Turabian StyleCen, Shihong, Xiaogai Lv, Beibei Xu, and Ying Xu. 2018. "The Effect of Gradient Bias Design on Electrochemistry and Tribology Behaviors of PVD CrN Film in a Simulative Marine Environment" Materials 11, no. 9: 1753. https://doi.org/10.3390/ma11091753




