Effect of CO2 Partial Pressure on the Corrosion Behavior of J55 Carbon Steel in 30% Crude Oil/Brine Mixture
Abstract
:1. Introduction
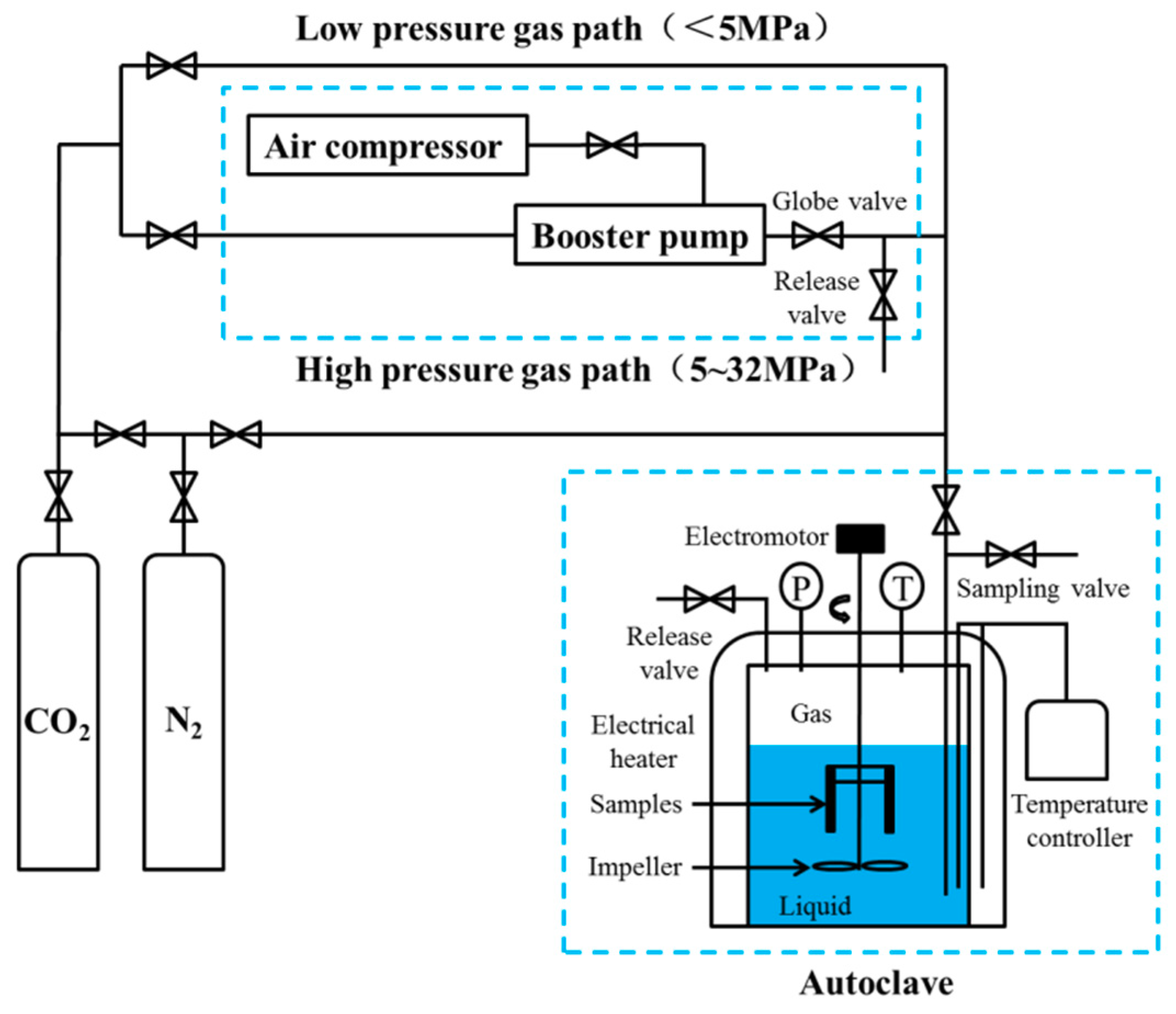
2. Materials and Methods
3. Results
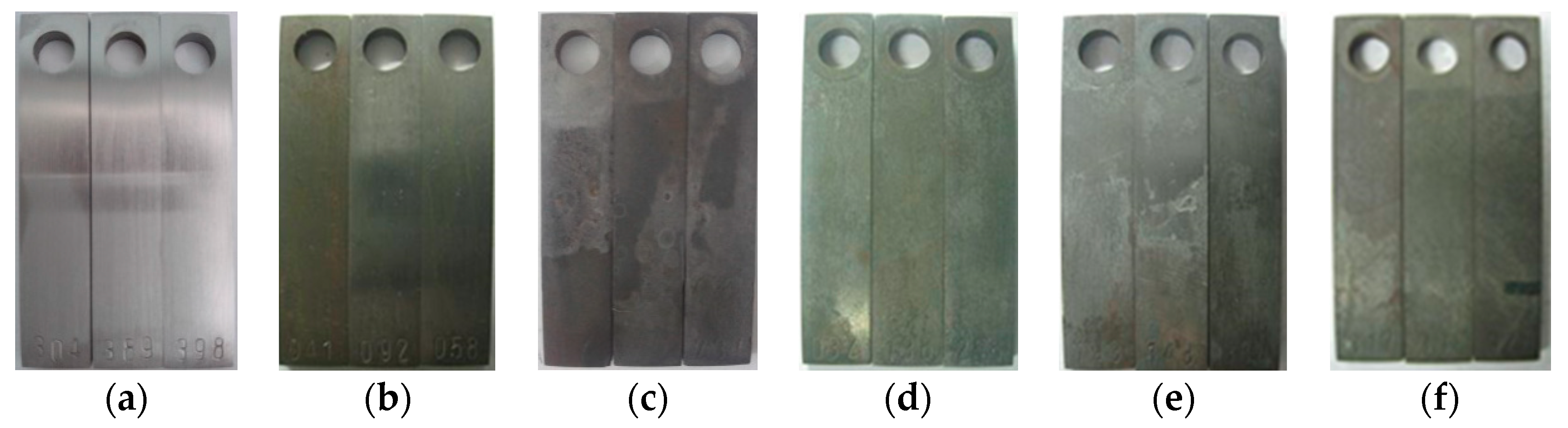
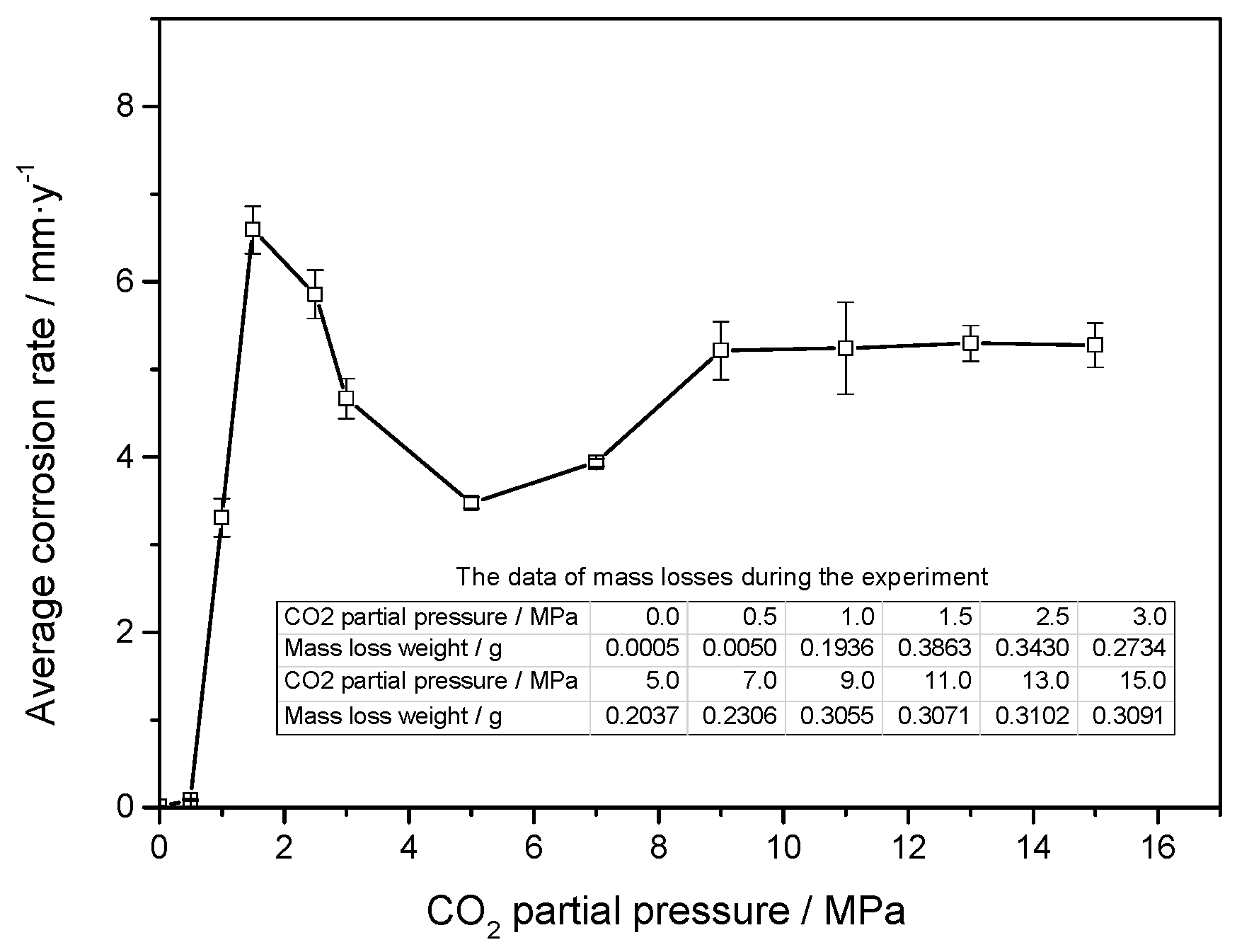
3.1. Weight Loss Tests
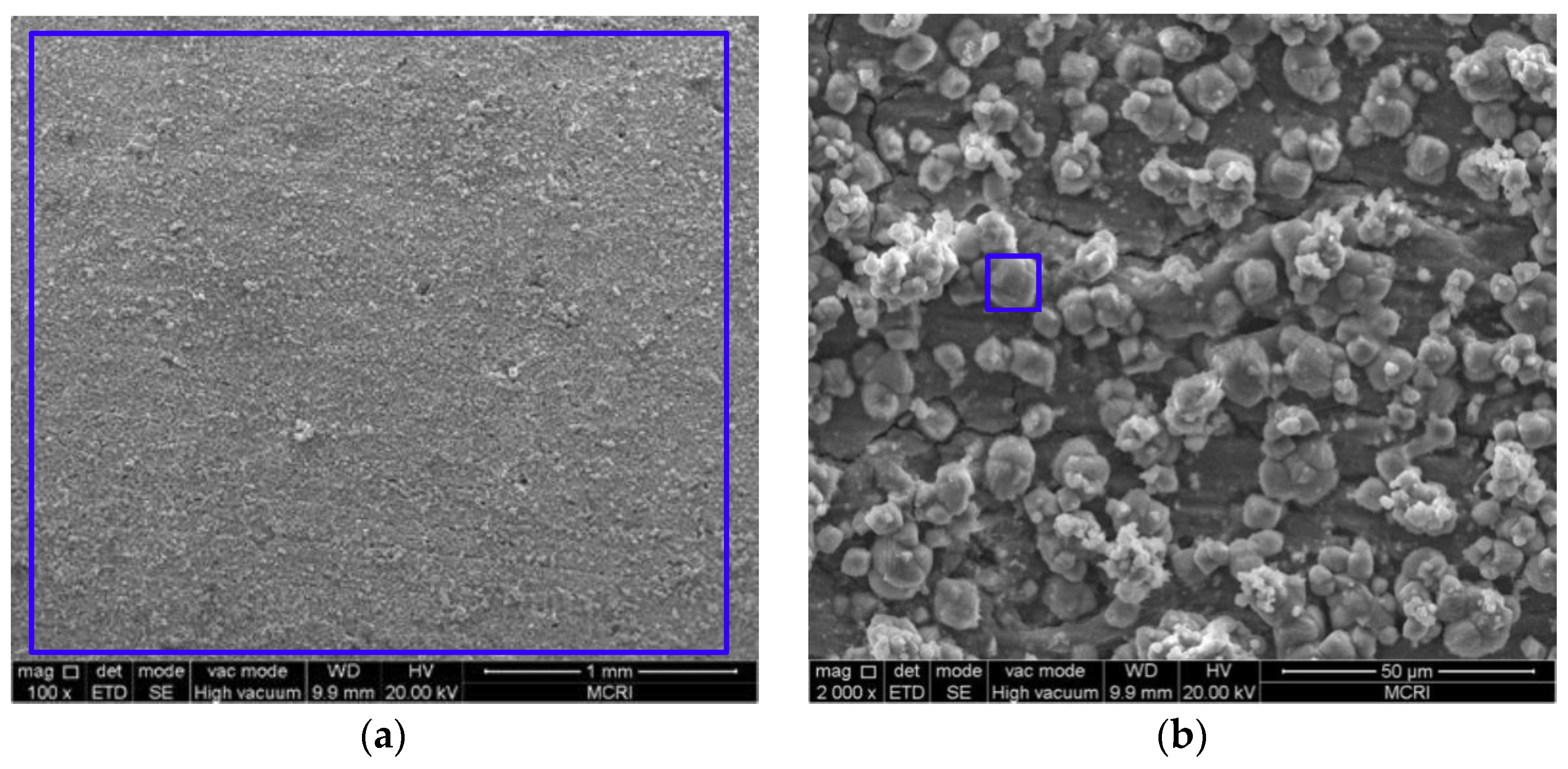
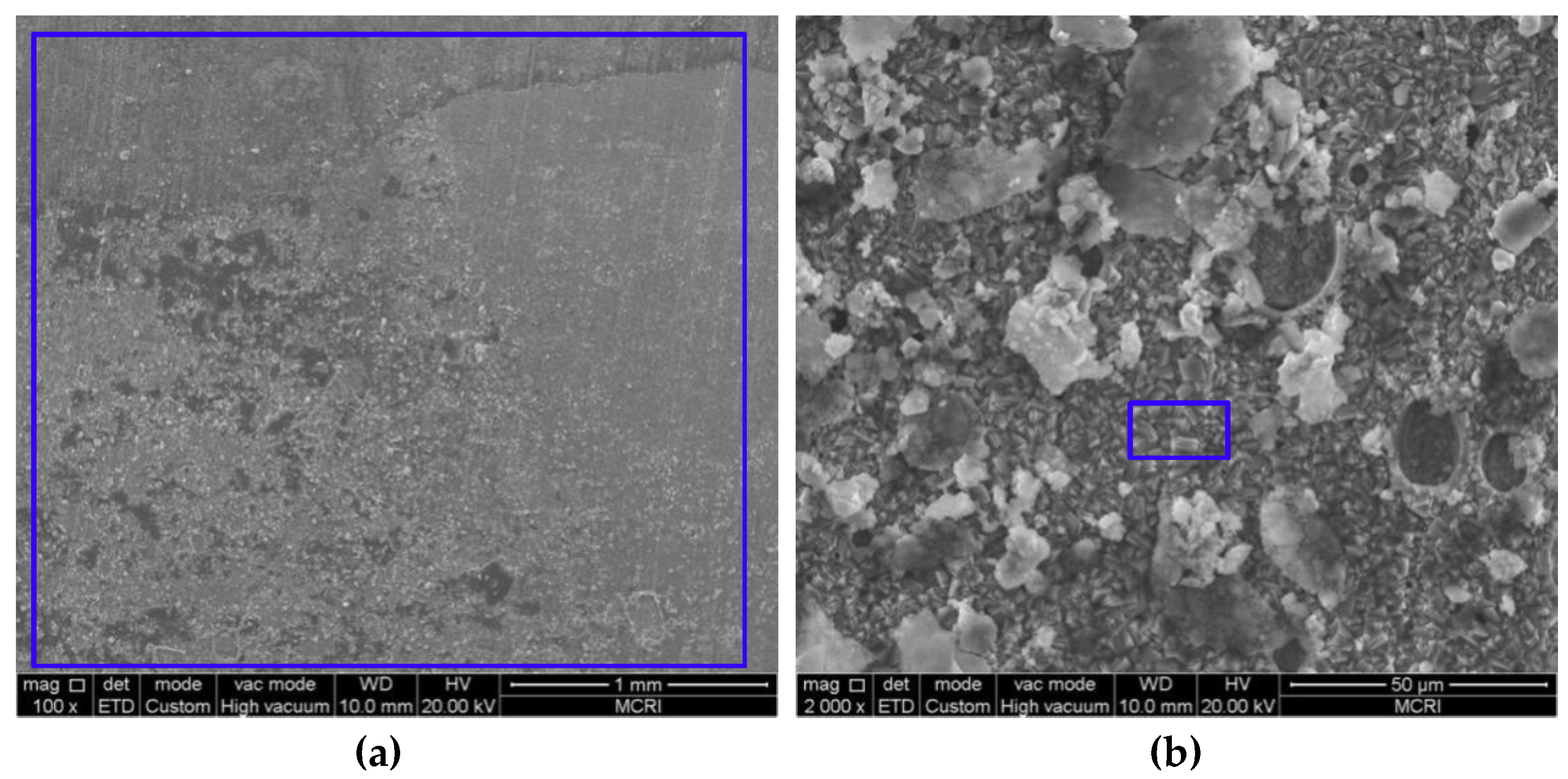
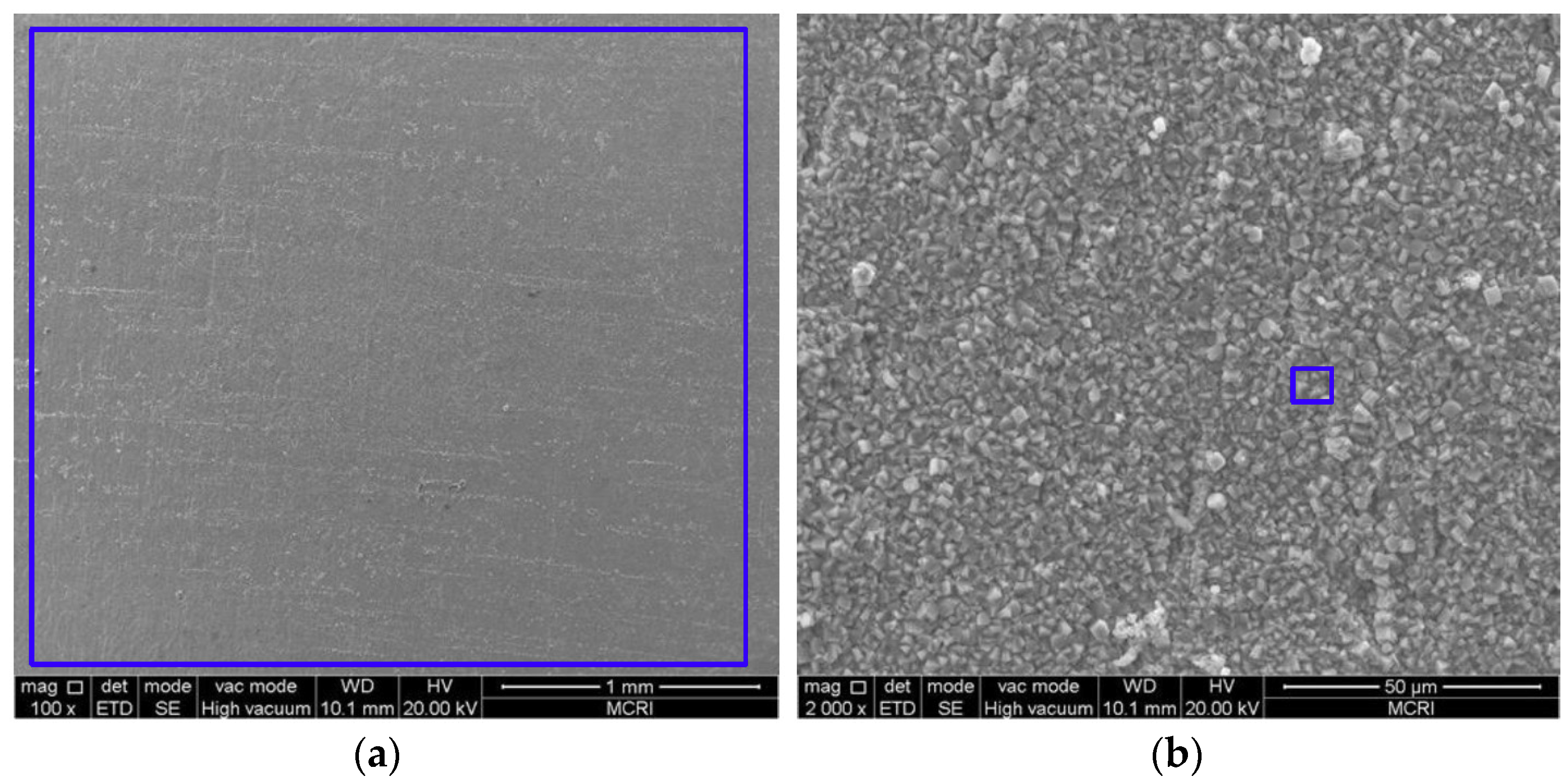
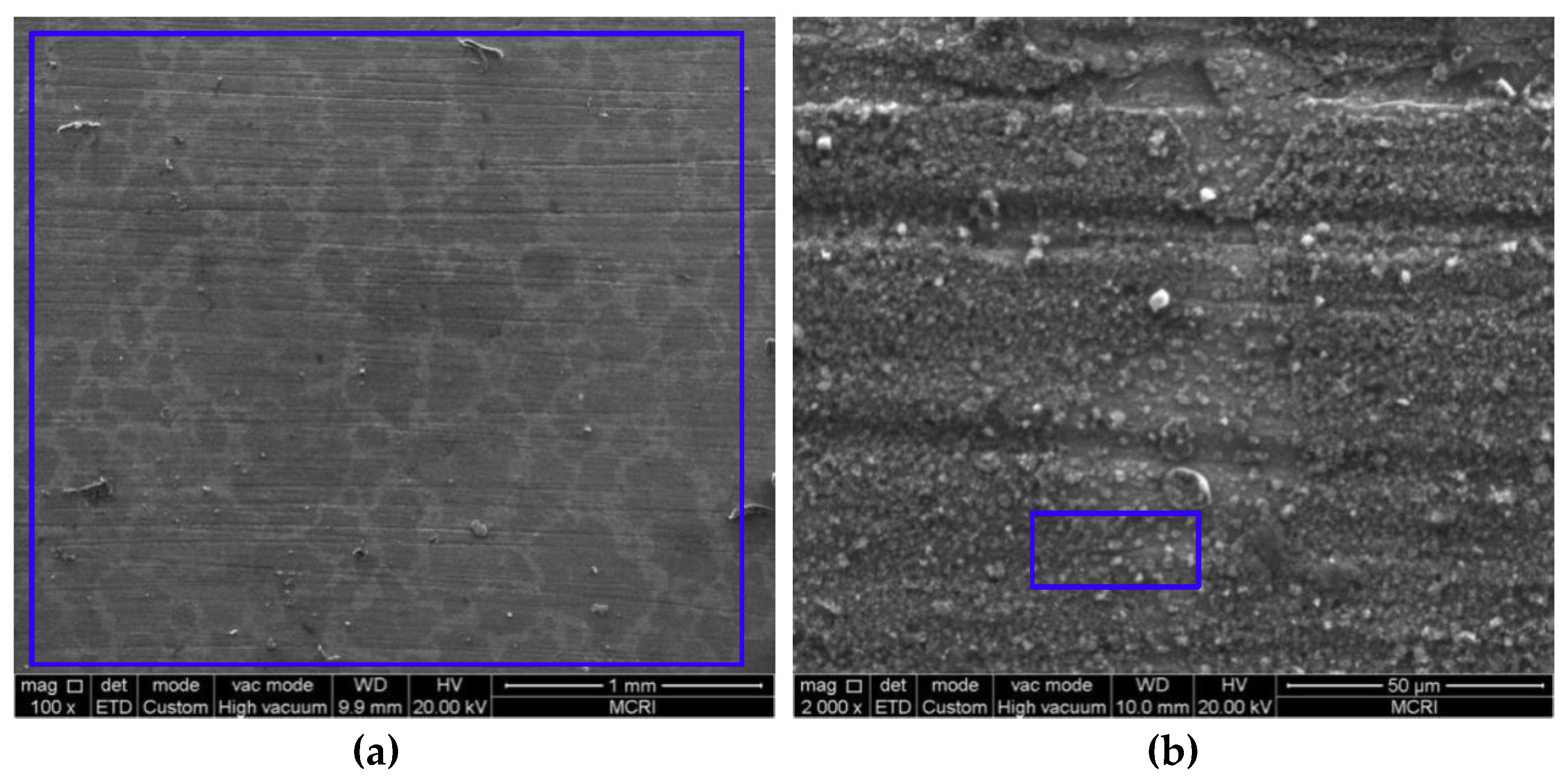
3.2. Microstructure and Composition of the Corrosion Scale
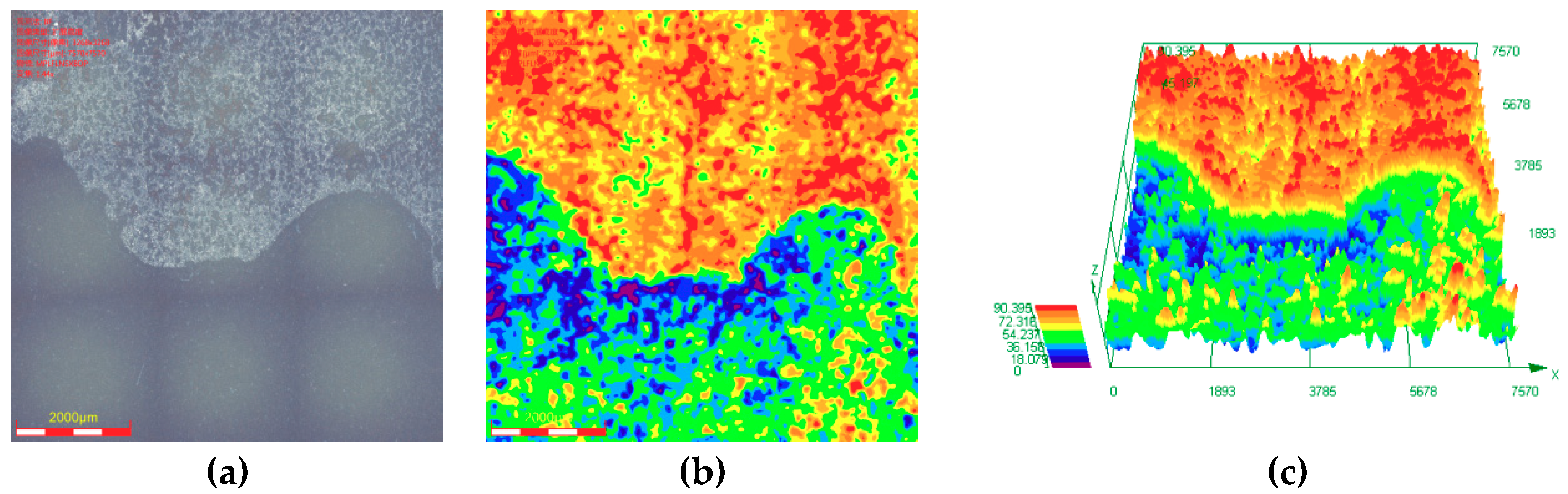
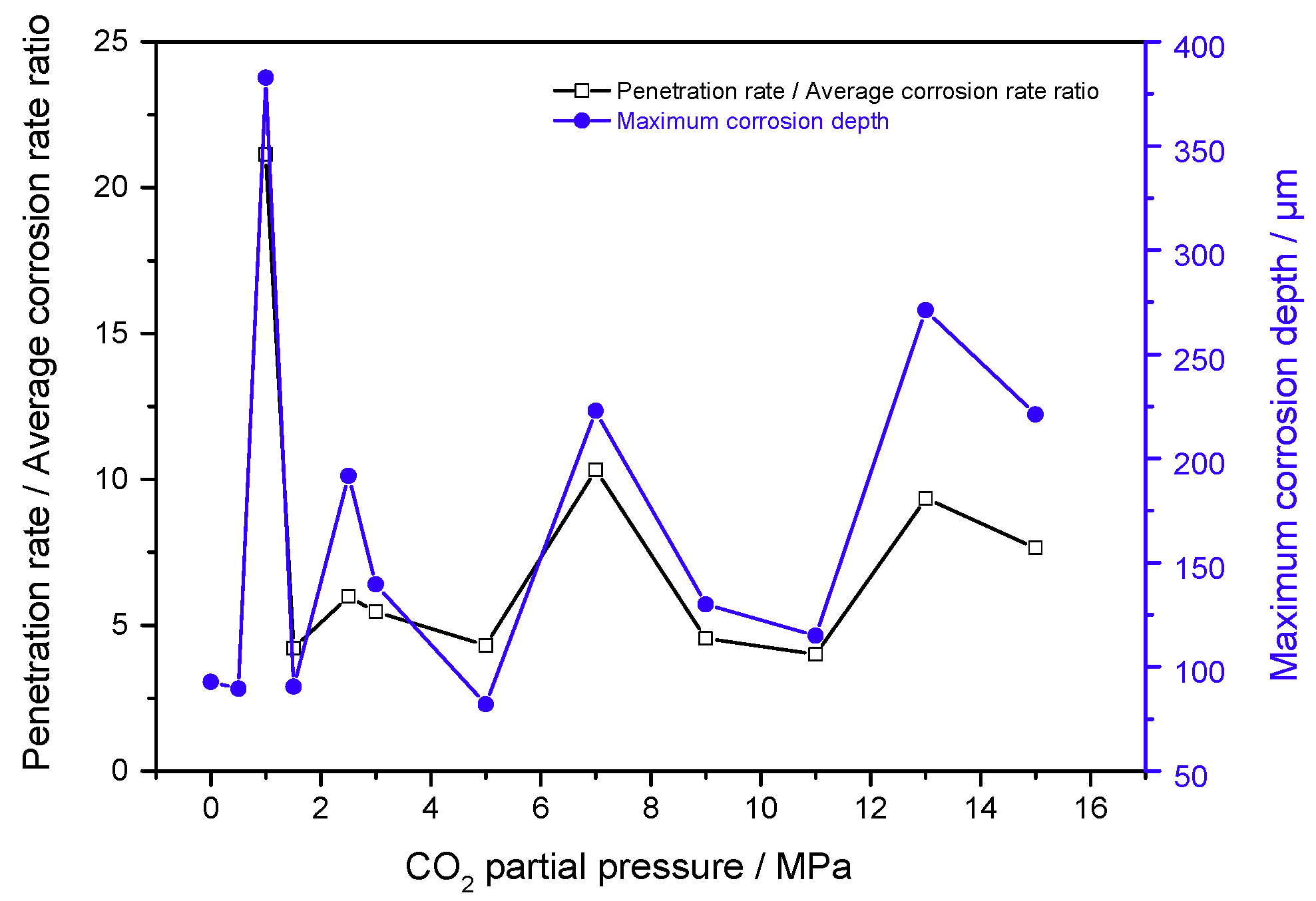
3.3. Maximum Corrosion Depth Tests
4. Discussion
4.1. Variation of pH with CO2 Partial Pressure
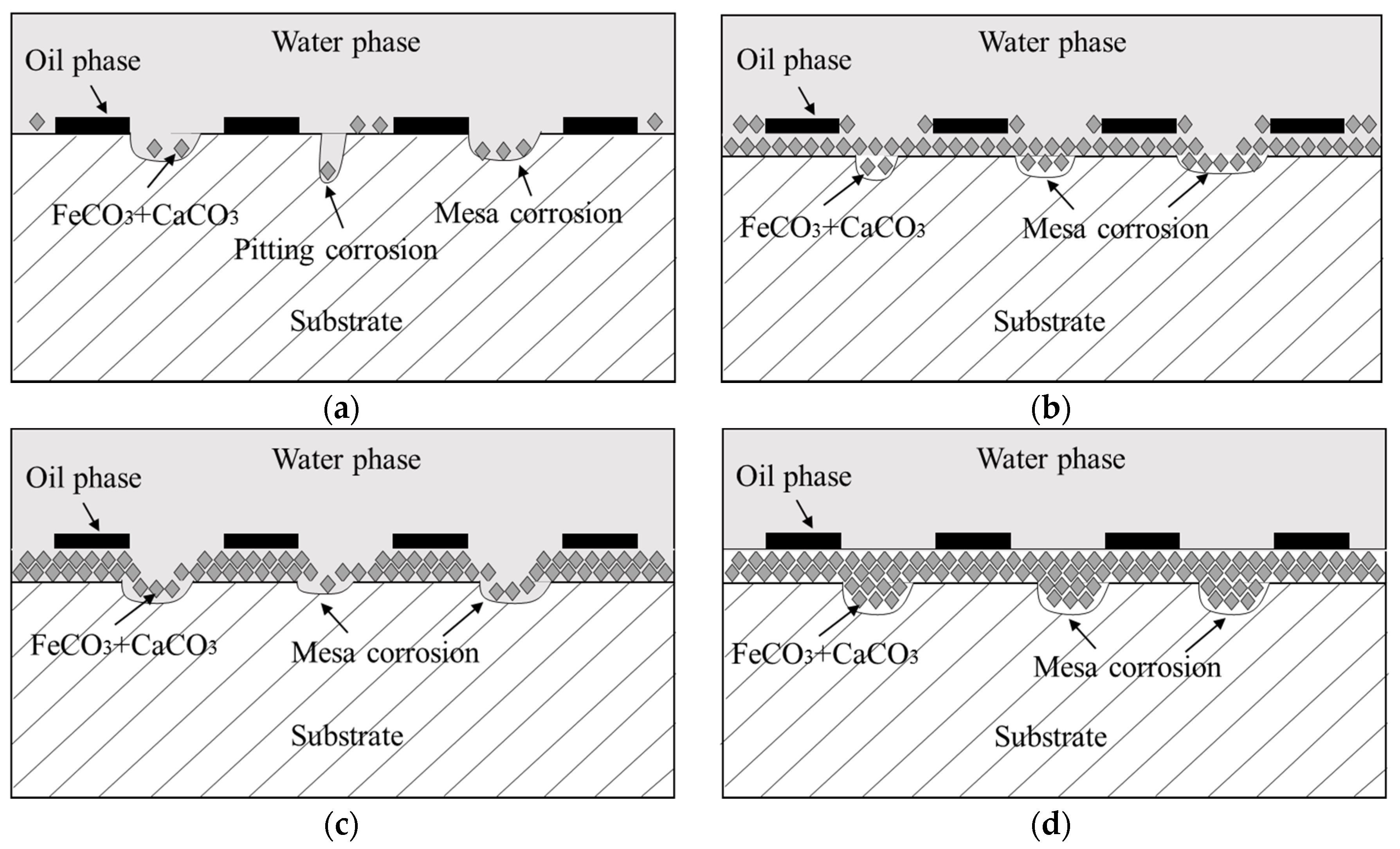
4.2. Formation Mechanism of Localized Corrosion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Uddin, M.; Jafari, A.; Perkins, E. Effects of mechanical dispersion on CO2 storage in Weyburn CO2-EOR field-numerical history match and prediction. Int. J. Greenh. Gas. Control 2013, 16, S35–S49. [Google Scholar] [CrossRef]
- Barnes, D.; Harrison, B.; Grammer, G.M.; Asmus, J. CO2-EOR and geological carbon storage resource potential in the Niagaran pinnacle reef trend, lower Michigan, USA. Energy Procedia 2013, 37, 6786–6799. [Google Scholar] [CrossRef]
- Choi, J.W.; Nicot, J.P.; Hosseini, S.A.; Clift, S.J.; Hovorka, S.D. CO2 recycling accounting and EOR operation scheduling to assist in storage capacity assessment at a U.S. gulf coast depleted reservoir. Int. J. Greenh. Gas. Control 2013, 18, 474–484. [Google Scholar] [CrossRef]
- Wang, Z.J.; Cates, M.E.; Langan, R.T. Seismic monitoring of a CO2 flood in a carbonate reservoir: A rock physics study. Geophysics 1998, 63, 1604–1617. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nesic, S. Effect of impurities on the corrosion behavior of CO2 transmission pipeline steel in supercritical CO2-water environments. Environ. Sci Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Water Quality Standard and Practice for Analysis of Oilfield Injecting Waters in Clastic Reservoirs; SY/T 5329-2012; National Energy Administration: Beijing, China, 2012.
- Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations; NACE RP0775-2005; NACE International: Houston, TX, USA, 2005.
- Farelas, F.; Choi, Y.S.; Nesic, S. Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: Part 2-effect of crude oil and flow. Corrosion 2013, 70, 137–145. [Google Scholar] [CrossRef]
- Sun, J.B.; Sun, C.; Zhang, G.A.; Zhao, W.B.; Wang, Y. Effect of water cut on the localized corrosion behavior of P110 tube steel in supercritical CO2/oil/water environment. Corrosion 2016, 72, 1470–1482. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.L.; Gao, K.W. Effects of crude oil on the corrosion behavior of pipeline steel under wet CO2 conditions. Mater. Perform. 2015, 54, 58–62. [Google Scholar]
- Efird, K.D.; Jasinski, R.J. Effect of the crude oil on corrosion of steel in crude oil/brine production. Corrosion 1989, 2, 165–171. [Google Scholar] [CrossRef]
- Lin, G.; Bai, Z.; Zhao, X. Effect of temperature and pressure on the morphology of carbon dioxide corrosion scales. Corrosion 2006, 62, 501–507. [Google Scholar] [CrossRef]
- Zhang, G.A.; Liu, D.; Li, Y.Z.; Guo, X.P. Corrosion behavior of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci. 2017, 120, 107–120. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Pang, X.L.; Qu, S.P.; Li, X.; Gao, K.W. The relationship between fracture toughness of CO2 corrosion scale and corrosion rate of X65 pipeline steel under supercritical CO2 condition. Int. J. Greenh. Gas. Control 2011, 5, 1643–1650. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Pang, X.L.; Qu, S.P.; Li, X.; Gao, K.W. Discussion of the CO2 corrosion mechanism between low partial pressure and supercritical condition. Corros. Sci. 2012, 59, 186–197. [Google Scholar] [CrossRef]
- Cui, Z.D.; Wu, S.L.; Li, C.F.; Zhu, S.L.; Yang, X.J. Corrosion behavior of oil tube steels under conditions of multiphase flow saturated with super-critical carbon dioxide. Mater. Lett. 2004, 58, 1035–1040. [Google Scholar] [CrossRef]
- Mustafa, A.H.; Angeles, C.B; Ismail, M.C. Inhibition of CO2 corrosion of X52 steel by imidazoline-based inhibitor in high pressure CO2 -water environment. J. Mater. Eng. Perform. 2013, 22, 1748–1755. [Google Scholar] [CrossRef]
- Choi, Y.S.; Farelas, F.; Neši, S.; Magalhães, A.A.O.; Andrade, C.D.A. Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: Part 1-effect of pressure and temperature. Corrosion 2014, 70, 38–47. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nešić, S. Determining the corrosive potential of co transport pipeline in high pco–water environments. Int. J. Greenh. Gas. Control 2011, 5, 788–797. [Google Scholar] [CrossRef]
- Nesic, S. Effects of multiphase flow on internal CO2 corrosion of mild steel pipelines. Energy Fuels 2012, 26, 4098–4111. [Google Scholar] [CrossRef]
- Nesic, S.; Lunde, L. Carbon dioxide corrosion of carbon steel in two-phase flow. Corrosion 1994, 50, 717–727. [Google Scholar] [CrossRef]
- Gozalpour, F.; Ren, S.R.; Tohidi, B. CO2 EOR and storage in oil reservoir. Oil Gas Sci. Technol. 2005, 60, 537–546. [Google Scholar] [CrossRef]
- Hao, H.; Hou, J.; Zhao, F.; Song, Z.; Hou, L.; Wang, Z. Gas channeling control during CO2 immiscible flooding in 3D radial flow model with complex fractures and heterogeneity. J. Pet. Sci. Eng. 2016, 146, 890–901. [Google Scholar] [CrossRef]
- Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM G-03; ASTM International: West Conshohocken, PA, USA, 1999.
- Roosen, C.; Ansorge, S.M.; Mang, T.; Leitner, W.; Greiner, L. Gaining pH-control in water/carbon dioxide biphasic systems. Green Chem. 2007, 9, 455–458. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Choi, Y.S.; Young, D.; Nesic, S. Effect of calcium on the formation and protectiveness of iron carbonate layer in CO2 corrosion. Corrosion 2013, 69, 912–920. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Young, D.; Brown, B.N.; Nesic, S. Effect of incorporation of calcium into Iron carbonate protective layers in CO2 corrosion of mild steel. Corrosion 2017, 73, 238–246. [Google Scholar] [CrossRef]
- Seiersten, M. Material selection for separation, transportation and disposal of CO2 corrosion. In Proceedings of the Corrosion Conference, Houston, TX, USA, 11–16 March 2001. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic chemistry: Chemical equilibria and rates in natural waters. J. Chem. Educ. 1996, 73, A277. [Google Scholar]
- Marshall, W.L.; Franck, E.U. Ion product of water substance, 0–1000 °C, 1–10000 bars new international formulation and its background. J. Phys. Chem. Ref. Data 1981, 10, 295–304. [Google Scholar] [CrossRef]
- Wiebe, R. The binary system carbon dioxide-water under pressure. Chem. Rev. 1941, 29, 475–481. [Google Scholar] [CrossRef]
- Harned, S.H.; Raymond, D.J. The ionization constant of carbonic acid in water and the solubility of carbon dioxide in water and aqueous salt solutions from 0 to 50°. J. Am. Chem. Soc. 1943, 65, 2030–2037. [Google Scholar] [CrossRef]
- Ziegler, K.J.; Hanrahan, J.P.; Glennon, J.D.; Holmes, J.D. Producing ‘pH switches’ in biphasic water-CO2 systems. J. Supercrit. Fluids 2003, 27, 109–117. [Google Scholar] [CrossRef]
- Farelas, F.; Galicia, M.; Brown, B.; Nesic, S.; Castaneda, H. Evolution of dissolution processes at the interface of carbon steel corroding in a CO2 environment studied by EIS. Corros. Sci. 2010, 52, 509–517. [Google Scholar] [CrossRef]
- Liu, Z.M.; Yang, G.Y.; Lu, Y.; Han, B.X.; Yan, H.K. Phase equilibria of the CO2—Jiangsu crude oil system and precipitation of heavy components induced by supercritical CO2. J. Supercrit. Fluids 1999, 16, 27–31. [Google Scholar] [CrossRef]
- Jalal, F.; Mahmoud, J. The physics of CO2 transfer during carbonated water injection into oil reservoirs: from non-equilibrium core-scale physics to field-scale implication. J. Petrol. Sci. Eng. 2018, 166, 798–805. [Google Scholar]





| Property | Unit | Value |
|---|---|---|
| Kinematic viscosity (65 °C) | mm2·s−1 | 7.254 |
| Acid value | mg KOH·g−1 | 0.107 |
| Sulfur content | wt.% | 0.08 |
| Wax content | wt.% | 12.86 |
| Colloid | wt.% | 2.31 |
| Asphaltene | wt.% | 0.60 |
| Property | Unit | Value |
|---|---|---|
| NaCl | g·L−1 | 18.5028 |
| CaCl2 | g·L−1 | 13.7338 |
| MgCl2 | g·L−1 | 0.5897 |
| Na2SO4 | g·L−1 | 0.2440 |
| NaHCO3 | g·L−1 | 0.0631 |
| salinity | g·L−1 | 33.0000 |
| Element (At. %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole | Local | Whole | Local | Whole | Local | Whole | Local | Whole | Local | |
| C K | 75.70 | 76.23 | 37.34 | 33.40 | 50.29 | 33.25 | 27.87 | 18.66 | 63.00 | 62.49 |
| O K | 0.34 | 1.50 | 44.87 | 55.80 | 36.73 | 45.10 | 46.48 | 53.98 | 23.68 | 25.52 |
| Si K | / | / | / | / | 0.21 | / | / | / | / | / |
| Cr K | / | / | 0.29 | / | / | / | / | / | / | / |
| Ca K | / | / | 3.49 | 3.17 | / | 0.20 | 1.84 | 2.29 | 0.44 | 0.59 |
| Cl K | 0.14 | / | 0.37 | 0.16 | 0.30 | 0.37 | / | / | / | / |
| Mn K | 0.40 | 0.30 | 0.54 | 0.35 | 0.14 | 0.28 | / | / | / | / |
| Fe K | 23.42 | 21.97 | 13.10 | 7.12 | 12.33 | 20.80 | 23.81 | 25.07 | 12.88 | 11.40 |
| total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Wang, Y.; Ma, Y.; Zhang, Q.; Zhang, N. Effect of CO2 Partial Pressure on the Corrosion Behavior of J55 Carbon Steel in 30% Crude Oil/Brine Mixture. Materials 2018, 11, 1765. https://doi.org/10.3390/ma11091765
Bai H, Wang Y, Ma Y, Zhang Q, Zhang N. Effect of CO2 Partial Pressure on the Corrosion Behavior of J55 Carbon Steel in 30% Crude Oil/Brine Mixture. Materials. 2018; 11(9):1765. https://doi.org/10.3390/ma11091765
Chicago/Turabian StyleBai, Haitao, Yongqing Wang, Yun Ma, Qingbo Zhang, and Ningsheng Zhang. 2018. "Effect of CO2 Partial Pressure on the Corrosion Behavior of J55 Carbon Steel in 30% Crude Oil/Brine Mixture" Materials 11, no. 9: 1765. https://doi.org/10.3390/ma11091765




