Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
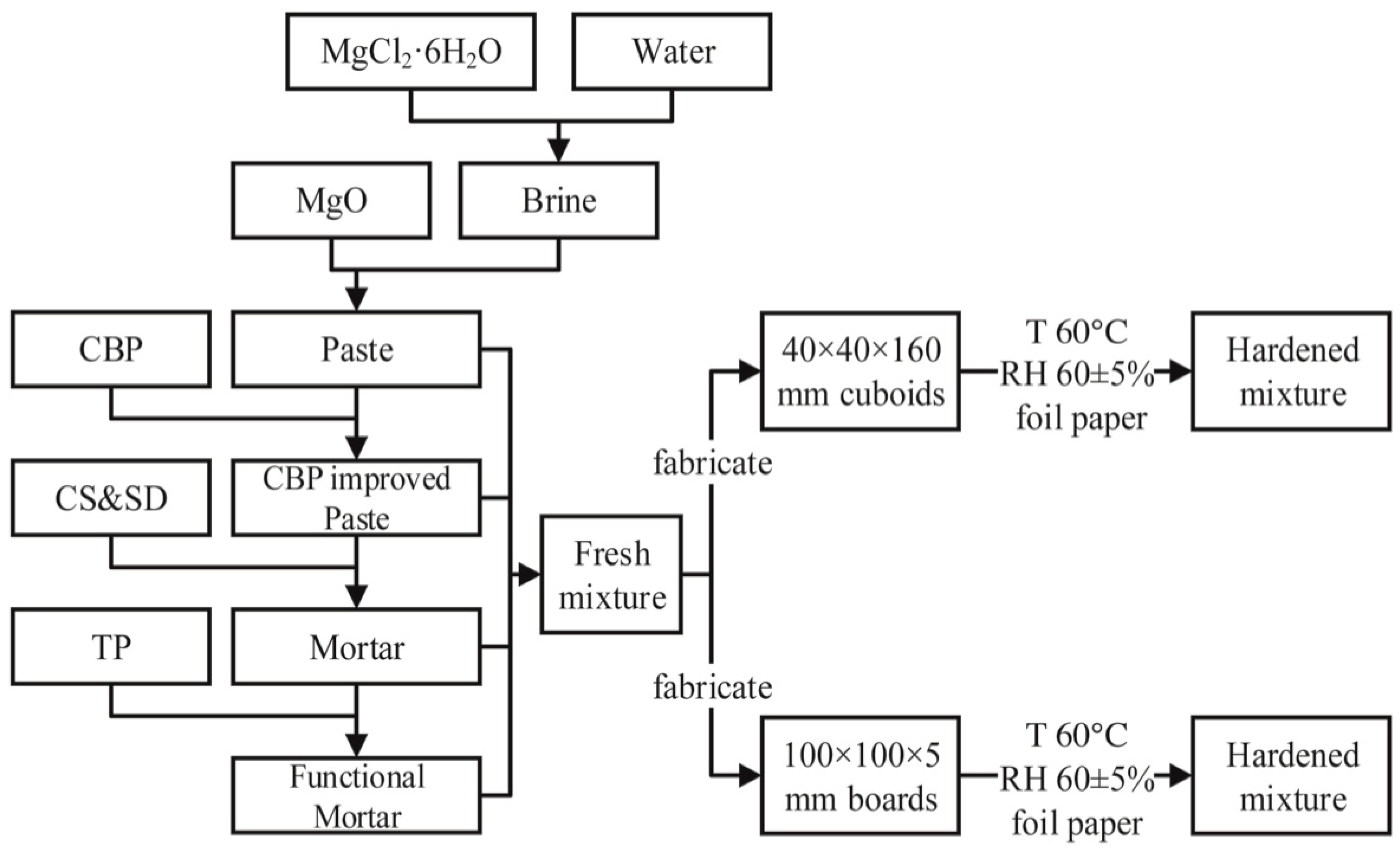
2.2. Sample Preparation
2.3. Test Methods
2.3.1. Fluidity
2.3.2. Specific Gravity
2.3.3. Water Absorption
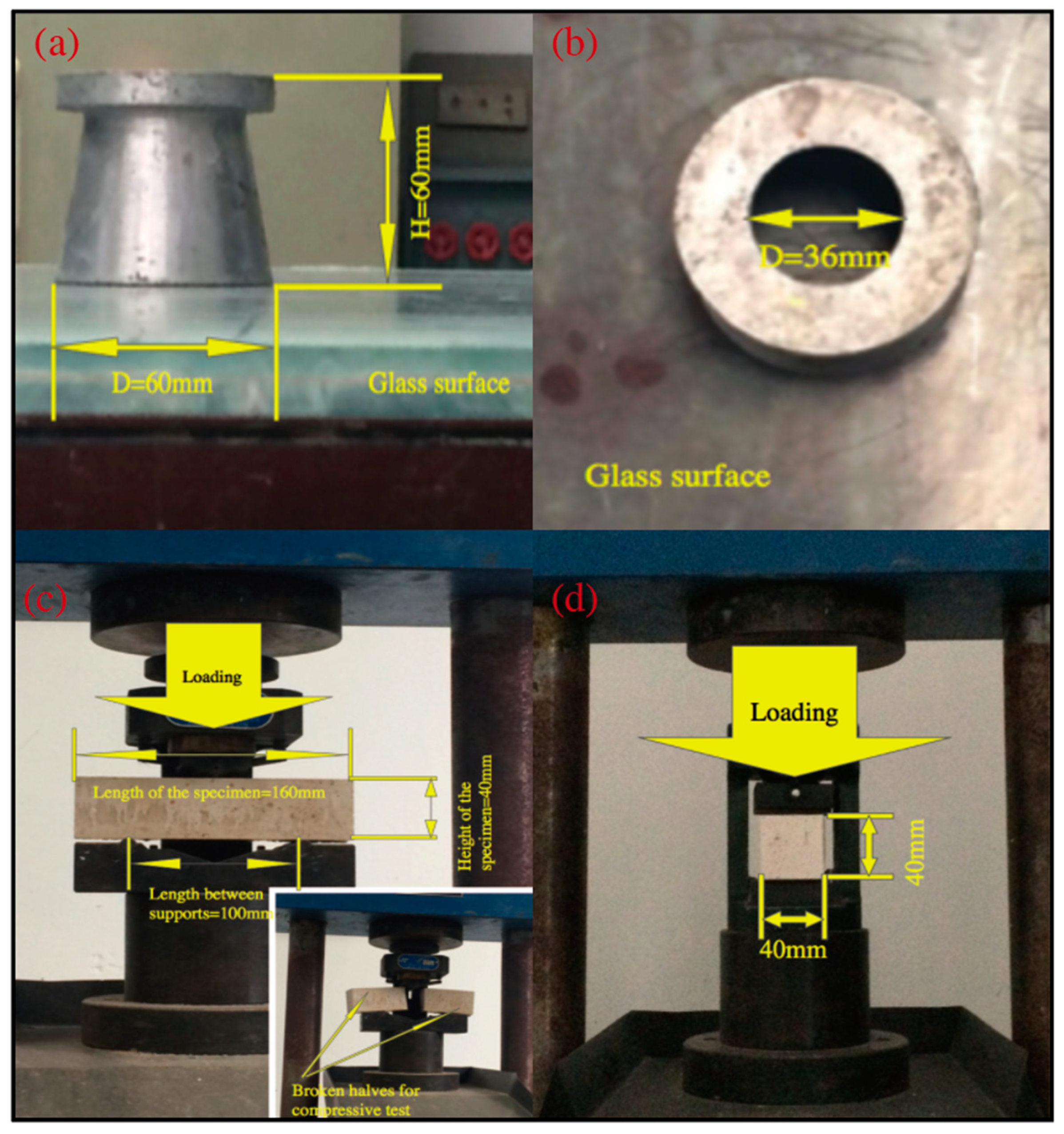
2.3.4. Flexural strength
2.3.5. Compressive Strength
2.3.6. Water Resistance
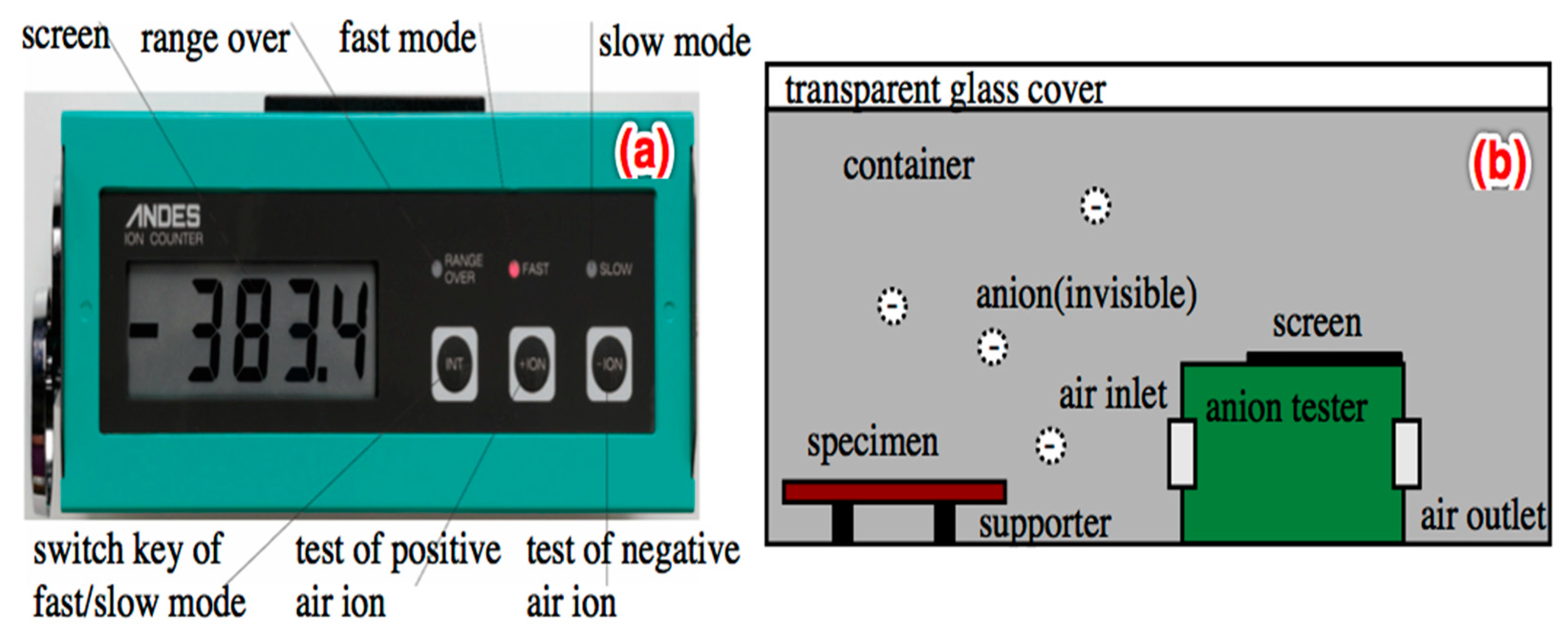
2.3.7. Negative Induction
3. Results and Discussion
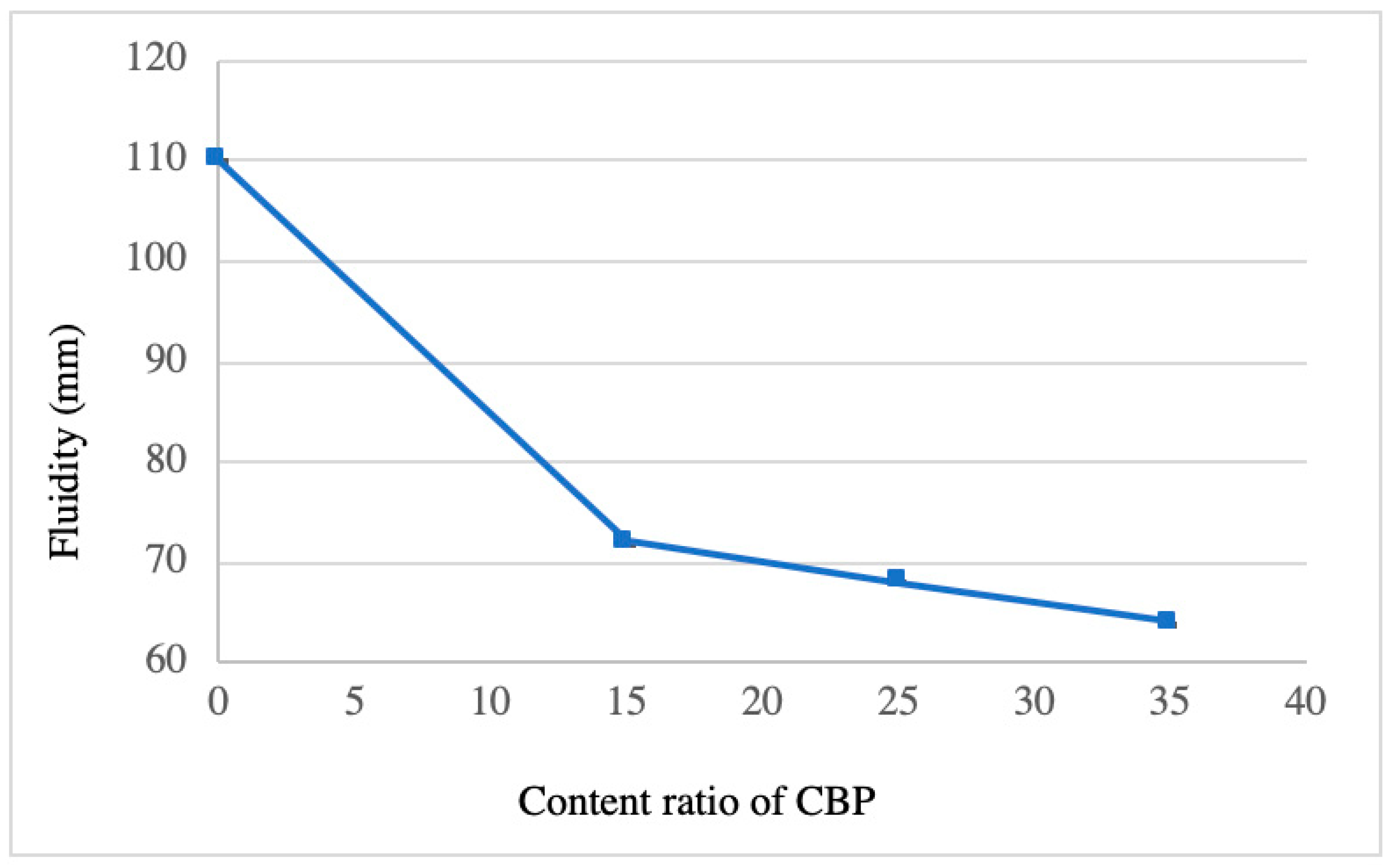
3.1. Fluidity
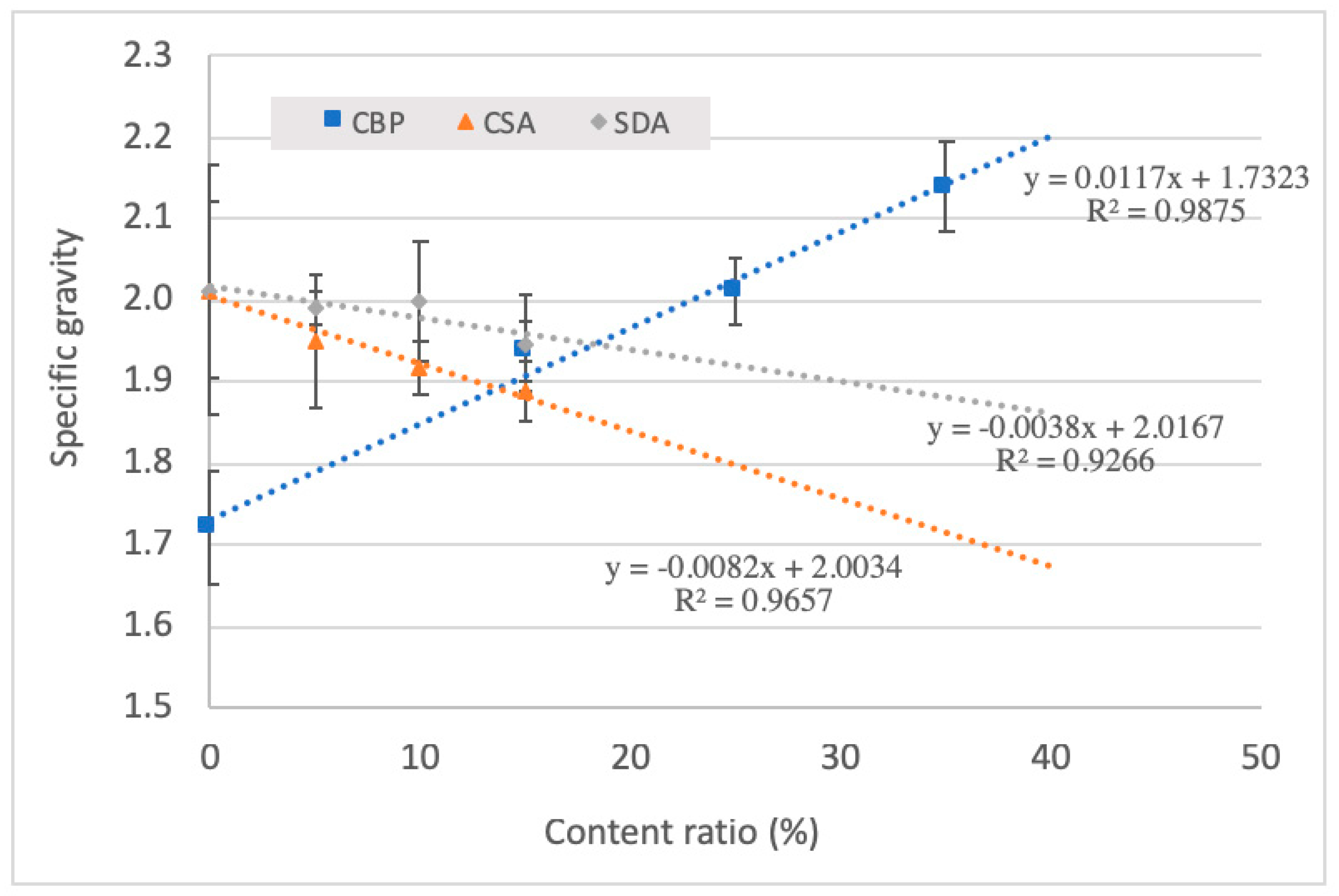
3.2. Specific Gravity
3.3. Water Absorption and Flexural Strength
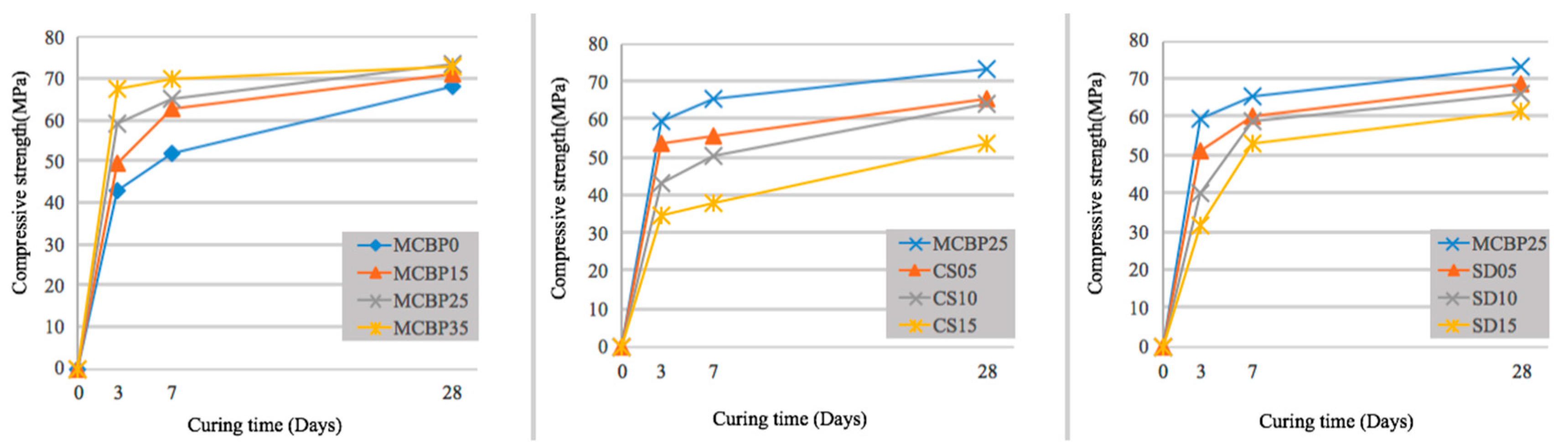
3.4. Compressive Strength
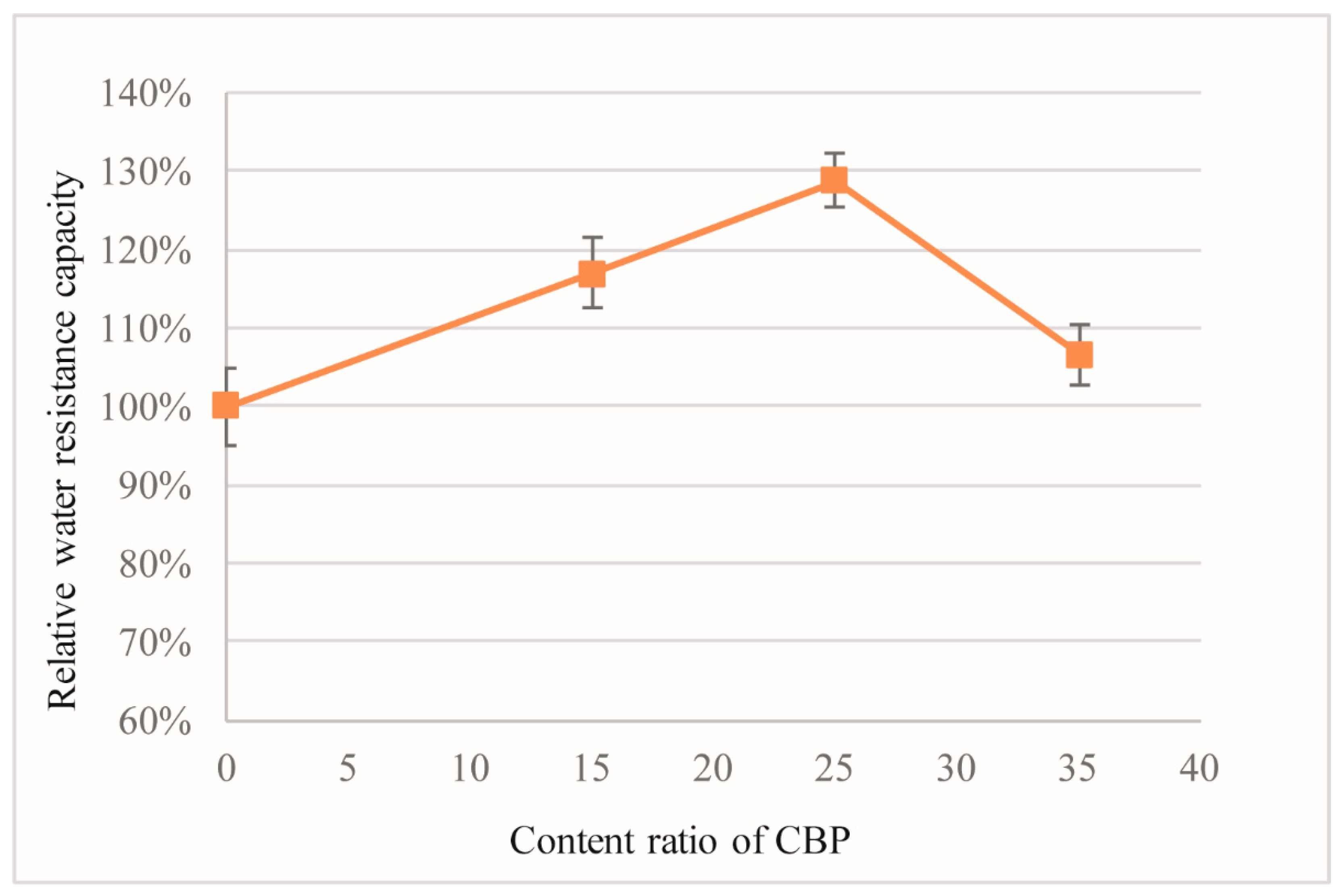
3.5. Water Resistance
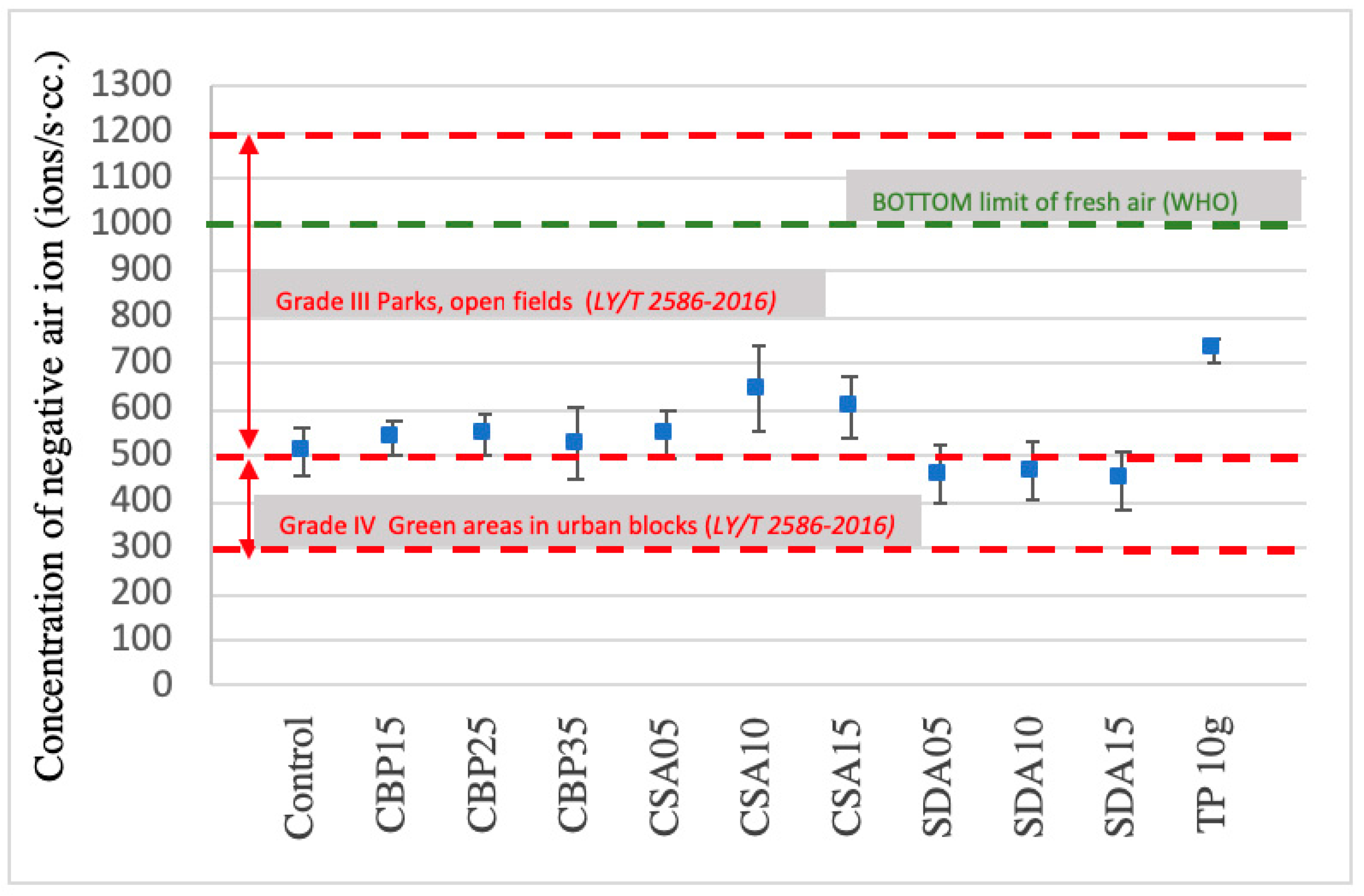
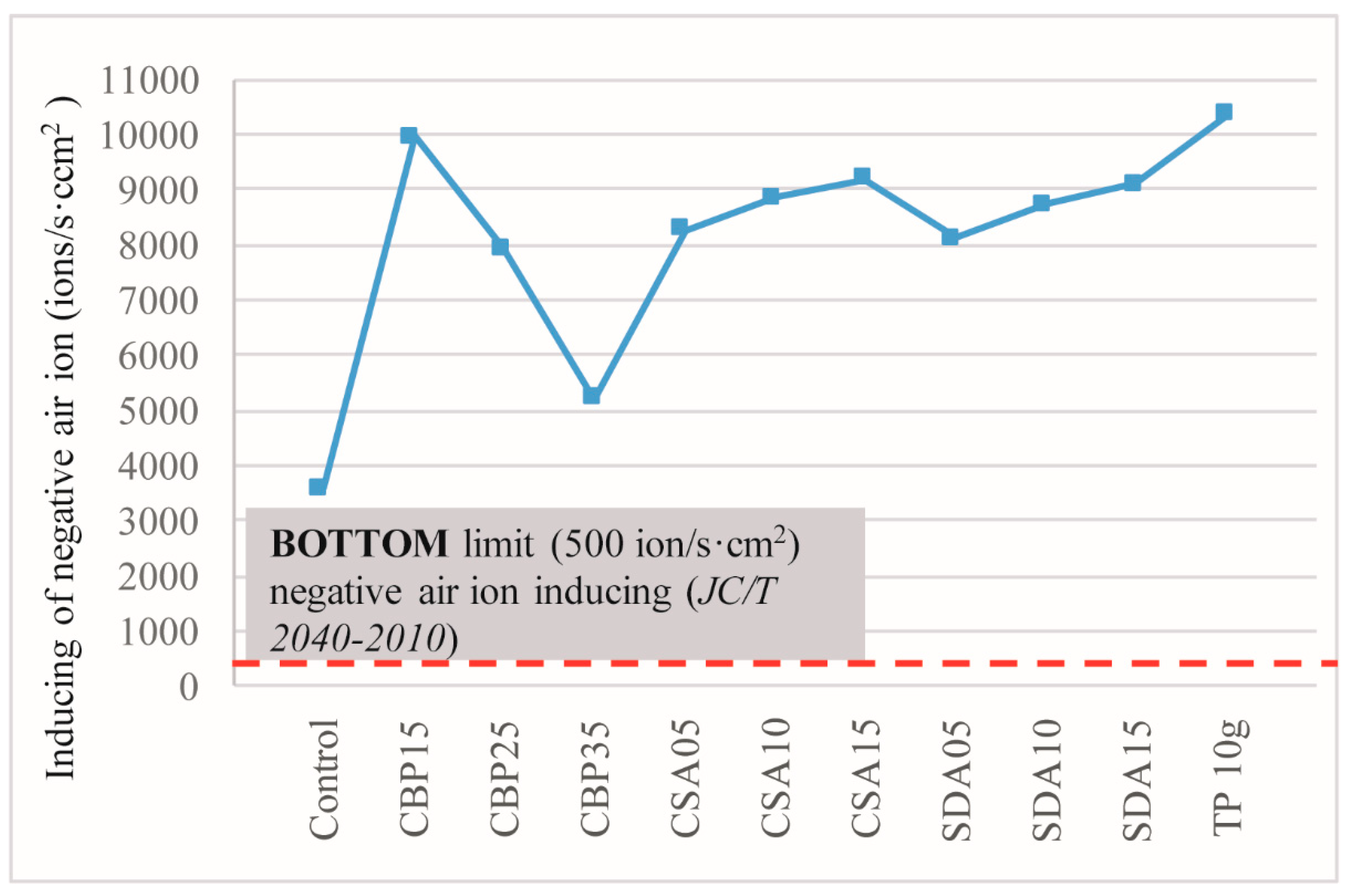
3.6. Negative Ion Induction
4. Conclusions
- (1)
- The incorporation of CS and SD in MOC is beneficial to the production of lightweight MOC board, and is also conducive to negative ion induction, because of the incompact structures, in which more voids are available to accommodate more air released by TP.
- (2)
- The incorporation of CBP in MOC improves the water resistance, but reduces the negative ion-inducing ability, due to the denser structure induced by the pore-filling effect and the pozzolanic reaction.
- (3)
- The TP-containing MOC board is able to induce negative ions, thereby improving the air quality of the surrounding environments to a level that is similar to the open fields.
Author Contributions
Funding
Conflicts of Interest
References
- Siddique, R.; Naik, T.R. Properties of concrete containing scrap-tire rubber—An overview. Waste Manag. 2004, 24, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawwad, H.A.; Khalil, K.A. Preparation and characterization of one-part magnesium oxychloride cement. Constr. Build. Mater. 2018, 189, 745–750. [Google Scholar] [CrossRef]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Chau, C.K.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–9465. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–618. [Google Scholar] [CrossRef]
- Qiao, H.; Cheng, Q.; Jinlei, W.; Yingying, S. The application review of magnesium oxychloride cement. J. Chem. Pharm. Res. 2014, 6, 180–185. [Google Scholar]
- He, P.; Poon, C.S.; Tsang, D.C.W. Effect of pulverized fuel ash and CO2 curing on the water resistance of magnesium oxychloride cement (MOC). Cem. Concr. Res. 2017, 97, 115–122. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C.W. Using incinerated sewage sludge ash to improve the water resistance of magnesium oxychloride cement (MOC). Constr. Build. Mater. 2017, 147, 519–524. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C.W. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]
- Cheng, H. Reuse Research Progress on Waste Clay Brick. Procedia Environ. Sci. 2016, 31, 218–296. [Google Scholar] [CrossRef]
- Heikal, M.; Zohdy, K.M.; Abdelkreem, M. Mechanical, microstructure and rheological characteristics of high performance self-compacting cement pastes and concrete containing ground clay bricks. Constr. Build. Mater. 2013, 38, 101–109. [Google Scholar] [CrossRef]
- Wu, H.; Duan, H.; Zheng, L.; Wang, J.; Niu, Y.; Zhang, G. Demolition waste generation and recycling potentials in a rapidly developing flagship megacity of South China: Prospective scenarios and implications. Constr. Build. Mater. 2016, 113, 618–1007. [Google Scholar] [CrossRef]
- Mao, R.; Duan, H.; Dong, D.; Zuo, J.; Song, Q.; Liu, G.; Hu, M.; Zhu, J.; Dong, B. Quantification of carbon footprint of urban roads via life cycle assessment: Case study of a megacity-Shenzhen, China. J. Clean. Prod. 2017, 166, 40–48. [Google Scholar] [CrossRef]
- Chen, X.-F.; Lin, S.-R.; Kou, S.-C. Effect of composite photo-catalysts prepared with recycled clay brick sands and nano-TiO2 on methy orange and NOx removal. Constr. Build. Mater. 2018, 171, 152–160. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, Z.; Wang, M.; Jia, W. Effects of corn stalk fiber content on properties of biomass brick. Constr. Build. Mater. 2016, 127, 11–17. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B.; Oderji, S.Y.; Mohsan, M. Development of a new bio-composite for building insulation and structural purpose using corn stalk and magnesium phosphate cement. Energy Build. 2018, 173, 719–733. [Google Scholar] [CrossRef]
- Binici, H.; Aksogan, O.; Demirhan, C. Mechanical, thermal and acoustical characterizations of an insulation composite made of bio-based materials. Sustain. Cities Soc. 2016, 20, 17–26. [Google Scholar] [CrossRef]
- Jarabo, R.; Monte, M.C.; Fuente, E.; Santos, S.F.; Negro, C. Corn stalk from agricultural residue used as reinforcement fiber in fiber-cement production. Ind. Crops Prod. 2013, 43, 832–839. [Google Scholar] [CrossRef]
- Jarabo, R.; Monte, M.C.; Blanco, A.; Negro, C.; Tijero, J. Characterisation of agricultural residues used as a source of fibres for fibre-cement production. Ind. Crops Prod. 2012, 36, 14–21. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Banthia, N. Plant-based natural fibre reinforced cement composites: A review. Cem. Concr. Compos. 2016, 68, 96–108. [Google Scholar] [CrossRef]
- Li, G.; Yu, Y.; Li, J.; Li, C.; Wang, Y. Research on adaptability between crop-stalk fibers and cement. Cem. Concr. Res. 2004, 34, 1081–1085. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Chen, K.; Gai, X.; Zhao, C.; Liao, L.; Shen, K.; Fan, Z.; Shan, Y. The origin of pyroelectricity in tourmaline at varying temperature. J. Alloys Compd. 2018, 744, 328–336. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, Y.; Huang, G.; Lai, A.C.K. Numerical and experimental study on airborne disinfection by negative ions in air duct flow. Build. Environ. 2018, 127, 204–210. [Google Scholar] [CrossRef]
- GB/T2419-2005. Test Method for Fluidity of Cement Mortar; Ministry of Construction: Beijing, China, 2005.
- GB/T17671-1999. Method of Testing Cements-Determination of Strength; The Quality and Techology Supervision Bureau: Beijing, China, 1999.
- Hou, X.; Sun, F.; Yan, D.; Xu, H.; Dong, Z.; Li, Q.; Yang, Y. Preparation of lightweight polypropylene composites reinforced by cotton stalk fibers from combined steam flash-explosion and alkaline treatment. J. Clean. Prod. 2014, 83, 454–462. [Google Scholar] [CrossRef]
- da Rosa, L.C.; Santor, C.G.; Lovato, A.; da Rosa, C.S.; Güths, S. Use of rice husk and sunflower stalk as a substitute for glass wool in thermal insulation of solar collector. J. Clean. Prod. 2015, 104, 90–97. [Google Scholar] [CrossRef]
- Pinto, J.; Vieira, B.; Pereira, H.; Jacinto, C.; Vilela, P.; Paiva, A.; Pereira, S.; Cunha, V.M.C.F.; Varum, H. Corn cob lightweight concrete for non-structural applications. Constr. Build. Mater. 2012, 34, 346–351. [Google Scholar] [CrossRef]
- Ortega, J.M.; Letelier, V.; Solas, C.; Moriconi, G.; Climent, M.Á.; Sánchez, I. Long-term effects of waste brick powder addition in the microstructure and service properties of mortars. Constr. Build. Mater. 2018, 182, 691–702. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.T. A review on dynamic mechanical properties of natural fibre reinforced polymer composites. Constr. Build. Mater. 2016, 106, 149–159. [Google Scholar] [CrossRef]
- Farinha, C.B.; de Brito, J.; Veiga, R. Assessment of glass fibre reinforced polymer waste reuse as filler in mortars. J. Clean. Prod. 2018. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, S.; Wang, H.; Chen, R. Effect of 5-phase seed crystal on the mechanical properties and microstructure of magnesium oxychloride cement. Constr. Build. Mater. 2017, 150, 409–417. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Cao, G.; Zhan, X.; Qiang, T.; Li, J. Visible-light activate Ag/WO3 films based on wood with enhanced negative oxygen ions production properties. Appl. Surf. Sci. 2017, 425, 889–895. [Google Scholar] [CrossRef]



| Materials | Cellulose | Hemicellulose | Lignin | Extractives | Ash | Tensile Strength | Young Modulus | Elongation at Break | Water Absorption | Bulk Density |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | MPa | GPa | (%) | (%) | (kg/m3) | |
| CS | 45.85 | 21.3 | 24.2 | 4 | 2.7 | 345 | 27.6 | 2.7 | 12 | 152.7 |
| SD | 37.57 | 28.56 | 23.4 | 8.2 | 1.2 | 248 | 3.2 | 25 | 8 | 195.2 |
| Materials | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | Loss on Ignition (LoI) | Water Absorption | Bulk Density |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (kg/m3) | |
| CBP | 53.3 | 7.4 | 16.6 | 6.7 | 2.5 | 1.1 | 6.2 | 19 | 1801 |
| TP | 40.5 | 20.42 | 34.12 | 0.39 | 0.59 | - | 3.98 | ~0 | 2500 |
| Mix. Notation | MgO | MgCl2·6H2O a | Water a | CBP a | CS a | SD a | TP a |
|---|---|---|---|---|---|---|---|
| M000000 (M0) | 1 | 0.4 | 0.3 | - | - | - | 0.10 |
| M150000 (CBP15) | 1 | 0.4 | 0.3 | 0.15 | - | - | 0.10 |
| M250000 (CBP25) | 1 | 0.4 | 0.3 | 0.25 | - | - | 0.10 |
| M350000 (CBP35) | 1 | 0.4 | 0.3 | 0.35 | - | - | 0.10 |
| M250500 (CS5) | 1 | 0.4 | 0.3 | 0.25 | 0.05 | - | 0.10 |
| M251000 (CS10) | 1 | 0.4 | 0.3 | 0.25 | 0.10 | - | 0.10 |
| M251500 (CS15) | 1 | 0.4 | 0.3 | 0.25 | 0.15 | - | 0.10 |
| M250005 (SD5) | 1 | 0.4 | 0.3 | 0.25 | - | 0.05 | 0.10 |
| M250010 (SD10) | 1 | 0.4 | 0.3 | 0.25 | - | 0.10 | 0.10 |
| M250015 (SD15) | 1 | 0.4 | 0.3 | 0.25 | - | 0.15 | 0.10 |
| Gradation | Concentration of Air Negative Ions | Reference Places | Air Freshness |
|---|---|---|---|
| I | n ≥ 3000 | forests, wetland | Best |
| II | 1200 ≤ n < 3000 | summits of mountains |  |
| III | 500 ≤ n < 1200 | parks, open fields | |
| IV | 300 ≤ n < 500 | green areas in urban blocks | |
| V | 100 ≤ n < 300 | common indoor areas | |
| VI | n < 100 | industrial plants | Worst |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-F.; Kou, S.-C.; Xing, F. Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder. Materials 2019, 12, 115. https://doi.org/10.3390/ma12010115
Chen X-F, Kou S-C, Xing F. Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder. Materials. 2019; 12(1):115. https://doi.org/10.3390/ma12010115
Chicago/Turabian StyleChen, Xue-Fei, Shi-Cong Kou, and Feng Xing. 2019. "Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder" Materials 12, no. 1: 115. https://doi.org/10.3390/ma12010115
APA StyleChen, X.-F., Kou, S.-C., & Xing, F. (2019). Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder. Materials, 12(1), 115. https://doi.org/10.3390/ma12010115






