Efficiency and Compatibility of Selected Alkoxysilanes on Porous Carbonate and Silicate Stones
Abstract
:1. Introduction
2. Materials and Methods
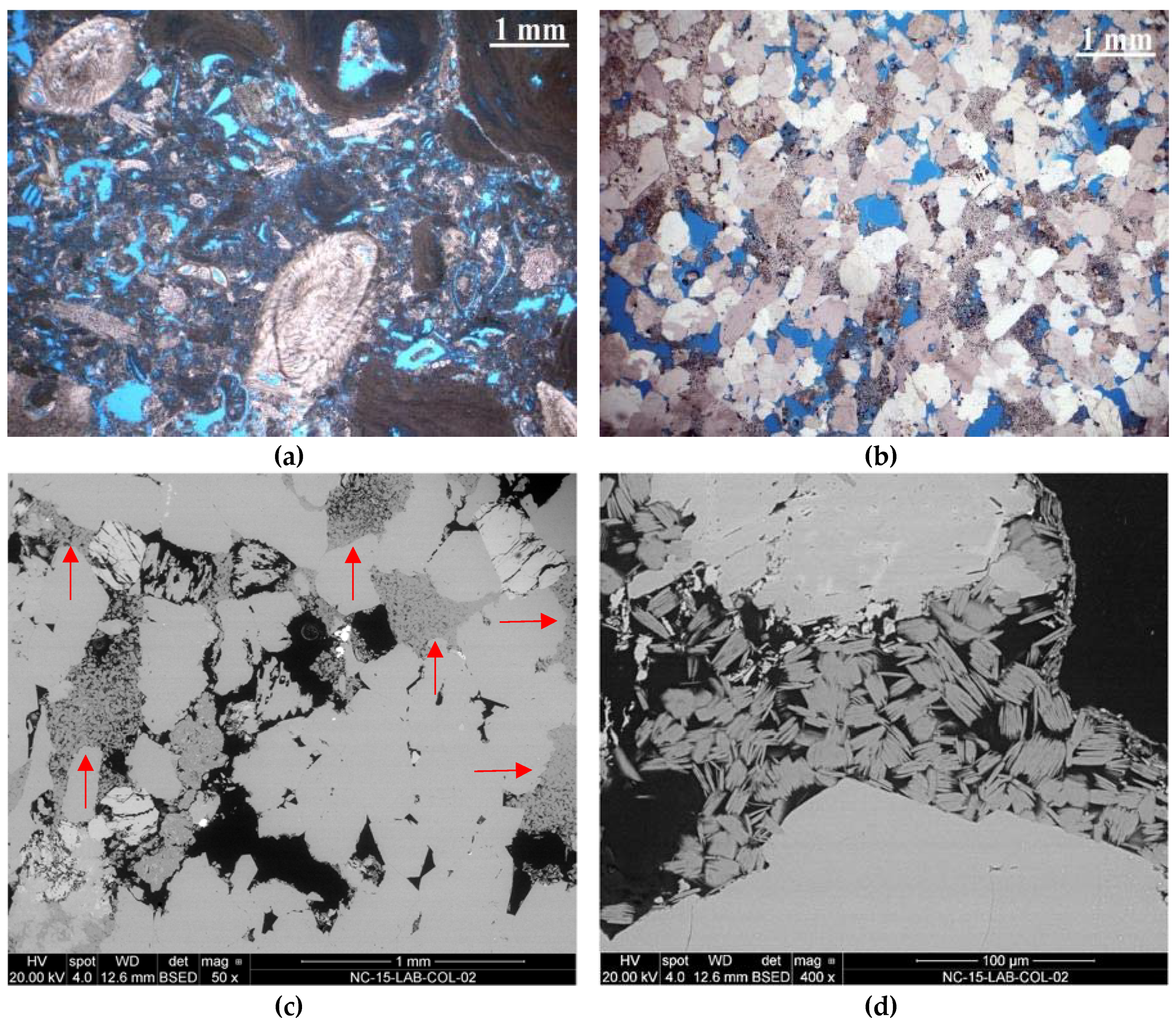
2.1. Petrographic Characterisation
2.2. Artificial Ageing by Thermal Treatment
2.3. Consolidation Treatment
2.4. Test Methods for Determining the Efficiency and Compatibility of Consolidants
- For the preparation of polished cross sections, laboratory treated specimens with dimensions 50 mm × 50 mm × 50 mm were vacuum impregnated with blue stained epoxy resin and cut perpendicular to the consolidated surface. The section size ensured that treatments which had reached the centre of the treated body could be traced by the analysis. The slices were then polished and examined by SEM. The instrument used was a field-emission scanning electron microscope of the type FEI Quanta 250 FEG. The working mode was low vacuum at 20 kV acceleration voltage. Contrast and brightness of the backscattered electron (BSE) images were adjusted to visualize the consolidant in the pores by distinct grey values. For visualisation purpose and image analysis, post processing of the micrographs was done with Adobe Photoshop by false-colour-mapping the silica gel inside the stone fabric. Furthermore, when calculations of e.g., the penetration depth to filling degree was to be obtained, the software ImageJ proved to be a valuable tool for such purposes.
- Mercury intrusion porosimetry (MIP) was performed with a Porosimeter Porotec Pascal 140/440. Changes in the pore radii distribution and open porosity determined by Hg-intrusion for the different conditions (sound, artificially aged and consolidated) were studied.
- The dynamic modulus of elasticity was determined with the longitudinal resonance frequency of an ultrasound signal in transmission, according to EN 14146 [49]. Prismatic specimens with dimensions 10 mm × 10 mm × 40 mm were used and mean values of three specimens were computed. The small specimen size was selected to ensure the consolidation of the entire body. The test was performed by an ultrasound pulse generator (CONOSONIC C2-GS), a pair of transducers (UP-DW), a clamping and pressure device for specimen’s assembly as well as and a notebook preinstalled with the Light House DW software, developed by Geotron-Elektronik, Germany. The UP-DW piezoelectric transducers, operating at a frequency band from 1 to 100 kHz, are specifically manufactured to determine the materials elastic parameter (DW stands for “dehnwelle” and translated from German it means “extensional wave”). This device is equipped with a built-in algorithm that calculates the longitudinal-, transverse-, surface- and extensional waves as well as E- and G Modulus and the Poisson’s ratio. However, the principle on how to obtain the dynamic modulus of elasticity (EdL) determined through the longitudinal fundamental resonance frequency (FL) is given by Equation (1):whereby (l) represents the specimens length and (ρ) the stones apparent density. As in our case the width of the specimen is four times its length, the correction factor (T) can be assumed to be 1 in which case the equation is simplified to Equation (2):EdL = 4 × 10−6·l2·FL2·ρ·T(FL) was recorded when the deviation of the measured fundamental resonance frequency stayed in a range of ±60 Hz, three times in a row. The dynamic modulus of elasticity is reported in GPa or kN/mm2. As for all non-destructive tests, measurements could be performed on the same specimen before and after treatment.EdL = 4 × 10−6·l2·FL2·ρ
- Splitting tensile strength was determined following the recommendations of ASTM D 3967-08 [50]. The electro-mechanical tension and compression-testing machine was a 150 kN Instron Model 4206, developed by Instron GmbH, in Germany. The apparatus consisted of a flat bearing block at the bottom and, to reduce the contact stresses, a curved bearing block on the top. Bearing strips with 0.6 mm thickness were used to reduce high stress concentrations. The loading rate was 100 N/s. 16 specimens per lithotype and condition (sound, aged and consolidated) were tested, each 60 mm in diameter and 30 mm in thickness. For the aged stone specimens and the reference product KSE 300, 10 out of the 16 specimens were tested in the frame of two master theses [51,52]. The test was executed in the direction perpendicular to the bedding plane, which was assessed through ultrasound pulse velocity. For the latter purposes, the frequency for both lithotypes was set to 80 kHz and the amplitude was adjusted according to the samples damping. Specimens were measured without a coupling medium. The splitting tensile strength was calculated with Equation (3) and is here reported in N/mm2.(P) is the maximum applied load indicated by the testing machine in newton [N], (L) the thickness, and (D) the diameter of the specimen, in mm.σt = 2 × π−1·P·L−1·D−1
- The three-point flexural strength was determined according to EN 12372 [53] with the load increased uniformly at a rate of 0.25 ± 0.05 MPa/s (or 41.67 N/s recalculated for the given dimensions) until the specimen broke. 10 specimens with 25 mm × 50 mm × 150 mm were tested, whereby the distance between the supporting rollers was 125 mm. The tests were performed with an electronic spindle-drive testing machine of the type Testomeric Quicktester 100 kN and evaluated by the Test & Motion software developed by DOLI Elektronik GmbH, Germany. The test was carried out in the direction perpendicular to the bedding plane, which was assessed through ultrasound pulse velocity. The flexural strength was calculated according to the following Equation (4):where (F) is the breaking load in newton, (l) the distance between the supporting rollers, (b) the width- and (h) the thickness of specimen adjacent to the plane of fracture, all reported in mm. The results are expressed in MPa or here in N/mm2 (1 MPa = 1 N/mm2).Rtf = 1.5·F·l·b−1·h−2
- Water absorption coefficient after one hour was determined according to standard EN 15801 [54] and is reported as kg·m−2·h−0.5. The test was carried out on three 30 mm × 30 mm × 30 mm specimens per stone and treatment. After a stage of pre-conditioning, samples were placed on water-soaked filter paper (Ahlstrom-Munktell laboratory filter paper, wet-strengthen grades) and the absorption of water was monitored gravimetrically. The test was performed on the same specimens before and after treatment.
- Contact angle of water was determined on the stone surface treated with the water repellent consolidants NC-27CP. Therefore, the Mobile Surface Analyzer from Krüss GmbH, Germany came to use.
- Water vapour permeability tests were performed according to EN 15803 [55] using the so-called “wet cup” method with a cup system Type 1 according to the standard. In this case, the cups were filled with water and placed in a climatic chamber at ambient conditions of 23 ± 1 °C and 50 ± 3% RH (Heraeus Vötsch Klimaprüfschrank VC3, model 4034). They were weighed every 24 h for one week. The results were plotted as mass change (Δm) against time (t) and the slope of the linear section of the curve (G, kg·s−1) was determined with the software OriginPro. (G) was further used to determine the water vapour permeance (Equation (5), in kg·m−2·s−1·Pa−1):where (A) represents the specimens surface area in m2 and ∆pv the water vapour pressure difference reported as Pa across the test specimen. The water vapour permeability reported in kg·m−1·s−1·Pa−1 was then determined with Equation (6):Wp = G·A−1·∆pv−1where (D) represents the average thickness of the test specimens in m. Three specimens per lithotype and treatment with dimensions of 50 mm × 50 mm × 10 mm were tested. The water vapour permeability is reported as the ratio of treated to untreated values.δp = Wp·D
- Finally, colour parameters were determined with a ColorLite sph850 spectrophotometer, according to standard EN 15886 [56]. The output of the measurements is reported as CIE (International Commission on Illumination) L*, a*, b* colour parameters, tested with a D65 illuminant at 10° standard observer with a reflectance spectrum in the range of 400 to 700 nm. ΔE* was reported and describes the metric difference or distance between two colours before and after treatment according to the standards of the International Commission on Illumination. Average (L*), (a*) and (b*) values were used to obtain the total colour difference (ΔE*) between treated (t) and untreated (nt) measurements with Equation (7).In the latter equation (ΔL*) corresponds to the lightness difference, (Δa*) to the red/green difference and (Δb*) to the yellow/blue difference of the tested stone specimens. Colour values measured for treated and untreated specimens were performed on sound stones, in order to exclude any possible impact induced by heat treatment. The results were calculated from an average of three measurements obtained at the same spot, with the help of stencils, before and after the treatment.
3. Results and Discussion
3.1. Spatial Distribution of Consolidants after Curing Assessed by Scanning Electron Microscopy
3.2. Porometric Characteristics Examined by Mercury Intrusion Porosimetry
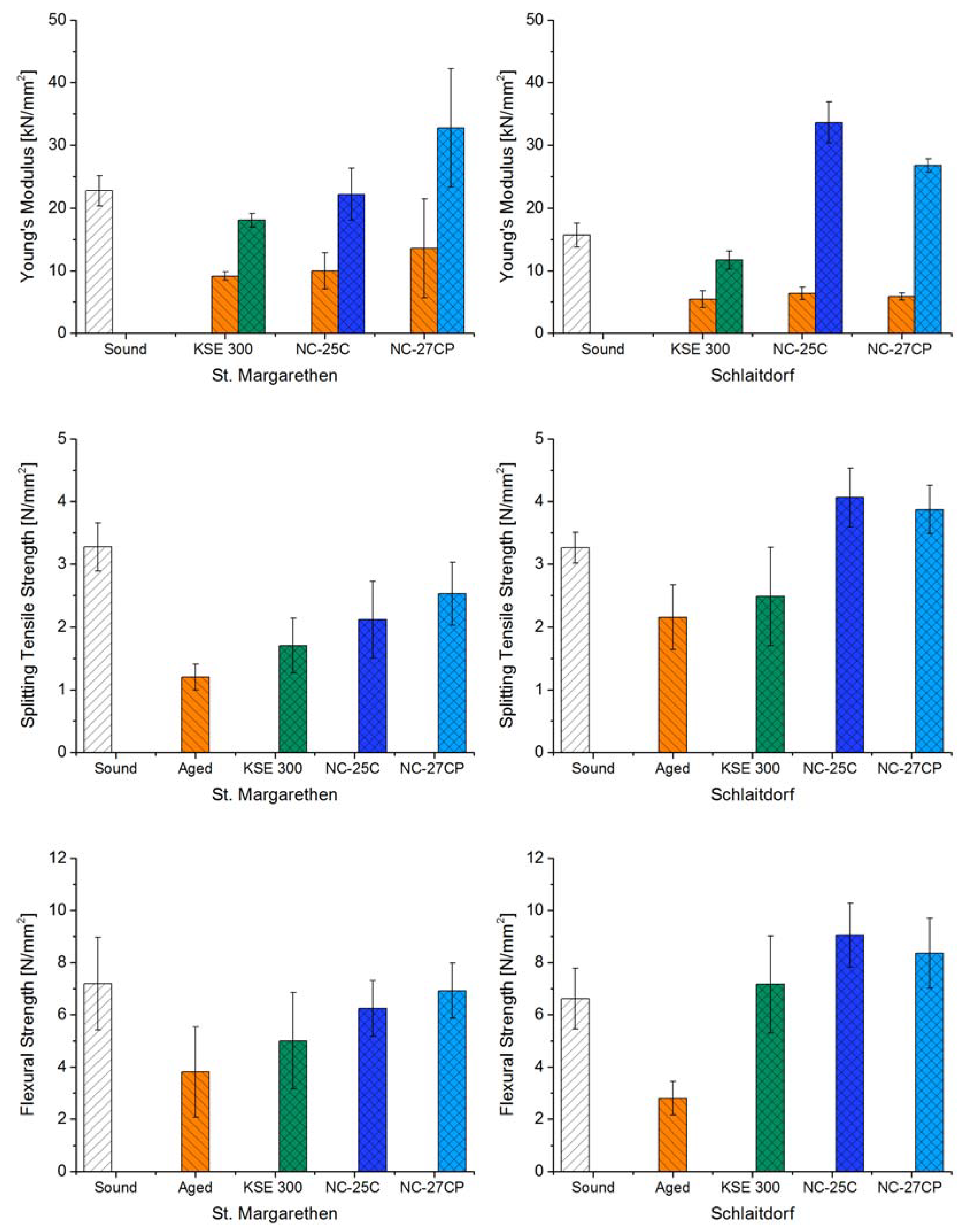
3.3. Evaluation of the Consolidation Efficiency (Mechanical Analysis)
3.3.1. Effects of Thermal Treatment Prior to Consolidation
3.3.2. Effects of Consolidation Treatment
3.4. Evaluation of the Compatibility (Moisture Related Properties and Visual Impact)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verges-Belmin, V. Illustrated Glossary on Stone Deterioration Patterns; ICOMOS-ISCS (International Scientific Committee for Stone): Paris, Fance, 2008. [Google Scholar]
- Ferreira Pinto, A.; Delgado Rodrigues, J. Stone consolidation: The role of treatment procedures. J. Cult. Herit. 2008, 9, 38–53. [Google Scholar] [CrossRef]
- Ahmed, H.T. Physical and mechanical characteristics of Helwan limestone: For conservation treatment of ancient Egyptian limestone monuments. J. Am. Sci. 2015, 11, 136–151. [Google Scholar]
- Pápay, Z.; Török, Á. Evaluation of the efficiency of consolidants on Hungarian porous limestone by non-destructive test methods. Cent. Eur. Geol. 2007, 50, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Franzoni, E.; Sassoni, E.; Scherer, G.W.; Naidu, S. Artificial weathering of stone by heating. J. Cult. Herit. 2013, 14, E85–E93. [Google Scholar] [CrossRef]
- Da Fonseca, B.S.; Ferreira Pinto, A.P.; Picarra, S.; Montemor, M.F. Artificial aging route for assessing the potential efficacy of consolidation treatments applied to porous carbonate stones. Mater. Des. 2017, 120, 10–21. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Mao, X.B.; Lu, A.H. Experimental study on the mechanical properties of rocks at high temperature. Sci. China Ser. E Technol. Sci. 2009, 52, 641–646. [Google Scholar] [CrossRef]
- Ruedrich, J.; Kirchner, D.; Siegesmund, S. Physical weathering of building stones induced by freeze-thaw action: A laboratory long-term study. Environ. Earth Sci. 2011, 63, 1573–1586. [Google Scholar] [CrossRef]
- Ban, M.; Baragona, A.; Ghaffari, E.; Weber, J.; Rohatsch, A. Artificial aging techniques on various lithotypes for testing of stone consolidants. In Science and Art: A Future for Stone, Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Volume 1; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, UK, 2016; pp. 253–260. [Google Scholar]
- Price, C.A. Stone Conservation: An Overview of Current Research; The J. Paul Getty Trust: Los Angeles, CA, USA, 1996. [Google Scholar]
- Delgado Rodrigues, J.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Sasse, H.; Snethlage, R. Evaluation of stone consolidation treatments. Sci. Technol. Cult. Herit. 1996, 5, 1996. [Google Scholar]
- Graziani, G.; Sassoni, E.; Franzoni, E. Consolidation of porous carbonate stones by an innovative phosphate treatment: Mechanical strengthening and physical-microstructural compatibility in comparison with TEOS-based treatments. Herit. Sci. 2015, 3. [Google Scholar] [CrossRef]
- Persia, F.; D’Amato, R.; Padella, F.; Pilloni, L.; Rinaldi, A.; Tatì, A. Performance of nanomaterials for the conservation of artistic stones. EAI Energ. Ambient. Innov. 2012, II-2012, 77–81. [Google Scholar]
- Siegesmund, S.; Snethlage, R. Stone in Architecture: Properties, Durability; Springer: Berlin/Heidelberg, Germany, 2011; pp. 415–550. ISBN 3642144756. [Google Scholar]
- Auras, M.; Meinhardt, J.; Snethlage, R. (Eds.) Leitfaden Naturstein-Monitoring, Nachkontrolle und Wartung als Zukunftsweisende Erhaltungsstrategien; Fraunhofer IRB Verlag: Stuttgart, Germany, 2011; ISBN 978-3-8167-8407-4. [Google Scholar]
- Tiano, P.; Filareto, C.; Ponticelli, S.; Ferrari, M.; Valentini, E. Drilling force measurement system, a new standardisable methodology to determine the stone cohesion: Prototype design and validation. Int. J. Restor. Build. Monum. 2000, 6, 115–132. [Google Scholar]
- Costa, D.; Delgado Rodrigues, J. Evaluation of consolidation treatments applied to granitic materials. Experience and critical overview of laboratory testing. In Proceedings of the International Symposium on Stone Consolidation in Cultural Heritage: Research and Practice, Lisbon, Portugal, 6–7 May 2008; Rodrigues, J.D., Mimoso, J.M., Eds.; LNEC: Lisbon, Portugal, 2008; pp. 389–398. [Google Scholar]
- Snethlage, R.; Ettl, H.; Sattler, L. Ultrasonic measurements on PMMA-impregnated marble sculptures. Zeitschrift der Deutschen Geologischen Gesellschaft 1999, 150, 387–396. [Google Scholar]
- Snethlage, R. Leitfaden Steinkonservierung, Planung von Untersuchungen und Maßnahmen zur Erhaltung von Denkmälern aus Naturstein; Fraunhofer IRB Verlag: Stuttgart, Germany, 2008; ISBN 978-3-8167-8407-4. [Google Scholar]
- Drdácký, M. Testing efficiency of stone conservation treatments. In Advanced Materials for the Conservation of Stone; Majid, H., Ioannis, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 175–184. ISBN 978-3-319-72259-7. [Google Scholar]
- Price, C.A.; Doehne, E. Stone Conservation: An Overview of Current Research, 2nd ed.; Getty Publications: Los Angeles, CA, USA, 2011. [Google Scholar]
- Clifton, J.R. Stone Consolidating Materials: A Status Report; NBS Technical Note 1118:46; U.S. Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1980.
- George, W.; Goins, E.S. Alkoxysilanes and the Consolidation of Stone; Getty Publications: Los Angeles, CA, USA, 2005. [Google Scholar]
- Ghaffari, E.; Köberle, T.; Weber, J. Methods of polarising microscopy and SEM to assess the performance of nano-lime consolidants in porous solids. In Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone, Columbia University, New York, NY, USA, 22–26 October 2012; pp. 22–26. [Google Scholar]
- Mascha, E.; Weber, J.; Ban, M. Erforschung von Nano-Materialien in der Gesteinsrestaurierung: Auswirkung von Festigungsmaßnahmen anhand von Laborversuchen innerhalb des EU-Projektes Nano-Cathedral. In Proceedings of the Natursteinsanierung Stuttgart 2018: Neue Natursteinrestaurierungsergebnisse und Messtechnische Erfassungen Sowie Sanierungsbeispiele, Stuttgart, Germany, 16 March 2018; Patitz, G., Grassegger-Schön, G., Wölbert, O., Eds.; Fraunhofer IRB Verlag: Stuttgart, Germany, 2018. [Google Scholar]
- Pintér, F.; Weber, J.; Bajnóczi, B. Visualisation of solid consolidants in pore space of porous limestone using microscopic method. In Proceedings of the 11th International Congress on Deterioration and Conservation of Stone, Torun, Poland, 15–20 September 2008; pp. 473–480. [Google Scholar]
- Zornoza-Indart, A.; Lopez-Arce, P.; Zoghlami, K.; Leal, N.; Simão, J. Marine Aerosol weathering of Mediterranean calcarenite stone: Durability of ethyl silicate, nano Ca(OH)2, nano SiO2, and nanostructured consolidating products. Stud. Conserv. 2018, 1–17. [Google Scholar] [CrossRef]
- Wendler, E.; Klemm, D.; Snethlage, R. Consolidation and hydrophobic treatment of natural stone. In Proceedings of the 5th International Conference on Durability of Building Materials and Components, Brighton, UK, 7–9 November 1990; Chapman & Hall: London, UK, 1990; pp. 203–212. [Google Scholar]
- Charola, A.E. Water repellents and other “protective” treatments: A critical review. Restor. Build. Monum. 2003, 9, 3–22. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, J.; Xu, R.F.; Ma, G.J. Hydrophilicity, photocatalytic activity and stability of tetraethyl orthosilicate modified TiO2 film on glazed ceramic surface. Appl. Surf. Sci. 2013, 266, 141–147. [Google Scholar] [CrossRef]
- Gherardi, F.; Goidanich, S.; Dal Santo, V.; Toniolo, L. Layered nano-TiO2 based treatments for the maintenance of natural stones in historical architecture. Angew. Chem. Int. Ed. Engl. 2018, 57, 7360–7363. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Kallithrakas-Kontos, N.; Korakaki, D.; Agioutantis, Z.; Maurigiannakis, S. Evaluation of silicon-based strengthening agents on porous limestones. Prog. Org. Coat. 2006, 57, 140–148. [Google Scholar] [CrossRef]
- Da Fonseca, B.S.; Picarra, S.; Ferreira Pinto, A.P.; Montemor, M.F. Development of formulations based on TEOS-dicarboxylic acids for consolidation of carbonate stones. New J. Chem. 2016, 40, 7493–7503. [Google Scholar] [CrossRef]
- Berto, T.; Godts, S.; De Clercq, H. The effects of commercial ethyl silicate based consolidation products on limestone. In Science and Art: A Future for Stone, Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Volume 1; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, UK, 2016; pp. 271–280. [Google Scholar]
- Sassoni, E.; D’Amen, E.; Roveri, N.; Scherer, G.W.; Franzoni, E. Photocatalytic hydroxyapatite-titania nanocomposites for preventive conservation of marble. IOP Conf. Ser. Mater. Sci. Eng. 2018, 364, 012073. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on solegel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Folk, R.L. Petrology of Sedimentary Rocks; Hemphill Publishing Company: Austin, TX, USA, 1980. [Google Scholar]
- Pettijohn, F.; Potter, P.; Siever, R. Sand and Sandstone, 2nd ed.; Springer: New York, NY, USA, 1987. [Google Scholar]
- Graue, B.J. Stone Deterioration and Replacement of Natural Building Stones at the Cologne Cathedral—A Contribution to the Preservation of Cultural Heritage. Ph.D. Thesis, Georg-August-Universität Göttingen, Göttingen, Germany, 2013. [Google Scholar]
- Graue, B.; Siegesmund, S.; Middendorf, B.; Oyhantcabal, P. Requirements for replacement stones at the Cologne cathedral—A systematic approach to general criteria of compatibility. In Proceedings of the 12th International Congress on Deterioration Conservation of Stone, Columbia University, New York, NY, USA, 22–26 October 2012. [Google Scholar]
- Grimm, W. Bildatlas wichtiger Denkmalgesteine der Bundesrepublik Deutschland; Bayerisches Landesamt fur Denkmalpflege: München, Germany, 1990; Volume 50. [Google Scholar]
- Gradstein, F.M.; Ogg, J.G.; Hilgen, F.J. On The Geologic Time Scale. Newsl. Stratigr. 2012, 45, 171–188. [Google Scholar] [CrossRef]
- Dunham, R.J. Classification of carbonate rocks according to depositional textures. Am. Assoc. Pet. Geol. 1962, 1, 108–121. [Google Scholar]
- Embry, A.F., III; Klovan, J.E. A late Devonian reef tract on northeastern Banks Island, NWT. Bull. Can. Pet. Geol. 1971, 19, 730–781. [Google Scholar]
- Moshammer, B.; Uhlir, C.; Rohatsch, A.; Unterwurzacher, M. Adnet ‘marble’, Untersberg ‘marble’and Leitha limestone—Best examples expressing Austria’s physical Cultural Heritage. Eng. Geol. Soc. Territ. 2015, 5, 253–257. [Google Scholar]
- Rohatsch, A. Neogene Bau-und Dekorgesteine Niederösterreichs und des Burgenlandes; Mitteilungen IAG BOKU: Wien, Austria, 2005. [Google Scholar]
- Ban, M.; Pliessnig, M. The Biedermeier cemetery of St Marx in Vienna: Planning, management and treatment implementation. In Conservation of Sculpture Parks; Archetype Publications Ltd.: London, UK, 2018; pp. 59–72. [Google Scholar]
- CEN. Standard EN 14146, Determination of Dynamic Elastic Modulus by Measuring the Fundamental Resonant Frequency; BSI: London, UK, 2004. [Google Scholar]
- ASTM International. ASTM D 3967–08, Standard Test Method for Splitting Tensile Strength of Intact Rock Core Specimens; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Baumgartner, M. Auswirkungen von Steinfestiger auf die Mechanischen Eigenschaften von Natursteinen historischer Bauwerke: Teil 3. Untersuchungen und Prüfungen von Gealterten und Gefestigten Steinproben. Master’s Thesis, Vienna University of Technology, Vienna, Austria, 2017. [Google Scholar]
- Ziniel, A. Auswirkungen von Steinfestiger auf die mechanischen Eigenschaften von Natursteinen historischer Bauwerke: Teil 2. Brazilian Test, Druck-, Biegezug-und direkter zweischnittiger Scherversuch. Master’s Thesis, Vienna University of Technology, Vienna, Austria, 2017. [Google Scholar]
- CEN. Standard EN 12372 Natural Stone Test Methods—Determination of Flexural Strength Under Concentrated Load; BSI: London, UK, 2007. [Google Scholar]
- CEN. Standard EN 15801, Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity; BSI: London, UK, 2010. [Google Scholar]
- CEN. Standard EN 15803, Conservation of Cultural Property—Test Methods—Determination of Water Vapour Permeability; BSI: London, UK, 2010. [Google Scholar]
- CEN. Standard EN 15886, Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces; BSI: London, UK, 2010. [Google Scholar]
- Remmers. Technical Guideline, KSE Modular System. Available online: http://www.remmers.co.uk/fileadmin/doc/pz/TL_0571_EN.pdf (accessed on 28 November 2018).
- Cnudde, V.; Cnudde, J.P.; Dupuis, C.; Jacobs, P.J.S. X-ray micro-CT used for the localization of water repellents and consolidants inside natural building stones. Mater. Charact. 2004, 53, 259–271. [Google Scholar] [CrossRef]
- Pesce, G.; Morgan, D.; Odgers, D.; Henry, A.; Allen, M.; Ball, R. Consolidation of weathered limestone using nanolime. Proc. Inst. Civil Eng. Constr. Mater. 2013, 166, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Alvarez De Buergo, M.; Fort, R.; Gomez-Heras, M. Contributions of scanning electron microscopy to the assessment of the effectiveness of stone conservation treatments. Scanning 2004, 26, 41–47. [Google Scholar] [CrossRef]
- Croveri, P.; Luigi, D.; Joann, C.; Chiantore, O. Porosimetric changes and consequences for damage phenomena induced by organic and inorganic consolidation treatments on highly porous limestone. In Science and Art: A Future for Stone, Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Volume 1; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, UK, 2016; pp. 67–74. [Google Scholar]
- Vutukuri, V.S.; Lama, R.D.; Saluja, S.S. Handbook on Mechanical Properties of Rocks: Testing Techniques and Results, 1st ed.; Trans Tech Publ.: Clausthal, Germany, 1974; Volume 1. [Google Scholar]
- Baumgartner, L. Das Festigkeits-und Verformungsverhalten von Postaer Sandstein bei Zugbeanspruchung. In Proceedings of the 19 Tagung für Ingenieurgeologie und des Forums für junge Ingenieurgeologen, Technische Universität, München, Germany, 13–16 March 2013; pp. 407–414. [Google Scholar]
- Coviello, A.; Lagioia, R.; Nova, R. On the measurement of the tensile strength of soft rocks. Rock Mech. Rock Eng. 2005, 38, 251–273. [Google Scholar] [CrossRef]
- Chen, R.; Stimpson, B. Interpretation of indirect tensile-strength tests when moduli of deformation in compression and in tension are different. Rock Mech. Rock Eng. 1993, 26, 183–189. [Google Scholar] [CrossRef]
- Perras, M.A.; Diederichs, M.S. A review of the tensile strength of rock: Concepts and testing. Geotechn. Geol. Eng. 2014, 32, 525–546. [Google Scholar] [CrossRef]
- Aydin, A.; Basu, A. The use of Brazilian test as a quantitative measure of rock weathering. Rock Mech. Rock Eng. 2006, 39, 77–85. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J. Swelling behaviour of stones and its interest in conservation. An appraisal. Mater. Constr. 2001, 51, 183–195. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, I.; Rodriguez-Navarro, C.; Scherer, G.W. Role of clay minerals in the physicomechanical deterioration of sandstone. J. Geophys. Res. Earth Sur. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Cherblanc, F.; Berthonneau, J.; Bromblet, P.; Huon, V. Influence of Water Content on the Mechanical Behaviour of Limestone: Role of the Clay Minerals Content. Rock Mech. Rock Eng. 2016, 49, 2033–2042. [Google Scholar] [CrossRef]
- Snethlage, R.; He, L.; Ma, T.; Wendler, E.; Sattler, L.; Simon, S. Der Sandstein von Dafosi-Untersuchungen zu den Ursachen der Schäden und zur Konservierung-The Sandstone of Dafosi Investigation into Causes of Deterioration and Conservation Methods. ICOMOS–Hefte des Deutschen Nationalkomitees 1996, 17, 220–239. [Google Scholar]
- Scherer, G.W.; Wheeler, G.S. Silicate consolidants for stone. Key Eng. Mater. 2009, 391, 1–25. [Google Scholar] [CrossRef]
- Milchin, M.; Weber, J.; Krist, G.; Ghaffari, E.; Karacsonyi, S. Ethyl-silicate consolidation for porous limestone coated with oil paint—A comparison of application methods. In Science and Art: A Future for Stone, Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Volume 2; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, UK, 2016; pp. 889–896. [Google Scholar]
- Park, H.D.; Shin, G.H. Geotechnical and geological properties of Mokattam limestones: Implications for conservation strategies for ancient Egyptian stone monuments. Eng. Geol. 2009, 104, 190–199. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 1: Effectiveness and compatibility in comparison with ethyl silicate. Constr. Build. Mater. 2016, 102, 918–930. [Google Scholar] [CrossRef]
- Martinho, E.; Mendes, M.; Dionisio, A. 3D imaging of P-waves velocity as a tool for evaluation of heat induced limestone decay. Constr. Build. Mater. 2017, 135, 119–128. [Google Scholar] [CrossRef]
- Jerome, P.S.; Weiss, N.R.; Gilbert, A.S.; Scott, J.A. Ethyl silicate as a treatment for marble: Conservation of St. John’s Hall, Fordham University. APT Bull. 1998, 29, 19–26. [Google Scholar] [CrossRef]
- Ksinopoulou, E.; Bakolas, A.; Moropoulou, A. Consolidation effectiveness of modified Si-based nanocomposites applied to limestones. Mater. Struct. 2018, 51. [Google Scholar] [CrossRef]
- Rohatsch, A.; Nimmrichter, J.; Chalupar, I. Physical properties of fine grained marble before and after conservation. In Proceedings of the 9th International Congress on Deterioration and Conservation of Stone, Venice, Italy, 19–24 June 2000; pp. 453–458. [Google Scholar]
- Stuck, H.; Forgo, L.Z.; Rudrich, J.; Siegesmund, S.; Torok, A. The behaviour of consolidated volcanic tuffs: Weathering mechanisms under simulated laboratory conditions. Environ. Geolol. 2008, 56, 699–713. [Google Scholar] [CrossRef]
- Pápay, Z.; Török, Á. Micro-Fabric, Pore-size distribution and water absorption of consolidated porous limestone. In Engineering Geology for Society and Territory-Volume 8; Springer: Cham, Switzerland, 2015; pp. 553–556. [Google Scholar] [CrossRef]
- Remmers. Technical Data Sheet, KSE 300. Available online: http://www.remmers.co.uk/fileadmin/doc/tm/TM1_0720_EN.pdf (accessed on 28 November 2018).
- Häupl, P.; Homann, M.; Kölzow, C.; Riese, O.; Maas, A.; Höfker, G.; Christian, N. Lehrbuch der Bauphysik: Schall-Wärme-Feuchte-Licht-Brand-Klima; Springer: Berlin, Germany, 2017; p. 215. ISBN 3658160748. [Google Scholar]
- Ballester, M.A.D.; Gonzalez, R.F. Basic methodology for the assessment and selection of water-repellent treatments applied on carbonatic materials. Prog. Org. Coat. 2001, 43, 258–266. [Google Scholar] [CrossRef]
- Tsakalof, A.; Manoudis, P.; Karapanagiotis, I.; Chryssoulakis, I.; Panayiotou, C. Assessment of synthetic polymeric coatings for the protection and preservation of stone monuments. J. Cult. Herit. 2007, 8, 69–72. [Google Scholar] [CrossRef]
- Charola, A.E. Water-repellent treatments for building stones: A practical overview. APT Bull. 1995, 26, 10–17. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M. Performances and coating morphology of a siloxane-based hydrophobic product applied in different concentrations on a highly porous stone. Coatings 2016, 6, 60. [Google Scholar] [CrossRef]
- Kronlund, D.; Bergbreiter, A.; Meierjohann, A.; Kronberg, L.; Lindén, M.; Grosso, D.; Smått, J.-H. Hydrophobization of marble pore surfaces using a total immersion treatment method–product selection and optimization of concentration and treatment time. Prog. Org. Coat. 2015, 85, 159–167. [Google Scholar] [CrossRef]
- Gherardi, F.; Roveri, M.; Goidanich, S.; Toniolo, L. Photocatalytic nanocomposites for the protection of European architectural heritage. Materials 2018, 11, 65. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Moreau, C.; Leroux, L.; Vergès-Belmin, V.; Fronteau, G.; Barbin, V. Which factors influence most the durability of water repellent treatments: Stone properties, climate or atmospheric pollution. In Proceedings of the Hydrophobe V, 5th International Conference on Water Repellent Treatment of Building Materials, Brussels, Belgium, 15–16 April 2008; pp. 129–142. [Google Scholar]
- Boutin, F. Comparative study of the efficiency of protective treatments applied to stone. In Proceedings of the Hydrophobe III, Third International Conference on Surface Technology with Water Repellent Agents, Universität Hannover, Hannover, Germany, 25–26 September 2001; pp. 233–244. [Google Scholar]
- De Clercq, H.; De Witte, E. Effectiveness of silicon based water repellent agents at different application conditions. II. Commercial water repellents. Int. J. Restor. Build. Monum. 2001, 7, 641–654. [Google Scholar]
- Poli, T.; Toniolo, L.; Sansonetti, A. Durability of protective polymers: The effect of UV and thermal ageing. Macromol. Symp. 2006, 238, 78–83. [Google Scholar] [CrossRef]
- Wendler, E.; von Plehwe-Leisen, E. Water repellent treatment of porous materials. A new edition of the WTA leaflet. Hydrophobe V Water Repel. Treat. Build. Mater. 2008, 155–168. [Google Scholar]
- Hansen, C.M. Water transport and condensation in fluoropolymer films. Prog. Org. Coat. 2001, 42, 167–178. [Google Scholar] [CrossRef]
- Garcia, O.; Malaga, K. Definition of the procedure to determine the suitability and durability of an anti-graffiti product for application on cultural heritage porous materials. J. Cult. Herit. 2012, 13, 77–82. [Google Scholar] [CrossRef]





| Porometric Characteristics of Materials | Conditions | |||||
|---|---|---|---|---|---|---|
| Sound | Aged | KSE 300 | NC-25C | NC-27CP | ||
| St. Margarethen Limestone | Total pore surface [m2/g] | 0.824 | 1.326 | 2.168 | 0.523 | 0.569 |
| Average pore diameter [µm] | 0.483 | 0.253 | 0.183 | 0.526 | 0.568 | |
| Total porosity [%] | 20.65 | 17.53 | 20.63 | 14.76 | 16.88 | |
| Schlaitdorf Sandstone | Total pore surface [m2/g] | 0.800 | 0.518 | 0.477 | 0.864 | 0.242 |
| Average pore diameter [µm] | 0.349 | 0.643 | 0.533 | 0.223 | 0.463 | |
| Total porosity [%] | 15.42 | 18.02 | 14.20 | 11.15 | 7.10 | |
| Product Specification | St. Margarethen Limestone | Schlaitdorf Sandstone | ||||
|---|---|---|---|---|---|---|
| Consolidant | KSE 300 | NC-25C | NC-27CP | KSE 300 | NC-25C | NC-27CP |
| Solid Content [%] | 31.62 | 47.82 | 59.73 | 32.12 | 46.59 | 59.98 |
| Standard Deviation | ±1.46 | ±0.57 | ±0.54 | ±1.04 | ±0.67 | ±0.50 |
| Consolidant | (S) Sound ± Std.N | (A) Aged ± Std.N | (C) Consolidated ± Std.N | Decrease (S-A, %) | Increase (A-C, %) | Magnitude (S-C, %) | ||
|---|---|---|---|---|---|---|---|---|
| St. Margarethen Limestone | Young’s Modulus (kN/mm2) | KSE 300 | 22.7 ± 2.4 | 9.2 ± 0.7 | 18.1 ± 1.1 | −60 | +97 | −21 |
| NC-25C | 10.0 ± 2.9 | 22.2 ± 4.1 | −56 | +123 | −2 | |||
| NC-27CP | 13.6 ± 7.9 | 32.7 ± 9.5 | −40 | +141 | +44 | |||
| Splitting Tensile Strength (N/mm2) | KSE 300 | 3.3 ± 0.4 | 1.2 ± 0.2 | 1.7 ± 0.4 | −63 | +42 | −48 | |
| NC-25C | 2.1 ± 0.6 | +76 | −35 | |||||
| NC-27CP | 2.5 ± 0.5 | +111 | −23 | |||||
| Flexural Strength (N/mm2) | KSE 300 | 7.2 ± 1.8 | 3.8 ± 1.7 | 5.0 ± 1.8 | −47 | +31 | −31 | |
| NC-25C | 6.2 ± 1.1 | +64 | −13 | |||||
| NC-27CP | 6.9 ± 1.1 | +82 | −4 | |||||
| Schlaitdorf Sandstone | Young’s Modulus (kN/mm2) | KSE 300 | 15.7 ± 1.9 | 5.5 ± 1.3 | 11.7 ± 1.4 | −65 | +114 | −26 |
| NC-25C | 6.4 ± 1.0 | 33.6 ± 3.3 | −60 | +429 | +114 | |||
| NC-27CP | 5.9 ± 0.6 | 26.8 ± 1.1 | −63 | +356 | +70 | |||
| Splitting Tensile Strength (N/mm2) | KSE 300 | 3.2 ± 0.2 | 2.2 ± 0.5 | 2.5 ± 0.8 | −34 | +16 | −24 | |
| NC-25C | 4.1 ± 0.5 | +89 | +25 | |||||
| NC-27CP | 3.9 ± 0.4 | +80 | +19 | |||||
| Flexural Strength (N/mm2) | KSE 300 | 6.6 ± 1.2 | 2.8 ± 0.6 | 7.2 ± 1.9 | −58 | +156 | +8 | |
| NC-25C | 9.0 ± 1.2 | +223 | +37 | |||||
| NC-27CP | 8.4 ± 1.3 | +198 | +26 | |||||
| Stone | Treatment | WAC(s) | WAC(a) | WAC(c) (6 w) | WAC(c) (6 m) | WVPc/a (6 w) |
|---|---|---|---|---|---|---|
| SM | KSE 300 | 4.49 ± 0.05 | 5.23 ± 0.02 | 4.52 ± 0.06 | 4.56 ± 0.03 | 0.89 ± 0.06 |
| NC-25C | 4.50 ± 0.18 | 5.05 ± 0.11 | 0.76 ± 0.27 | 2.99 ± 0.28 | 0.84 ± 0.03 | |
| NC-27CP | 4.51 ± 0.15 | 5.17 ± 0.18 | 0.47 ± 0.51 | 0.54 ± 0.26 | 0.55 ± 0.02 | |
| S | KSE 300 | 3.29 ± 0.30 | 3.66 ± 0.28 | 0.18 ± 0.15 | 2.14 ± 0.50 | 1.75 ± 0.45 |
| NC-25C | 2.74 ± 0.72 | 3.57 ± 0.25 | 1.65 ± 0.15 | 1.21 ± 0.28 | 0.81 ± 0.03 | |
| NC-27CP | 2.74 ± 0.59 | 3.53 ± 0.16 | 0.08 ± 0.04 | 0.20 ± 0.04 | 0.35 ± 0.00 |
| Stone | Treatments | ΔL* (6 w) | ΔL* (12 m) | Δa* (6 w) | Δa* (12 m) | Δb* (6 w) | Δb* (12 m) | ΔE* (6 w) | ΔE* (12 m) |
|---|---|---|---|---|---|---|---|---|---|
| SM | KSE 300 | 4.51 | 6.62 | 0.87 | 0.45 | 5.30 | 4.53 | 7.01 | 8.03 |
| NC-25C | 0.87 | 2.71 | 1.29 | 0.92 | 5.15 | 3.98 | 5.38 | 4.90 | |
| NC-27CP | −1.41 | 0.44 | 2.10 | 1.51 | 7.31 | 6.15 | 7.74 | 6.35 | |
| S | KSE 300 | −5.49 | 2.46 | 1.10 | 0.81 | 5.80 | 3.84 | 8.06 | 4.63 |
| NC-25C | 0.30 | 2.77 | 0.76 | 1.39 | 2.86 | 5.04 | 2.97 | 5.92 | |
| NC-27CP | −2.57 | 1.63 | 0.61 | 0.75 | 0.76 | 0.66 | 2.75 | 1.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, M.; Mascha, E.; Weber, J.; Rohatsch, A.; Delgado Rodrigues, J. Efficiency and Compatibility of Selected Alkoxysilanes on Porous Carbonate and Silicate Stones. Materials 2019, 12, 156. https://doi.org/10.3390/ma12010156
Ban M, Mascha E, Weber J, Rohatsch A, Delgado Rodrigues J. Efficiency and Compatibility of Selected Alkoxysilanes on Porous Carbonate and Silicate Stones. Materials. 2019; 12(1):156. https://doi.org/10.3390/ma12010156
Chicago/Turabian StyleBan, Matea, Elisabeth Mascha, Johannes Weber, Andreas Rohatsch, and José Delgado Rodrigues. 2019. "Efficiency and Compatibility of Selected Alkoxysilanes on Porous Carbonate and Silicate Stones" Materials 12, no. 1: 156. https://doi.org/10.3390/ma12010156





