Calculation of the Intermetallic Layer Thickness in Cold Metal Transfer Welding of Aluminum to Steel
Abstract
:1. Introduction
2. Experimental Methods
3. Numerical Methods
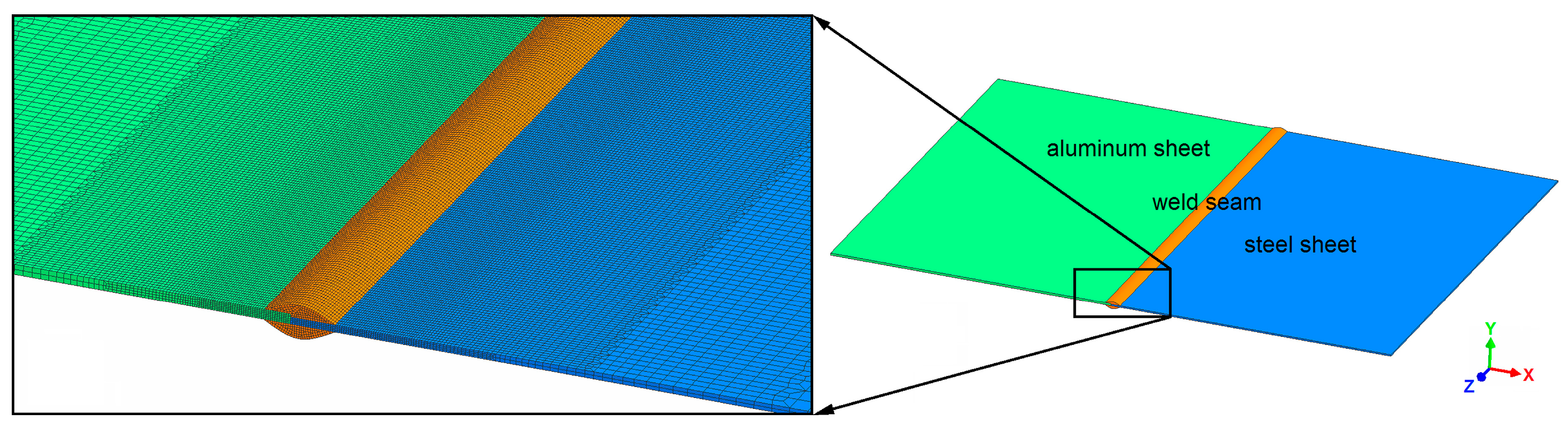
3.1. Geometry and Mesh
3.2. Material Properties
3.3. Process Definition and Boundary Conditions
3.4. Calculation of the IM Layer Thickness
4. Results and Discussion
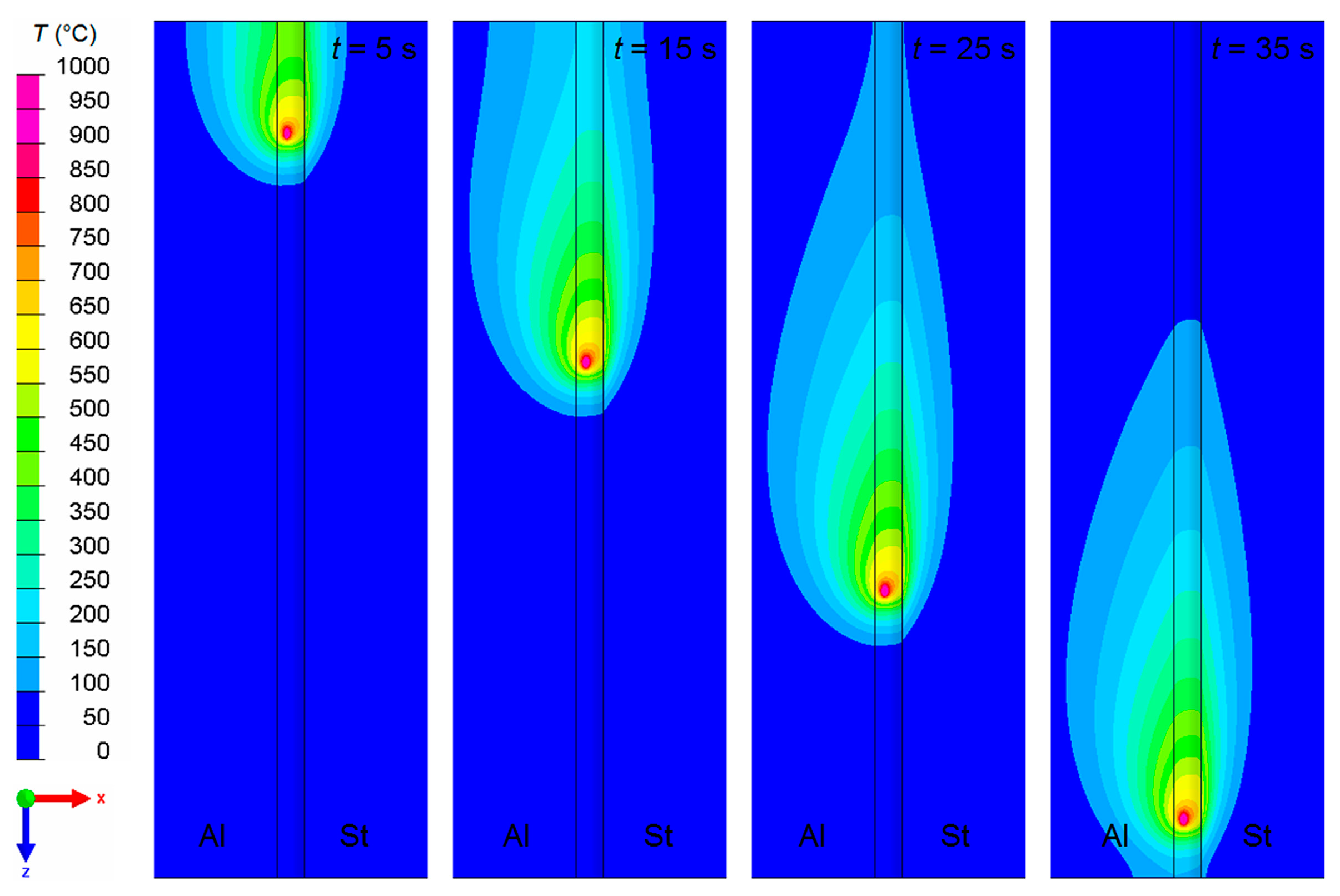
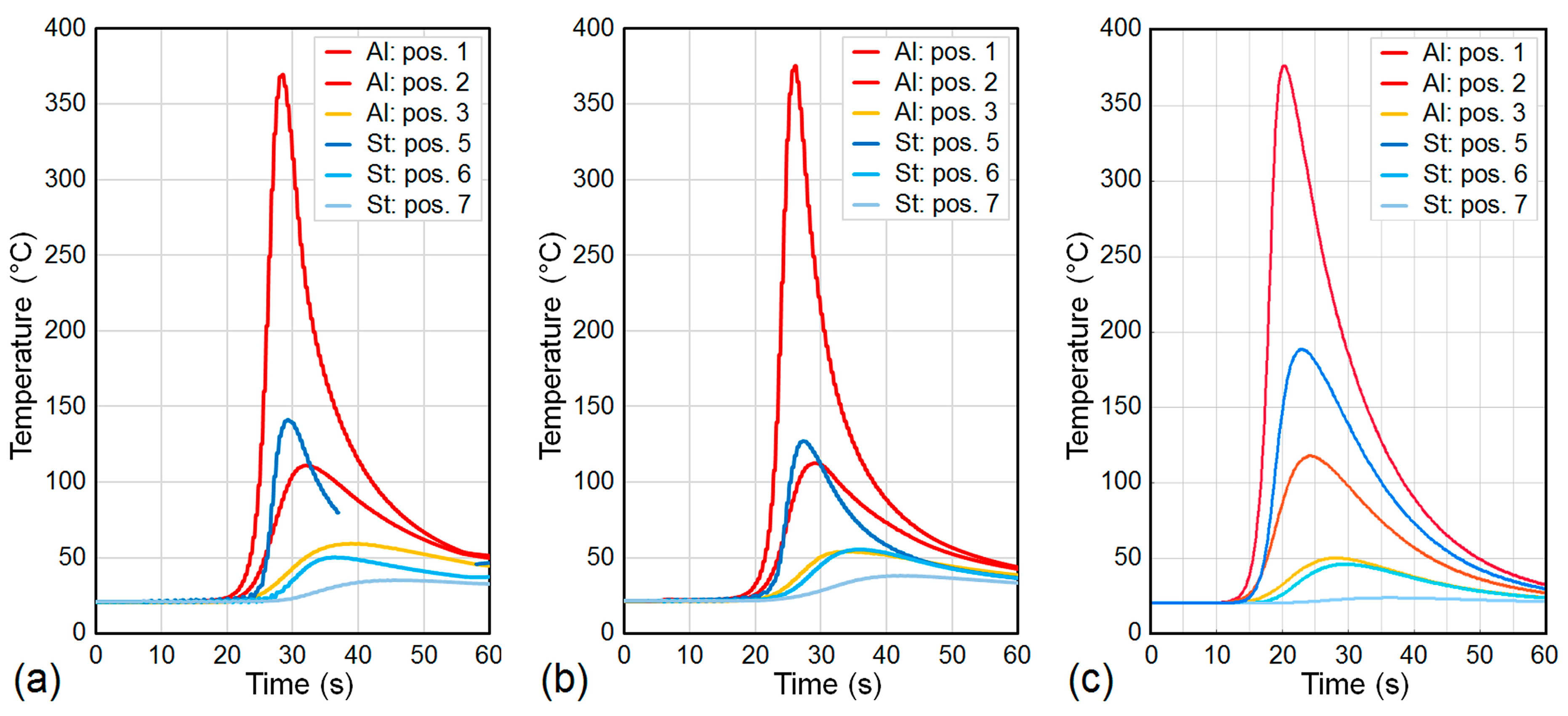
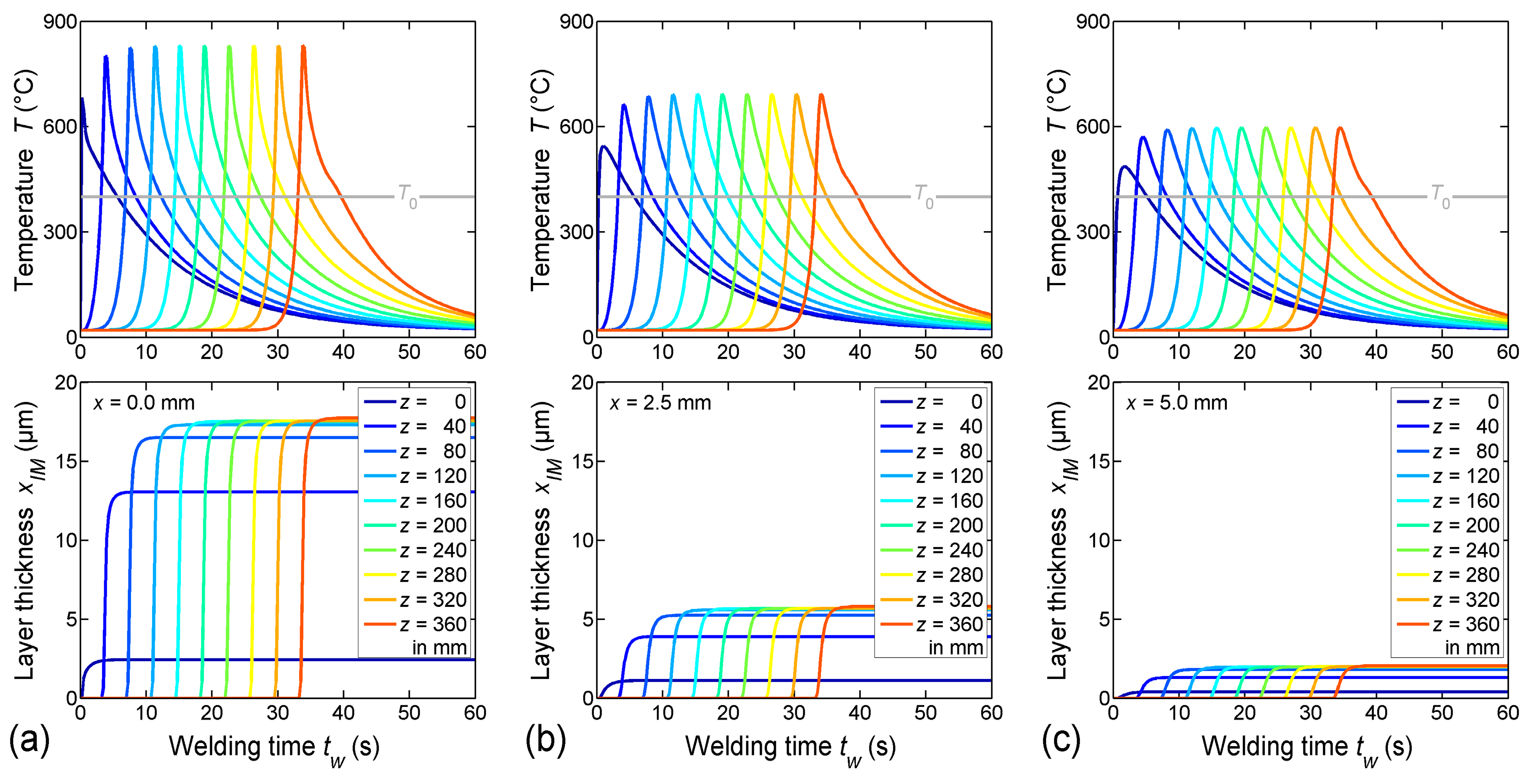
4.1. Temperature Field
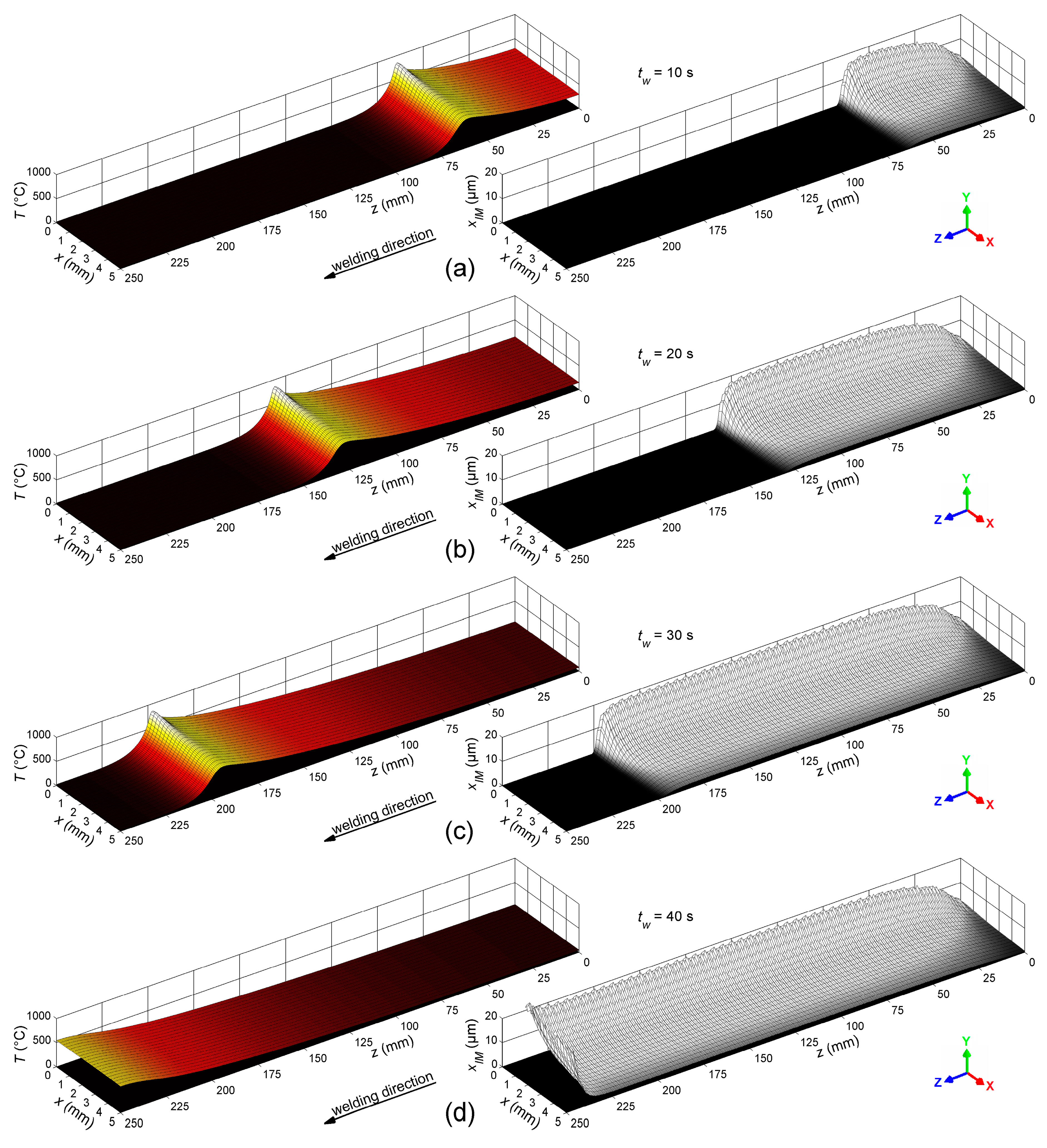
4.2. Thickness of the Intermetallic Layer
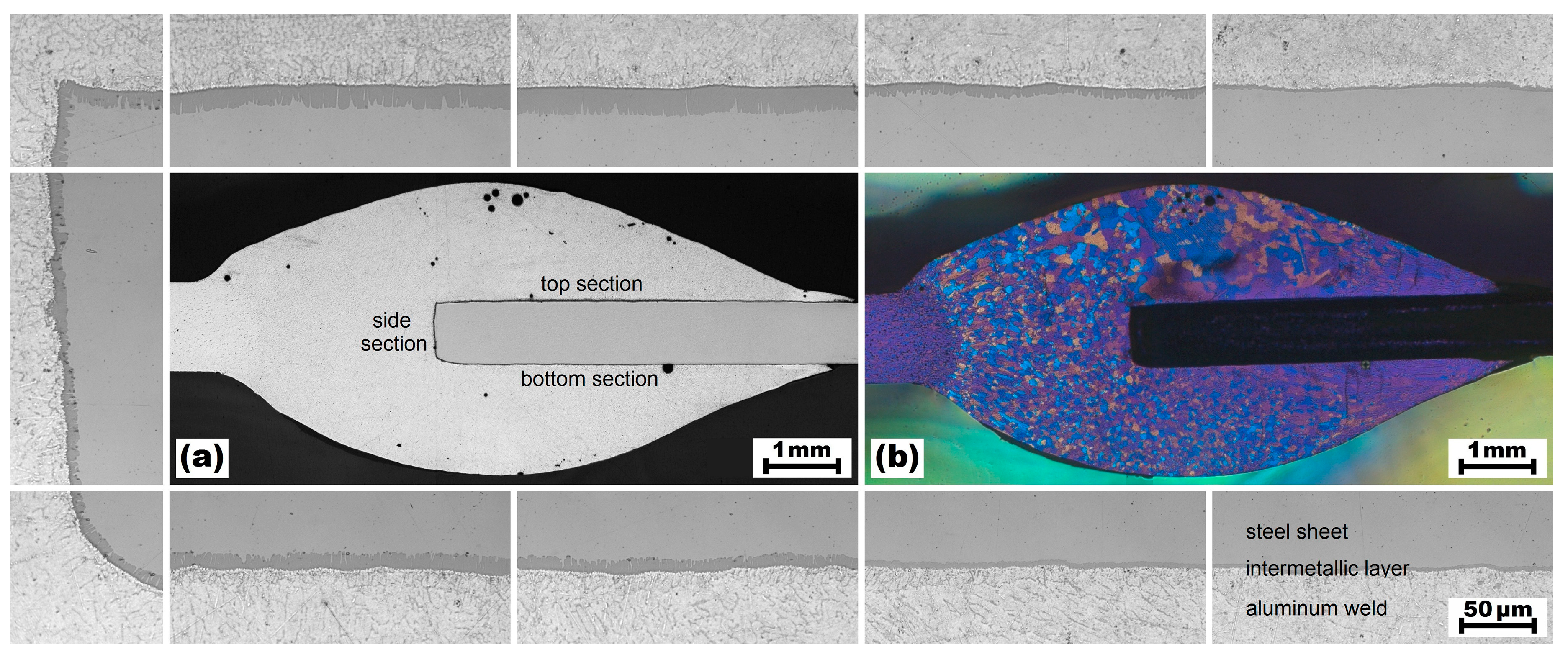
4.3. Experimental Validation
5. Conclusions
- (1)
- Due to the steep temperature gradient perpendicular to the welding direction, the thickness of the IM layer varies distinctly. A thickness of about 17 µm was calculated directly at the weld butt, where the peak temperature was about 800 °C, but a thickness of less than 2 µm was calculated at the base corner of the weld seam, where the peak temperature was about 600 °C. These results corresponded well with experimental microstructure investigations.
- (2)
- Excessive formation of the layer was observed at the end zone of the weld seam, where a thermal hot spot occurred. Hence, this region of the weld seam was identified to be critical regarding brittleness of the joint. In comparison, suppressed formation of the layer was observed at the start zone of the weld seam, where the molten filler alloy is deposited onto the initially cold metal sheets. Hence, this weld seam region was identified to be critical regarding insufficient bonding.
- (3)
- In order to obtain a more constant layer thickness, increasing the heat input at the start position of the welding trajectory and decreasing the heat input at the end position of the welding trajectory is advisable. For this reason, automated in-line control of main process parameters, in particular of welding current and welding voltage, is required.
- (4)
- The presented numerical method is based on the temperature field occurring directly at the aluminum-steel interface where the IM layer forms. Thus, the exact experimental or numerical determination of this temperature field is crucial for the subsequent calculation of the layer thickness.
- (5)
- This method represents an engineering approach which is of practical interest for designing dissimilar aluminum-steel weldments, because it enables estimation and/or validation of the basic growth parameters (i.e., and ) required for calculating the thickness of the IM layer even for weldments with complex shapes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Achar, D.R.G.; Ruge, J.; Sundaresan, S. Verbinden von Aluminium mit Stahl, besonders durch Schweißen (I), (II), (III). Aluminium 1980, 56, 147–293. [Google Scholar]
- Atabaki, M.M.; Nikodinovski, M.; Chenier, P.; Ma, J.; Harooni, M.; Kovacevic, R. Welding of aluminum alloys to steels: An overview. J. Manuf. Sci. Prod. 2014, 14, 59–78. [Google Scholar] [CrossRef]
- Jank, N.; Staufer, H.; Bruckner, J. Schweißverbindungen von Stahl mit Aluminium-eine Perspektive für die Zukunft. BHM 2008, 153, 189–192. [Google Scholar] [CrossRef]
- Gebhardt, E.; Obrowski, W. Reaktion von festem Eisen mit Schmelzen aus Aluminium und Aluminium-legierungen. Z. Metallk. 1953, 44, 154–160. [Google Scholar]
- Heumann, T.; Dittrich, S. Über die Kinetik der Reaktion von festem und flüssigem Aluminium mit Eisen. Z. Metallk. 1959, 50, 617–625. [Google Scholar]
- Yeremenko, V.N.; Natanzon, Y.V.; Dybkov, V.I. The effect of dissolution on the growth of the Fe2Al5 inter-layer in the solid iron-liquid aluminium system. J. Mater. Sci. 1981, 16, 1748–1756. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Denner, S.G.; Jones, R.D. Kinetic interactions between aluminumliquid and iron/steelsolid for conditions applicable to hot-dip aluminizing. Met. Technol. 1977, 4, 167–174. [Google Scholar] [CrossRef]
- Eggeler, G.; Vogel, H.; Friedrich, J.; Kaesche, H. Target preparation for the transmission electron micro-scopic identification of the Al3Fe (θ-Phase) in hot-dip aluminised low alloyed steel. Prakt. Metallogr. 1985, 22, 163–170. [Google Scholar]
- Eggeler, G.; Auer, W.; Kaesche, H. On the influence of silicon on the growth of the alloy layer during hot dip aluminizing. J. Mater. Sci. 1986, 21, 3348–3350. [Google Scholar] [CrossRef]
- Eggeler, G.; Auer, W.; Kaesche, H. Reactions between low alloyed steel and initially pure as well as iron-saturated aluminium melts between 670 and 800 °C. Z. Metallk. 1986, 77, 239–244. [Google Scholar]
- Dybkov, V.I. Interaction of 18Cr-10Ni stainless steel with liquid aluminium. J. Mater. Sci. 1990, 25, 3615–3633. [Google Scholar] [CrossRef]
- Bahadur, A.; Mohanty, O.N. Structural studies of hot dip aluminized coatings on mild steel. Mater. Trans. JIM 1991, 32, 1053–1061. [Google Scholar] [CrossRef]
- Bahadur, A.; Mohanty, O.N. Aluminium diffusion coatings on medium carbon steel. Mater. Trans. JIM 1995, 36, 1170–1175. [Google Scholar] [CrossRef]
- Shyam, A.; Suwas, S.; Bhargava, S. Microstructural features of iron aluminides formed by the reaction between solid iron and liquid aluminium. Prakt. Metallogr. 1997, 34, 264–277. [Google Scholar]
- Shin, D.; Lee, J.-Y.; Heo, H.; Kang, C.-Y. Formation procedure of reaction phases in Al hot dipping process of steel. Metals 2018, 8, 820. [Google Scholar] [CrossRef]
- Bouayad, A.; Gerometta, C.; Belkebir, A.; Ambari, A. Kinetic interactions between solid iron and molten aluminium. Mater. Sci. Eng. A 2003, 363, 53–61. [Google Scholar] [CrossRef]
- Bouché, K.; Barbier, F.; Coulet, A. Intermetallic compound layer growth between solid iron and molten aluminium. Mater. Sci. Eng. A 1998, 249, 167–175. [Google Scholar] [CrossRef]
- Shahverdi, H.R.; Ghomashchi, M.R.; Shabestari, S.; Hejazi, J. Microstructural analysis of interfacial reaction between molten aluminium and solid iron. J. Mater. Process. Technol. 2002, 124, 345–352. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Growth of intermetallic layer in the aluminide mild steel during hot-dipping. Surf. Coat. Technol. 2009, 204, 824–828. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Study of microstructure and phase evolution of hot-dipped aluminide mild steel during high-temperature diffusion using electron backscatter diffraction. Appl. Surf. Sci. 2011, 257, 4663–4668. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajihara, M. Morphology of compounds formed by isothermal reactive diffusion between solid Fe and liquid Al. Mater. Trans. 2009, 50, 2212–2220. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajihara, M. Kinetics of isothermal reactive diffusion between solid Fe and liquid Al. J. Mater. Sci. 2010, 45, 5676–5684. [Google Scholar] [CrossRef]
- Pasche, G.; Scheel, M.; Schäublin, R.; Hébert, C.; Rappaz, M.; Hessler-Wyser, A. Time-resolved X-Ray microtomography observation of intermetallic formation between solid Fe and liquid Al. Metallogr. Mater. Trans. A 2013, 44, 4119–4123. [Google Scholar] [CrossRef]
- Van Alboom, A.; Lemmens, B.; Breitbach, B.; De Grave, E.; Cottenier, S.; Verbeken, K. Multi-method identification and characterization of the intermetallic surface layers of hot-dip Al-coated steel: FeAl3 or Fe4Al13 and Fe2Al5 or Fe2Al5 + x. Surf. Coat. Technol. 2017, 324, 419–428. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Crystallography of Fe2Al5 phase at the interface between solid Fe and liquid Al. Intermetallics 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Morphology and formation of Fe-Al intermetallic layers on iron hot-dipped in Al-Mg-Si alloy melt. Intermetallics 2014, 54, 136–142. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Growth of Fe2Al5 phase on pure iron hot-dipped in Al-Mg-Si alloy melt with Fe in solution. ISIJ Int. 2015, 55, 1454–1459. [Google Scholar] [CrossRef]
- Yin, F.; Zhao, M.; Liu, Y.; Han, W.; Li, Z. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron and molten aluminum. Trans. Nonferrous Met. Soc. China 2013, 23, 556–561. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Lemmens, B.; Springer, H.; Duarte, M.J.; De Graeve, I.; De Strycker, J.; Raabe, D.; Verbeken, K. Atom probe tomography of intermetallic phases and interfaces formed in dissimilar joining between Al alloys and steel. Mater. Charact. 2016, 120, 268–272. [Google Scholar] [CrossRef]
- Lemmens, B.; Springer, H.; De Graeve, I.; De Strycker, J.; Raabe, D.; Verbeken, K. Effect of silicon on the microstructure and growth kinetics of intermetallic phases formed during hot-dip aluminizing of ferritic steel. Surf. Coat. Technol. 2017, 319, 104–109. [Google Scholar] [CrossRef]
- Dangi, B.; Brown, T.W.; Kulkarni, K.N. Effect of silicon, manganese and nickel present in iron on the inter-metallic growth at iron-aluminum alloy interface. J. Alloys Compd. 2018, 769, 777–787. [Google Scholar] [CrossRef]
- Springer, H.; Szczepaniak, A.; Raabe, D. On the role of zinc on the formation and growth of intermetallic phases during interdiffusion between steel and aluminium alloys. Acta Mater. 2015, 96, 203–211. [Google Scholar] [CrossRef]
- Agudo, L.; Eyidi, D.; Schmaranzer, C.H.; Arenholz, E.; Jank, N.; Bruckner, J.; Pyzalla, A.R. Intermetallic FexAly-phases in a steel/Al-alloy fusion weld. J. Mater. Sci. 2007, 42, 4205–4214. [Google Scholar] [CrossRef]
- Agudo, L.; Weber, S.; Pinto, H.; Arenholz, E.; Wagner, J.; Hackl, H.; Bruckner, J.; Pyzalla, A. Study of microstructure and residual stresses in dissimilar Al/Steels welds produced by cold metal transfer. Mater. Sci. Forum 2008, 571, 347–353. [Google Scholar] [CrossRef]
- Agudo, L.; Jank, N.; Wagner, J.; Weber, S.; Schmaranzer, C.; Arenholz, E.; Bruckner, J.; Hackl, H.; Pyzalla, A. Investigation of microstructure and mechanical properties of steel-aluminium joints produced by metal arc joining. Steel Res. Int. 2008, 79, 530–535. [Google Scholar] [CrossRef]
- Agudo Jácome, L.; Weber, S.; Leitner, A.; Arenholz, E.; Bruckner, J.; Hackl, H.; Pyzalla, A.R. Influence of filler composition on the microstructure and mechanical properties of steel-aluminum joints produced by metal arc joining. Adv. Eng. Mater. 2009, 11, 350–358. [Google Scholar] [CrossRef]
- Zhang, H.T.; Feng, J.C.; He, P.; Hackl, H. Interfacial microstructure and mechanical properties of aluminium-zinc-coated steel joints made by a modified metal inert gas welding-brazing process. Mater. Charact. 2007, 58, 588–592. [Google Scholar] [CrossRef]
- Zhang, H.T.; Feng, J.C.; He, P. Interfacial phenomena of cold metal transfer (CMT) welding of zinc coated steel and wrought aluminium. Mater. Sci. Technol. 2008, 24, 1346–1349. [Google Scholar] [CrossRef]
- Madhavan, S.; Kamaraj, M.; Vijayaraghavan, L. Microstructure and mechanical properties of cold metal transfer welded aluminium/dual phase steel. Sci. Technol. Weld. Joi. 2016, 21, 194–200. [Google Scholar] [CrossRef]
- Madhavan, S.; Kamaraj, M.; Vijayaraghavan, L.; Srinivasa Rao, K. Microstructure and mechanical proper- ties of aluminium/steel dissimilar weldments: Effect of heat input. Mater. Sci. Technol. 2017, 33, 200–209. [Google Scholar] [CrossRef]
- Cao, R.; Yu, G.; Chen, J.H.; Wang, P.-C. Cold metal transfer joining aluminum alloys-to-galvanized mild steel. J. Mater. Process. Technol. 2013, 213, 1753–1763. [Google Scholar] [CrossRef]
- Ünel, E.; Taban, E. Properties and optimization of dissimilar aluminum steel CMT welds. Weld. World 2017, 61, 1–9. [Google Scholar] [CrossRef]
- Silvayeh, Z.; Vallant, R.; Sommitsch, C.; Götzinger, B.; Karner, W. Experimental investigations on single-sided CMT welding of hybrid aluminum-steel blanks. In Proceedings of the 10th International Conference on Trends in Welding Research, Tokyo, Japan, 11–14 October 2016. [Google Scholar]
- Silvayeh, Z.; Vallant, R.; Sommitsch, C.; Götzinger, B.; Karner, W.; Hartmann, M. Influence of filler alloy composition and process parameters on the intermetallic layer thickness in single-sided cold metal transfer welding of aluminum-steel blanks. Metall. Mater. Trans. A 2017, 48, 5376–5386. [Google Scholar] [CrossRef]
- Bruckner, J. Cold Metal Transfer Has a Future Joining Steel to Aluminum. Weld. J. 2005, 84, 38–40. [Google Scholar]
- Selvi, S.; Vishvaksenan, A.; Rajasekar, E. Cold metal transfer (CMT) technology—An overview. Def. Technol. 2018, 14, 28–44. [Google Scholar] [CrossRef]
- Götzinger, B.; Karner, W.; Hartmann, M.; Silvayeh, Z. Optimised process for steel-aluminium welding. Lightweight Des. Worldw. 2017, 10, 42–46. [Google Scholar] [CrossRef]
- Akdeniz, M.V.; Mekhrabov, A.O.; Yilmaz, T. The role of Si addition on the interfacial interaction in Fe-Al diffusion layer. Scr. Metal. Mater. 1994, 31, 1723–1728. [Google Scholar] [CrossRef]
- Akdeniz, M.V.; Mekhrabov, A.O. The effect of substitutional impurities on the evolution of Fe-Al diffusion layer. Acta Mater. 1998, 46, 1185–1192. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Song, J.-H.; Kim, Y.-S. Effects of carbon content of carbon steel on its dissolution into a molten aluminum alloy. Mater. Sci. Eng. A 2005, 390, 437–443. [Google Scholar] [CrossRef]
- Sasaki, T.; Yakou, T.; Mochiduki, K.; Ichinose, K. Effects of carbon contents in steels on alloy layer growth during hot-dip aluminum coating. ISIJ Int. 2005, 45, 1887–1892. [Google Scholar] [CrossRef]
- Shibata, K.; Morozumi, S.; Koda, S. Formation of the alloy layer in the Fe-Al diffusion couple. J. Japan Inst. Met. 1966, 30, 382–388. [Google Scholar] [CrossRef]
- Jindal, V.; Srivastava, V.C.; Das, A.; Ghosh, R.N. Reactive diffusion in the roll bonded iron-aluminum system. Mater. Lett. 2006, 60, 1758–1761. [Google Scholar] [CrossRef]
- Kajihara, M. Quantitative Evaluation of Interdiffusion in Fe2Al5 during reactive diffusion in the binary Fe-Al system. Mater. Trans. 2006, 47, 1480–1484. [Google Scholar] [CrossRef]
- Naoi, D.; Kajihara, M. Growth behavior of Fe2Al5 during reactive diffusion between Fe and Al at solid-state temperatures. Mater. Sci. Eng. A 2007, 459, 375–382. [Google Scholar] [CrossRef]
- Zhe, M.; Dezellus, O.; Gardiola, B.; Braccini, M.; Viala, J.C. Chemical changes at the interface between low carbon steel and an Al-Si alloy during solution heat treatment. J. Phase Equil. Diffus. 2011, 32, 486–497. [Google Scholar] [CrossRef]
- Xu, L.; Robson, J.D.; Wang, L.; Prangnell, P.B. The influence of grain structure on intermetallic compound layer growth rates in Fe-Al dissimilar welds. Metall. Mater. Trans. A 2018, 49, 515–526. [Google Scholar] [CrossRef]
- Rong, J.; Kang, Z.; Chen, S.; Yang, D.; Huang, J.; Yang, J. Growth kinetics and thickness prediction of interfacial intermetallic compounds between solid steel and molten aluminum based on thermophysical simulation in a few seconds. Mater. Charact. 2017, 132, 413–421. [Google Scholar] [CrossRef]
- Das, A.; Shome, M.; Goecke, S.-F.; De, A. Numerical modelling of gas metal arc joining of aluminium alloy and galvanised steels in lap joint configuration. Sci. Technol. Weld. Joi. 2016, 21, 303–309. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, M.J.; Shi, Y.; Huang, J.; Yang, F. Analysis and modeling of the growth of intermetallic compounds in aluminum-steel joints. RSC Adv. 2017, 7, 37797–37805. [Google Scholar] [CrossRef]
- Silvayeh, Z.; Vallant, R.; Sommitsch, C.; Götzinger, B.; Karner, W.; Hartmann, M. Numerical estimation of the intermetallic layer thickness in aluminum-steel welding. In Proceedings of the European Congress on Advanced Materials and Processes (EUROMAT 2017), Thessaloniki, Greece, 17–22 September 2017. [Google Scholar]
- Borrisutthekul, R.; Yachi, T.; Miyashita, Y.; Mutoh, Y. Suppression of intermetallic reaction layer formation by controlling heat flow in dissimilar joining of steel and aluminum alloy. Mater. Sci. Eng. A 2007, 467, 108–113. [Google Scholar] [CrossRef]
- Mezrag, B.; Deschaux Beaume, F.; Rouquette, S.; Benachour, M. Indirect approaches for estimating the efficiency of the cold metal transfer welding process. Sci. Technol. Weld. Join. 2018, 23, 508–519. [Google Scholar] [CrossRef]
- Haelsig, A.; Kusch, M.; Mayr, P. New findings on the efficiency of gas shielded arc welding. Weld. World 2012, 56, 98–104. [Google Scholar] [CrossRef]
- Pépe, N.; Egerland, S.; Colegrove, P.A.; Yapp, D.; Leonhartsberger, A.; Scotti, A. Measuring the process efficiency of controlled gas metal arc welding processes. Sci. Technol. Weld. Joi. 2011, 16, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Goldak, J.A.; Chakravarti, A.; Bibby, M. A New Finite Element Model for Welding Heat Sources. Metall. Trans. B 1984, 15, 200–305. [Google Scholar] [CrossRef]
- Goldak, J.A.; Akhiaghi, M. Computational Welding Mechanics; Springer: New York, NY, USA, 2005; pp. 30–33. [Google Scholar]




| Ref. | Researcher | T (°C) | Material Combination | Q (kJ/mol) | k0 (m2/s) |
|---|---|---|---|---|---|
| [5] | Heumann and Dittrich | 700–960 | pure Fe (s) pure Al (l) | 55 | - |
| [8] | Denner and Jones | 673–826 | 0.05 wt % C steel (s) pure Al (l) | 170 | - |
| 0.17 wt % C steel (s) pure Al (l) | 195 | - | |||
| [11] | Eggeler, Auer and Kaesche | 670–800 | low C steel (s) pure Al (l) | 134 * 155 ** | - |
| low C steel (s) Fe-saturated Al (l) | 87 * 104 ** | - | |||
| [17] | Bouayad, Gerometta, Belkebir and Ambari | 700–900 | pure Fe (s) pure Al (l) | 73 * 74 ** | - |
| [30] | Springer, Kostka, Payton, Raabe, Kaysser-Pyzalla and Eggeler | 600–675 | 0.08 wt % C steel (s) pure Al (s,l) | 190 | - |
| 0.08 wt % C steel (s) Al + 5 wt % Si (s,l) | 17 | - | |||
| [34] | Springer, Szczepaniak and Raabe, includes data from [4,30,54,57] | 400–750 | 0.08 wt % C steel (s) pure Al (s,l) | 190 | - |
| 0.08 wt % C steel (s) Al + 2.5 wt % Zn (s,l) | 165 | - | |||
| [32] | Lemmens, Springer, De Graeve, De Strycker, Raabe and Verbeken | 670–725 | 0.01 wt % C steel (s) pure Al (l) | 224 | - |
| 0.01 wt % C steel (s) Al + 1 wt % Si (l) | 142 | - | |||
| 0.01 wt % C steel (s) Al + 3 wt % Si (l) | 149 | - | |||
| 0.01 wt % C steel (s) Al + 10 wt % Si (l) | -72 | - | |||
| [23] | Tanaka and Kajihara | 780–820 | pure Fe (s) pure Al (l) | 248 | 1.26 × 102 |
| [29] | Yin, Zhao, Liu, Han and Li | 700–800 | pure Fe (s) pure Al (l) | 207 | 1.10 |
| pure Fe (s) Al + 1 wt % Si (l) | 169 | 3.68 × 10−3 | |||
| pure Fe (s) Al + 2 wt % Si (l) | 167 | 1.46 × 10−3 | |||
| pure Fe (s) Al + 3 wt % Si (l) | 172 | 1.38 × 10−3 | |||
| [54] | Shibata, Morozumi and Koda | 605–655 | pure Fe (s) pure Al (s) | 226 | - |
| [55] | Jindal, Srivastava, Das and Ghosh | 500–600 | IF steel (s) pure Al (s) | 85 | 3.82 × 10−8 |
| [56] | Kajihara | 550–640 | pure Fe (s) pure Al (s) | 281 | 1.32 × 102 |
| [57] | Naoi and Kajihara | ||||
| [58] | Zhe, Dezellus, Gardiola, Braccini and Viala | 535 | 0.03 wt % C steel (s) Al + 7 wt % Si (s) | 153 | 4.37 × 10−4 |
| [59] | Xu, Robson, Wang and Prangnell | 400–480 | 0.08 wt % C steel (s) Al + 0.8 wt % Si (s) | 116 | - |
| 480–570 | 248 | - | |||
| 400–570 | 160 | - |
| Parameter | Symbol | Value |
|---|---|---|
| Welding current (mean value) | 71 A | |
| Welding voltage (mean value) | 8.1 V | |
| Welding speed | 0.4 m/min | |
| Feeding rate of the filler wire | 3.9 m/min | |
| Diameter of the filler wire | 1.2 mm | |
| Distance between the torch and the workpiece | 6 mm | |
| Angle between the torch and the workpiece | 90° | |
| Flow rate of the argon shielding gas | 12 l/min |
| Material | Al | Fe | Mg | Mn | Si | Cu | Zn | Ti | Cr | V | C | P | S | Zr | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild steel sheet DC04 (1.0338) | - | bal. | - | max. 0.4 | - | - | - | - | - | - | max. 0.08 | max. 0.03 | max. 0.03 | - | - |
| Aluminum alloy sheet EN AW-6014 | bal. | max. 0.35 | 0.4–0.8 | 0.05–0.2 | 0.3–0.6 | max. 0.25 | max. 0.1 | max. 0.1 | max. 0.2 | 0.05–0.2 | - | - | - | - | - |
| Aluminum filler wire Al-0.3Mg-0.5Sc-0.4Zr | bal. | 0.02–0.04 | 0.2–0.3 | 0.03–0.05 | 0.03–0.05 | - | - | - | - | - | - | - | - | 0.3–0.5 | 0.4–0.6 |
| Parameter | Symbol | Value |
|---|---|---|
| Nominal energy input | 86.3 J/mm | |
| Efficiency of the welding process | 1 | |
| Width of the weld pool | 2 | 2.0 mm |
| Penetration depth of the weld pool | 1.5 mm | |
| Length of the weld pool | + | 3.0 mm |
| Duration of the welding process | 37.5 s | |
| Duration of the post-welding cooling period | 22.5 s | |
| Time increment at time step | 0.25 s | |
| Ambient temperature (= initial sheet temperature) | 20 °C | |
| Stefan-Boltzmann constant | 5.67 × 10−8 W/m2K4 | |
| Thermal emission coefficient | 1 | |
| Convective heat transfer coefficient at the weld seam | 10 W/m2K | |
| Conductive heat transfer coefficient at the metal sheets | 200 W/m2K |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvayeh, Z.; Götzinger, B.; Karner, W.; Hartmann, M.; Sommitsch, C. Calculation of the Intermetallic Layer Thickness in Cold Metal Transfer Welding of Aluminum to Steel. Materials 2019, 12, 35. https://doi.org/10.3390/ma12010035
Silvayeh Z, Götzinger B, Karner W, Hartmann M, Sommitsch C. Calculation of the Intermetallic Layer Thickness in Cold Metal Transfer Welding of Aluminum to Steel. Materials. 2019; 12(1):35. https://doi.org/10.3390/ma12010035
Chicago/Turabian StyleSilvayeh, Zahra, Bruno Götzinger, Werner Karner, Matthias Hartmann, and Christof Sommitsch. 2019. "Calculation of the Intermetallic Layer Thickness in Cold Metal Transfer Welding of Aluminum to Steel" Materials 12, no. 1: 35. https://doi.org/10.3390/ma12010035





